Abstract
Background
Musculoskeletal pain is a societal epidemic because it is highly prevalent and a leading contributor to disability; however, physical therapists are still challenged when predicting which patients are at high risk for persistent symptoms.
Objective
The objectives of this study were to identify patient characteristics predictive of persistent musculoskeletal pain 12 months following physical therapist care and to determine the influence of anatomical region.
Design
The design included a secondary analysis of a cohort study.
Methods
Participants ranged in age from 18 to 65 years, had a primary report of knee, shoulder, back, or neck pain, were receiving physical therapy, and were enrolled in the Orthopedic Physical Therapy Investigative Network (OPT-IN) Optimal Screening for Prediction of Referral and Outcome (OSPRO) validation cohort study. Candidate predictor variables included demographic and clinical characteristics, comorbidities, and OSPRO Review of Systems (OSPRO-ROS) and OSPRO Yellow Flag (OSPRO-YF) tool scores. Persistent musculoskeletal pain was assessed by self-report responses to questions on the duration of pain and activity limitation. Logistic regression was used for completed cases to identify predictors of persistent pain at 12 months in full and parsimonious models.
Results
Follow-up assessment at 12 months was performed for 63.4% of participants (279/440). Participants with persistent pain at 12 months (n = 101; 36.2%) had more comorbidities, higher numerical pain rating scale scores, and higher OSPRO-ROS and OSPRO-YF tool scores at baseline than those without persistent pain, and the findings were independent of anatomical region. The number of comorbidities (odds ratio [OR] range = 0.30–0.46), numerical pain rating scale scores (OR at baseline = 1.44–1.75; OR at 4 weeks = 1.37–1.39), and OSPRO-ROS (plus additional items) scores (OR = 1.33–1.54) were predictors in full and parsimonious models.
Limitations
Convenience sampling was used, with a follow-up rate at 12 months (63.4%) that was lower than anticipated, and an operational definition for chronic low back pain was applied to persistent musculoskeletal pain in other body regions.
Conclusions
The OSPRO-ROS tool may be used to improve the prediction of persistent musculoskeletal pain at 12 months in conjunction with comorbidities and pain intensity (baseline and 4 weeks). These are potentially important findings because persistent pain was not commonly evaluated in previous screening studies; however, it is a relevant outcome in an era of front-line nonpharmacological pain management.
Musculoskeletal pain conditions are highly prevalent and rank among the leading causes of years lived with disability worldwide.1 In the United States, chronic musculoskeletal pain is an epidemic because it is highly prevalent, with indications of increasing prevalence and is a leading contributor to disability.1,2 However, physical therapists are still challenged when predicting which patients with musculoskeletal pain are at high risk for persistent symptoms.
Previous prognostic research has primarily focused on pain and functional outcomes. For example, a recent systematic review of 78 studies to identify generic prognostic indicators across a wide range of musculoskeletal pain conditions in primary care settings predominantly consisted of studies reporting on physical function (n = 37) or pain severity (n = 23) outcome measures.3 Interestingly, persistence of symptoms was not commonly evaluated (n = 11; 14.1%) as an outcome domain in the studies included in this review.3 As a result, there is a knowledge gap in the ability to predict which patients with musculoskeletal pain conditions may be at high risk for persistent pain.
Accurate prediction of persistent musculoskeletal pain is challenging for a variety of reasons. In population-based research, extensive heterogeneity in how persistent pain is defined has been implicated as a reason for variability in reported prevalence estimates.4 Frequently, symptom duration based on single temporal thresholds (eg, >3 months) are used to operationally define chronicity; however, such methodology does not alone provide an indication of chronic pain.5–8 To account for these limitations, the National Institutes of Health Pain Consortium Task Force has provided recommendations specifically for chronic low back pain that distinguish between episodic and ongoing symptoms by asking questions to assess the duration of symptoms over time and the duration of persistent, ongoing symptoms over consecutive days.9 In addition, the Institute of Medicine's Interagency Pain Research Coordinating Committee has recommended a 2-tiered approach in the National Pain Strategy consisting of screening for chronic pain based on duration and severity followed by assessment of participation restrictions to identify high-impact chronic pain.10 These definitions have been proposed for low back pain but also provide parameters applicable for other musculoskeletal pain conditions.11
Recent clinical practice guidelines from the American College of Physicians and Centers for Disease Control and Prevention recommend nonpharmacologic interventions as front-line treatment options for acute and chronic pain conditions.12,13 Health care providers (including physical therapists) will be responsible for accurately predicting responders to nonpharmacologic interventions delivered. From a health services research perspective, there is a need to understand what patient-level factors are predictive of persistent pain to inform the planning of future investigations evaluating the effectiveness of treatment options for nonpharmacologic care pathways. This prognostic information could also be used to inform patient and clinician preferences, improve efficiency of referral patterns to other health care providers when appropriate, and identify patients at higher risk for escalated health care utilization including unwarranted exposure to interventions associated with increased harm (eg, opioid medications). Consequently, early identification of those likely to have persistent pain provides multiple opportunities for physical therapists to improve musculoskeletal pain management strategies.
Therefore, the purpose of this analysis is to identify patient-level factors predictive of persistent musculoskeletal pain 12 months after an episode of physical therapy. Specifically, we were interested in determining whether newly developed assessment tools for pain associated psychological distress and review of systems improved prediction of persistence of pain in combination with other patient-level factors. Results from this analysis would provide information on whether these tools are useful measurement adjuncts in combination with other patient-level factors to identify those at risk for persistent pain 12 months after seeking physical therapist treatment.
Methods
This is a secondary analysis from the Optimal Screening for Prediction of Referral and Outcome (OSPRO) validation cohort.14 A convenience sample (ie, without tracking percentage eligible) was gathered between December 2014 and December 2015 with 12-month follow-up assessment occurring through January 2017 and included 9 clinical sites distributed across several different geographical regions in the United States. The primary goal from this cohort study was to determine predictive validity of the newly developed assessment tools—OSPRO Review of Systems (OSPRO-ROS) and OSPRO Yellow Flags (OSPRO-YF)—for self-report of clinical outcomes for pain, function, comorbidity change, and health care utilization. The OSPRO-ROS and OSPRO-YF tools are currently in the process of being validated for clinical outcomes and health care utilization, which will constitute the primary validation analyses. The purpose of this planned secondary analysis was to supplement the primary analyses investigating whether the newly developed OSPRO-ROS and OSPRO-YF tools improved prediction of a separate outcome of interest—persistent musculoskeletal pain. Details on the rationale, design, and methods for the cohort study are available in a prior publication that describes the methods in more details and identifies the outcomes of interest for the primary validation analyses.14
Participants
People between the ages of 18 and 65 years were eligible to participate in this study if they were seeking outpatient physical therapist treatment for musculoskeletal pain; had primary reports of pain involving the cervical spine, lumbar spine, shoulder, or knee; and were able to read and comprehend the English language (necessary because of self-report forms). People were excluded from study participation for any diagnosis indicative of widespread chronic pain syndrome (eg, fibromyalgia or irritable bowel syndrome), neuropathic pain syndrome (eg, complex regional pain syndrome or diabetic neuropathy), psychiatric history (currently in care of mental health care provider or taking ≥2 prescription psychiatric medications), cancer (currently receiving treatment for active cancer), or neurological disorder (eg, stroke, spinal cord injury, or traumatic brain injury). For this analysis, we included only people for whom complete follow-up data were available because our primary objective was to test the newly developed OSPRO-ROS and OSPRO-YF tools for predicting persistent musculoskeletal pain at 12 months.
Predictive Measures
Demographic and historical variables
Participants completed a standard intake form previously used in our clinical studies15,16 including age, sex, race, household income, education level, and geographic region. Historical data included anatomical location of the pain, previous episodes, and surgical history.
Pain-related clinical variables.
Baseline pain status was assessed on the basis of established definitions that account for the duration of pain and activity limitations17,18 using the following 2 questions: “How long have you been experiencing your current painful symptoms?” and “Have you experienced ANY pain and activity limitations every day for the past 3 months?” Open-ended responses to question 1 of “greater than 90 days” or a response to question 2 of “Yes” were then used to create a categorical variable classifying participants as having persistent pain at baseline (Yes or No). Baseline pain intensity was assessed with a numerical pain rating scale (with scores from 0 to 10), and participants rated their current pain intensity as well as their best (lowest) and worst (highest) pain intensity over the past 24 hours.19–21 The average of these 3 ratings was used to represent baseline pain intensity for this analysis.
Comorbidities
Health history was determined with the Charlson Comorbidity Index and the Functional Comorbidity Index.22,23 The Charlson Comorbidity Index lists 19 medical conditions that participants are asked to indicate whether they “have ever been diagnosed with by a physician.” Similarly, the Functional Comorbidity Index lists 18 medical conditions that participants are asked to indicate whether they “have ever been diagnosed with by a physician.” These indexes were selected because they assess different medical conditions and inclusion of both would allow for full consideration of comorbidities. A composite comorbidity count (potential range = 0–30) was derived by adding number of comorbidities reported in the Charlson Comorbidity Index to the number of unique comorbidities reported in the Functional Comorbidity Index (ie, similar comorbidities reported in both indexes were counted only once) and categorized (0, 1, or ≥2) for this analysis.
OSPRO tools
The OSPRO-ROS tool was administered at baseline.24 This measure includes standard symptom descriptors previously used to assist with identification of systemic involvement consisting of questions related to symptoms of the cardiovascular, gastrointestinal, endocrine, nervous, integumentary, pulmonary, and musculoskeletal systems. The OSPRO-ROS tool was scored by summing the positive responses, providing a potential range of 0 to 23 if all 23 items are used with higher scores indicating higher levels of systemic symptom reports.24 Predictive capacity of the short version (10 items) and additional items (13 separate items) of the OSPRO-ROS tool were evaluated separately.
The OSPRO-YF tool was administered at baseline, 4 weeks, and 6 and 12 months later.25 This measure includes items from pain vulnerability domains (negative affect and fear-avoidance) and pain resilience domains (positive affect and self-efficacy) to assist with identification of pain associated psychological distress. The OSPRO-YF tool was scored by summing all item responses from the original parent questionnaires on the original scale, with positive coping items reverse scored, providing a potential range of 6 to 89 if all 17 items are used with higher scores indicating higher levels of psychological distress.25 Predictive capacity of the short form (10 items) and predictive capacity of the additional items (7 separate items) of the OSPRO-YF tool were evaluated separately.
Outcome
Persistent musculoskeletal pain was assessed by self-report responses.9 Participants provided responses to questions on duration of pain and activity limitation in regard to the musculoskeletal pain condition they had received physical therapist services. At the 6- and 12-month follow-up assessments, the following 2 questions were asked: “How long has your musculoskeletal pain condition been an ongoing problem for you?” and “How often has your musculoskeletal pain condition been an ongoing problem for you over the past 6 months?” Responses to question 1 of “greater than or equal to 3 to 6 months” and responses to question 2 of “every day or nearly every day” or “at least half the days” were used to create a variable that classified pain as “persistent” at 6 and 12 months (Yes or No).9 The 12-month response was used as the endpoint of interest for our regression analyses, and responses to the 6-month follow-up questions allowed for the potential to distinguish between episodic and ongoing symptoms consistent with previous recommendations.
Data Analysis
Means and standard deviations were calculated for all baseline continuous variables and frequency counts with percentages were calculated for categorical variables. These baseline descriptive statistics are presented for the entire study sample comparing those classified as having and not having persistent musculoskeletal pain at the 12-month follow-up assessment. All analyses were performed at an alpha level of .05.
Predictive analysis
Our primary analysis assessed the additional variance explained by the OSPRO-ROS and OSPRO-YF tools after considering other patient-level factors for predicting persistent musculoskeletal pain at 12 months using logistic regression with completed cases as described in Table 1. This full model consisted of several separate blocks to account for all planned covariates (blocks 1 and 2) prior to considering OSPRO tools (blocks 3 and 4). After block 4, changes in OSPRO-YF tool and pain intensity scores at 4-weeks were included to investigate potential use for treatment monitoring purposes (block 5). After block 5, interaction terms for OSPRO-ROS and OSPRO-YF tool by baseline persistent pain were included to investigate influence of tools based on baseline persistent pain status (block 6). Regression diagnostics were performed to assess for multicollinearity between predictor variables in all logistic regression analyses. Predictive performance of models for discrimination (ie, ability to separate participants with and without persistent musculoskeletal pain at 12 months)26 was assessed using the area under the receiver operating characteristic curve (AUC). AUC estimates have the potential to range from 0.5 to 1.0, with 0.5 indicating that the model is no better than chance at discriminating people who have persistent musculoskeletal pain at 12 months from those who do not. For consistency with previous studies,27,28 AUC estimates were interpreted as follows: <0.6 = noninformative, 0.6 to 0.7 = (poor) discrimination, 0.7 to 0.8 = acceptable, 0.8 to 0.9 = excellent, and >0.9 = outstanding.
Table 1.
Description of and Rationale for Predictors Entered Into Logistic Regression Models for Predicting Musculoskeletal Pain Persistent for 12 Monthsa.
| Model | Predictors Entered | Rationale |
|---|---|---|
| Block 1 | Demographic | To account for relevant demographic (age, sex, race, household income, education level, and geographic region) variables. |
| Clinical | To account for relevant clinical (anatomical location of pain, previous episodes, and surgical history) variables. | |
| Comorbidityb | To account for no. of medical comorbidities. | |
| Block 2 | Baseline persistent pain | To account for pain-related clinical variables. |
| Baseline pain intensity | ||
| Block 3 | OSPRO-ROS (10 items) | To account for brief versions of OSPRO tools. |
| OSPRO-YF (10 items) | ||
| Block 4 | OSPRO-ROS + additional 13 itemsc | To account for additional items in longer versions of OSPRO tools. |
| OSPRO-YF + additional 7 itemsc | ||
| Block 5 | Pain intensity (4-wk change) | To account for changes in baseline pain intensity at 4 wk. |
| OSPRO-YF (10 items) (4-wk change) | To account for changes in OSPRO-YF tool at 4 wk. | |
| OSPRO-YF + additional 7 itemsc (4-wk change) | ||
| Block 6 | Baseline persistent pain interactions | To account for influence of OSPRO-ROS and OSPRO-YF tools on the basis of baseline persistent pain status. |
| All models reported with completed cases | ||
| Full model (with all covariates) | To determine predictive capabilities of OSPRO tools after accounting for planned covariates. | |
| Parsimonious model 1 (after block 4) | To determine best-fit model after accounting for planned covariates. | |
| Parsimonious model 2 (after block 6) | To determine best-fit model after accounting for planned covariates, change scores, and OSPRO tool × baseline persistent pain interactions. |
aOSPRO = Optimal Screening for Prediction of Referral and Outcome, OSPRO-ROS = OSPRO Review of Systems, OSPRO-YF = OSPRO Yellow Flag.
bComorbidity data included composite counts (0, 1, or ≥2) from the Charlson Comorbidity Index and the Functional Comorbidity Index. Pain intensity was measured with a numerical pain rating scale from 0 to 10.
cAdditional items were from longer versions of the corresponding tool.
Exploratory predictive analysis
Exploratory analyses identified predictors that could be used to inform future risk model development.27,29–31 Specifically, to search for simpler and more clinically applicable models that optimize predictive power with fewer predictors, we identified 2 parsimonious models using backward selection methods. Parsimonious model 1 was constructed by applying backward selection to the model that included all variables up to block 4; while parsimonious model 2 resulted from the full model including all variables to block 6. The significance level for deletion of a variable from the model was set at .05.
Role of the Funding Source
This study was funded by a 2013 Clinical Research Network grant from the Orthopaedic Section of the American Physical Therapy Association. J.M. Beneciuk received funding support from the National Institutes of Health Rehabilitation Research Career Development Program (K12-HD055929). T.A. Lentz received support from the Foundation for Physical Therapy with Promotion of Doctoral Studies (PODS) I and II Awards. The funders played no role in the conduct of this study.
Results
Participant Data
These analyses included data from 279 of 440 participants (63.4%) who completed a follow-up assessment at 12 months. Compared with participants who did not complete the 12-month follow-up assessment, those who did complete this assessment were older (mean = 46.5 [SD = 16.0] versus 43.1 [SD = 15.3] years) and had lower OSPRO-YF 10-item (16.9 [SD = 6.3] versus 18.4 [SD = 7.2]) and additional 7-item (14.3 [SD = 4.9] versus 15.9 [SD = 6.3]) tool scores (P < .05). Compared with participants who completed the follow-up assessment, those with incomplete data were more likely to be unemployed (19.3% versus 10.8%), have a household income of less than 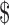 20,000 (18.0% versus 10.8%), and have less than a high school education (6.2% versus 0.4%). At baseline and the 12-month follow-up, 79.6% (222/279) and 36.2% (101/279) of participants had persistent musculoskeletal pain, respectively.
20,000 (18.0% versus 10.8%), and have less than a high school education (6.2% versus 0.4%). At baseline and the 12-month follow-up, 79.6% (222/279) and 36.2% (101/279) of participants had persistent musculoskeletal pain, respectively.
Baseline Differences
Differences in baseline characteristics for persistent musculoskeletal pain groups are provided in Table 2. Participants with persistent musculoskeletal pain at 12 months were associated with more previous episodes in the same anatomical area over the past year and had higher intake pain intensity scores (P < .05). Other intake differences for those with persistent pain included increased number of comorbidities and, higher OSPRO-ROS and OSPRO-YF scores (P < .05). No other baseline differences were observed, including comparisons across body regions for which participants were receiving physical therapist care (Tab. 2).
Table 2.
Descriptive Baseline Characteristics of Study Participants With and Those Without Persistent Musculoskeletal Pain at 12 Monthsa.
| Variable | Total Sample (n = 279) | Participants With Persistent Pain (n = 101) | Participants Without Persistent Pain (n = 178) | P |
|---|---|---|---|---|
| Demographic | ||||
| Age, y, mean (SD) | 46.5 (S) | 48.3 (15.1) | 45.4 (16.5) | .152 |
| Sex, women | 183 (65.6) | 73 (72.3) | 110 (61.8) | .173 |
| Race | .276 | |||
| American Indian/Alaska native | 2 (0.7) | 1 (1.0) | 1 (0.6) | |
| Asian | 17 (6.1) | 2 (2.0) | 15 (8.4) | |
| Black or African American | 30 (10.8) | 11 (10.9) | 19 (10.7) | |
| White | 226 (81.0) | 86 (85.1) | 140 (78.7) | |
| Prefer not to answer | 4 (1.4) | 1 (1.0) | 3 (1.7) | |
| Household income | .004 | |||
<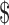 20,000 20,000 |
30 (10.8) | 20 (19.8) | 10 (5.6) | |
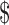 20,000– 20,000–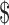 35,000 35,000 |
39 (14.0) | 16 (15.8) | 23 (12.9) | |
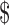 35,001– 35,001–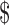 50,000 50,000 |
33 (11.8) | 8 (7.9) | 25 (14.0) | |
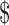 50,001– 50,001–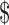 70,000 70,000 |
32 (11.5) | 12 (11.9) | 20 (11.2) | |
>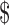 70,000 70,000 |
112 (40.1) | 32 (31.7) | 80 (44.9) | |
| Prefer not to answer | 33 (11.8) | 13 (12.9) | 20 (11.2) | |
| Employment | .055 | |||
| Employed full-time | 149 (53.4) | 48 (47.5) | 101 (56.7) | |
| Employed part-time | 41 (14.7) | 10 (9.9) | 31 (17.4) | |
| Unemployed | 30 (10.8) | 15 (14.9) | 15 (8.4) | |
| Retired | 47 (16.8) | 22 (21.8) | 25 (14.0) | |
| Prefer not to answer | 12 (4.3) | 6 (5.9) | 6 (3.4) | |
| Education | .208 | |||
| High school or lower | 18 (6.5) | 6 (5.9) | 12 (6.7) | |
| Some college | 63 (22.6) | 29 (28.7) | 34 (19.1) | |
| Graduated from college | 79 (28.3) | 32 (31.7) | 47 (26.4) | |
| Some postgraduate course work | 41 (14.7) | 14 (13.9) | 27 (15.2) | |
| Completed postgraduate degree | 74 (26.5) | 19 (18.8) | 55 (30.9) | |
| Prefer not to answer | 4 (1.4) | 1 (1.0) | 3 (1.7) | |
| Geographical region | .095 | |||
| Portland, OR | 16 (5.7) | 4 (4.0) | 12 (6.7) | |
| Los Angeles, CA | 43 (15.4) | 10 (9.9) | 33 (18.5) | |
| Greenville, SC | 58 (20.8) | 24 (23.8) | 34 (19.1) | |
| Boulder, CO | 16 (5.7) | 6 (5.9) | 10 (5.6) | |
| Jacksonville, FL | 29 (10.4) | 9 (8.9) | 20 (11.2) | |
| Gainesville, FL | 80 (28.7) | 37 (36.6) | 43 (24.2) | |
| Philadelphia, PA | 13 (4.7) | 1 (1.0) | 12 (6.7) | |
| Chicago, IL | 11 (3.9) | 5 (5.0) | 6 (3.4) | |
| Terra Haute, IN | 13 (4.7) | 5 (5.0) | 8 (4.5) | |
| Clinical | ||||
| Body region | .403 | |||
| Neck | 59 (21.1) | 23 (22.8) | 36 (20.2) | |
| Low back | 72 (25.8) | 31 (30.7) | 41 (23.0) | |
| Shoulder | 66 (23.7) | 21 (20.8) | 45 (25.3) | |
| Knee | 82 (29.4) | 26 (25.7) | 56 (31.5) | |
| Surgery, yes | 46 (16.5) | 16 (15.8) | 30 (16.9) | .827 |
| Persistent pain at baseline, yes | 222 (79.6) | 84 (83.2) | 138 (77.5) | .261 |
| Episodes over past year, yes | 143 (51.3) | 62 (61.4) | 81 (45.5) | .036 |
| Pain intensity, NPRS, mean (SD) | 3.9 (1.7) | 4.7 (1.5) | 3.4 (1.6) | <.0001 |
| Comorbidityb | <.0001 | |||
| 0 | 79 (28.3) | 17 (16.8) | 62 (34.8) | |
| 1 | 54 (19.4) | 14 (13.9) | 40 (22.5) | |
| ≥2 | 144 (51.6) | 70 (69.3) | 74 (41.6) | |
| OSPRO-ROS, 10 items, mean (SD) | 2.6 (2.3) | 3.4 (2.7) | 2.1 (1.9) | <.0001 |
| OSPRO-ROS + additional 13 items,c mean (SD) | 1.2 (1.6) | 1.8 (1.9) | 0.8 (1.2) | <.0001 |
| OSPRO-YF, 10 items, mean (SD) | 16.9 (6.3) | 19.4 (7.1) | 15.5 (5.3) | <.0001 |
| OSPRO-YF + additional 7 items,c mean (SD) | 14.3 (4.9) | 16.0 (5.3) | 13.4 (4.4) | <.0001 |
aData are reported as number (percentage) of participants unless otherwise indicated. NPRS = numerical pain rating scale (potential range = 0–10, with higher scores indicating higher pain intensity), OSPRO-ROS = Optimal Screening for Prediction of Referral and Outcome Review of Systems; OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag.
bComorbidity data included composite counts (0, 1, or ≥2) from the Charlson Comorbidity Index and the Functional Comorbidity Index.
cAdditional items were from longer versions of the corresponding tool.
Prediction of persistent musculoskeletal pain at 12 months: primary “full-model” performance
Variance explained for the full model (with all covariates) in blocks 1 through 6 increased from 0.215 to 0.382; the Nagelkerke maximum rescaled R2 increased from 0.294 to 0.521; and the AUC increased from 0.777 to 0.878. The incremental details of the above statistics are summarized in Table 3. As expected, after accounting for demographic, clinical, and comorbidity variables (block 1), the addition of baseline persistent pain status and pain intensity (block 2) provided the largest incremental change in variance explained (R2 = 0.063) for the prediction of persistent musculoskeletal pain at 12 months. The subsequent addition of baseline OSPRO-ROS and OSPRO-YF tools (10-item versions) (block 3) and additional item versions (block 4) provided less incremental change in variance explained, with R2ranging from 0.015 to 0.016. Changes in OSPRO-YF tool and pain intensity scores at 4 weeks (block 5) still contributed additional variance (R2 = 0.059); however, OSPRO-ROS and OSPRO-YF tool × baseline persistent pain interactions (block 6) did not contribute to the prediction of persistent musculoskeletal pain at 12 months.
Table 3.
Model Performance for Predicting 12-Month Persistent Musculoskeletal Paina.
| Model | R2 | Maximum Rescaled R2 | AUC (95% CI) |
|---|---|---|---|
| Complete, full model | |||
| Block 1 | 0.215b | 0.294 | 0.777 (0.723–0.832) |
| Demographic, clinical, and comorbidities | |||
| Block 2 | 0.278c | 0.380 | 0.824 (0.773–0.876) |
| Baseline persistent pain | |||
| Baseline pain intensity | |||
| Block 3 | 0.294c | 0.402 | 0.829 (0.779–0.879) |
| OSPRO-ROS (10 items) | |||
| OSPRO-YF (10 items) | |||
| Block 4 | 0.310c | 0.424 | 0.841 (0.793–0.888) |
| OSPRO-ROS + additional 13 itemsd | |||
| OSPRO-YF + additional 7 itemsd | |||
| Block 5 | 0.369c | 0.502 | 0.870 (0.826–0.914) |
| Pain intensity (4-wk change) | |||
| OSPRO-YF (10 items) (4-wk change) | |||
| OSPRO-YF + additional 7 itemsd (4-wk change) | |||
| Block 6 | 0.382 | 0.521 | 0.878 (0.835–0.920) |
| Baseline persistent pain interactions | |||
| Parsimonious models (backward selection) | |||
| Parsimonious model 1 (after block 4) | 0.211b | 0.289 | 0.778 (0.723–0.833) |
| Comorbidities | |||
| Baseline pain intensity | |||
| OSPRO-YF (10 items) | |||
| OSPRO-ROS + additional 13 itemsd | |||
| Parsimonious model 2 (after block 6) | 0.246b | 0.335 | 0.802 (0.748–0.856) |
| Comorbidities | |||
| Baseline pain intensity | |||
| Pain intensity (4-wk change) | |||
| OSPRO-ROS + additional 13 itemsd |
aComorbidity data included composite counts (0, 1, or ≥2) from the Charlson Comorbidity Index and the Functional Comorbidity Index. Pain intensity was measured with a numerical pain rating scale (potential range = 0–10, with higher scores indicating higher pain intensity). AUC = area under the receiver operating characteristic curve, OSPRO-ROS = Optimal Screening for Prediction of Referral and Outcome Review of Systems, OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag.
b R2 P < .05 (first block of model).
c R2 incremental change P < .05 (compared with previous block).
dAdditional items were from longer versions of the corresponding tool.
Exploratory analysis
Parsimonious model 1 (fit after block 4 to determine influence of OSPRO tools after accounting for planned covariates) explained 0.211 of variance (Nagelkerke maximum rescaled R2 = 0.289; AUC = 0.778 [95% CI = 0.723–0.833]) in persistent musculoskeletal pain at 12 months with comorbidities, baseline pain intensity, OSPRO-YF (10 items), and OSPRO-ROS (additional 13 items) as best-fit predictors (Tab. 3). Parsimonious model 2 (fit after block 6 to account for changes in OSPRO-YF tool and pain intensity at 4 weeks and baseline persistent pain interactions) explained 0.246 of the variance (Nagelkerke maximum rescaled R2 = 0.335; AUC = 0.802 [95% CI = 0.748–0.856]) with comorbidities, pain intensity (baseline and 4-week changes), and OSPRO-ROS (additional 13 items) as best-fit predictors (Tab. 3).
Discussion
The purpose of this analysis was to evaluate the predictive capacity of newly developed screening tools in combination with patient-level factors for persistent musculoskeletal pain 12 months after an episode of physical therapy. Our results indicated that number of comorbidities, pain intensity (baseline and 4 week changes) and OSPRO-ROS (additional 13 items) were consistent predictors for persistent musculoskeletal pain at 12 months in full and parsimonious models. These are important findings because persistent pain has not been commonly evaluated in previous predictive studies using concise screening tools16,32,33; however, it is a relevant outcome in an era of front-line nonpharmacological pain management.12,13 These findings suggest that prediction of persistent musculoskeletal pain can improve statistically when a review of systems tool is used in conjunction with comorbidities and baseline pain intensity.
Primary Musculoskeletal Pain Site
Although not a primary aim, the findings relative to anatomical body region are noteworthy for 2 reasons. First, when comparing participants with and without persistent musculoskeletal pain at 12 months, distribution across body regions (neck, low back, shoulder and knee) were similar (Tab. 2). Second, anatomical location of musculoskeletal pain (when combined with other variables) did not contribute variance to persistent musculoskeletal pain at 12 months in any logistic regression models (Tab. 4). These findings are consistent with previous work from our group and others indicating primary anatomical location of musculoskeletal pain site not being associated with clinical outcomes in physical therapy settings.34–36 For example, George et al34 observed similar prevalence rates for depressive symptoms with consistent detrimental influence on clinical outcomes across neck, lumbar, upper and lower extremity body regions. As a result, the authors suggested screening for depressive symptoms should be expanded in physical therapy settings to include all body regions impacted by musculoskeletal pain.34
Table 4.
Parameters for Individual Predictors of Persistent Musculoskeletal Pain at 12 Monthsa.
| Model | OR (95% CI)a | P |
|---|---|---|
| Model 5 (full model excluding interaction) | ||
Household income (reference = <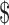 20,000) 20,000) |
||
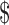 20,000– 20,000–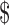 35,000 35,000 |
0.36 (0.08–1.63) | .185 |
 35,001– 35,001– 50,000 50,000 |
0.06 (0.01–0.31) | .001 |
 50,001– 50,001– 70,000 70,000 |
0.14 (0.03–0.72) | .019 |
> 70,000 70,000 |
0.18 (0.04–0.78) | .022 |
| Do not know/prefer not to answer | 0.23 (0.05–1.13) | .070 |
| Education (reference = high school or lower) | ||
| Some college | 8.03 (1.26–51.27) | .027 |
| Graduated from college | 14.60 (2.20–96.78) | .006 |
| Some postgraduate course work | 12.63 (1.69–94.55) | .014 |
| Completed postgraduate degree | 5.26 (0.75–36.63) | .094 |
| Prefer not to answer | >99.9 (<0.001–>99.9) | .991 |
| Musculoskeletal pain location (reference = knee) | ||
| Neck | 1.60 (0.50–5.15) | .434 |
| Low back | 1.18 (0.44–3.17) | .749 |
| Shoulder | 0.62 (0.21–1.87) | .396 |
| Comorbidities (reference = 2 + ) | ||
| 0 | 0.30 (0.11–0.81) | .018 |
| 1 | 0.47 (0.17–1.31) | .151 |
| Baseline pain intensity | 1.75 (1.30–2.36) | <.001 |
| OSPRO-ROS (10 items) | 0.92 (0.74–1.14) | .445 |
| OSPRO-YF (10 items) | 1.09 (0.98–1.21) | .099 |
| OSPRO-ROS + additional 13 itemsb | 1.54 (1.13–2.10) | .006 |
| OSPRO-YF + additional 7 itemsb | 0.94 (0.82–1.07) | .340 |
| Pain intensity (4-wk change) | 1.37 (1.05–1.79) | .019 |
| OSPRO-YF (10 items) (4-wk change) | 1.07 (0.97–1.18) | .176 |
| OSPRO-YF + additional 7 itemsb (4-wk change) | 0.91 (0.82–1.02) | .104 |
| Parsimonious model 1 | ||
| Comorbidities (reference = 2 + ) | ||
| 0 | 0.50 (0.25–1.01) | .054 |
| 1 | 0.53 (0.25–1.13) | .101 |
| Baseline pain intensity | 1.44 (1.18–1.75) | <.001 |
| OSPRO-YF (10 items) | 1.06 (1.01–1.11) | .032 |
| OSPRO-ROS + additional 13 itemsb | 1.33 (1.09–1.64) | .006 |
| Parsimonious model 2 | ||
| Comorbidities (reference = 2 + ) | ||
| 0 | 0.46 (0.22–0.96) | .039 |
| 1 | 0.50 (0.22–1.12) | .091 |
| Baseline pain intensity | 1.72 (1.39–2.12) | <.0001 |
| Pain intensity (4-wk change) | 1.39 (1.15–1.69) | .001 |
| OSPRO-ROS + additional 13 itemsb | 1.44 (1.15–1.80) | .001 |
aComorbidity data included composite counts (0, 1, or ≥2) from the Charlson Comorbidity Index and the Functional Comorbidity Index. Pain intensity was measured with a numerical pain rating scale (potential range = 0–10, with higher scores indicating higher pain intensity). OR = odds ratio, OSPRO-ROS = Optimal Screening for Prediction of Referral and Outcome Review of Systems, OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag.
bAdditional items were from longer versions of the corresponding tool.
Similarly, findings from this current study suggest that the OSPRO-ROS tool additional items may be used across anatomically different musculoskeletal pain conditions for improving the prediction of persistent musculoskeletal pain at 12 months after comorbidities and baseline pain intensity are considered. While these findings are encouraging, future studies incorporating more sophisticated analytical strategies are required before we can advocate for clinical use of the OSPRO-ROS tool additional items for the prediction of persistent musculoskeletal pain.
OSPRO Tools
Conceptually, the OSPRO-ROS and OSPRO-YF tools were developed to assist with the identification of systemic involvement and pain-associated psychological distress, respectively. Although we did not test their independent, unique predictive capacity in the primary analysis, the OSPRO ROS and OSPRO-YF tools together were found to contribute additional variance to persistent musculoskeletal pain at 12 months when combined with other predictors in the full model (Tab. 3). The variance explained by the addition of these tools was smaller than other blocks in the model, and as a result, the clinical implications of using the OSPRO tools to predict persistence of pain are not immediately clear. However, the amount of variance explained is comparable to how other commonly used screening tools perform in multivariate analyses.16,37–39
Consistent contributions to persistent musculoskeletal pain were provided by the OSPRO-ROS tool additional items in full and parsimonious models, indicating that tool merits further attention in future predictive models. Contributions provided by the OSPRO-YF tool were not as consistent, potentially as a result of the inclusion of 4-week pain intensity change scores in our predictive models (ie, parsimonious model 2); however, this notion is purely speculative, and future analyses will provide further clarification regarding OSPRO-YF tool clinical utility.
Using both OSPRO tools to screen for systemic involvement and pain associated psychological distress yellow flags when combined with other patient-level factors may potentially improve prediction of persistent musculoskeletal pain, which is a novel aspect of this study as previous musculoskeletal pain screening studies have tended to focus on pain related psychological distress27,32,33 for prediction of pain intensity, disability, or return to work outcomes in patients with low back pain.16,32,33
Comorbidities
Compared to individuals with ≥2 comorbidities; those with no comorbidities were less likely to have persistent musculoskeletal pain at 12 months (Tab. 4). These findings are consistent with previous studies indicating the adverse influence comorbidities have on musculoskeletal pain related outcomes,40–42 which has important implications as many individuals with musculoskeletal pain are also characterized with nonmusculoskeletal comorbidity.43–45 These implications may have a more profound effect for individuals with multisite musculoskeletal pain based on the strong positive relationship between number of pain sites and nonmusculoskeletal symptoms.46 Therefore, physical therapists should strongly consider routine comorbidity assessment to enhance clinical decision making for outcome prediction because this approach may not be part of standard clinical practice and because patients with musculoskeletal pain and simultaneous comorbidities collectively tend to have poor functional status and a poor prognosis and to respond less favorably to treatment.41
Comparison With Other Musculoskeletal Pain Outcome Prediction Models
Direct quantitative comparison of our predictive models with others that have included concise screening tools is beyond the scope of this analysis; however, providing context for similarities and differences is worth mentioning. First, previous predictive studies incorporating concise screening tools16,27,32,33,47 have primarily involved spinal pain, but our sample included a variety musculoskeletal pain conditions—a novel aspect. Second, comorbidities and systemic involvement are not commonly assessed using validated (ie, Charlson Comorbidity Index and Functional Comorbidity Index)22,23 or standardized (ie, OSPRO-ROS)24 measures, therefore these data provide preliminary support for predictive capacity of these measures for persistent pain. Third, comparable previous models including concise screening tools27,32,33 have undergone separate development and validation phases as per previous recommendations.29,30 Finally, OSPRO tools added relatively small amounts of variance to our full model containing several covariates, similar to previous studies that have used multivariate methods.16,37–39 Therefore, risk model development resulting in a smaller, clinically applicable model provides future direction for this line of research with our parsimonious model findings offering preliminary indication of individual factor predictive capacity.
Opportunities for Physical Therapy
In the United States, we are embarking on an era in health care where nonpharmacological alternatives are being recommended as the primary treatment options for common musculoskeletal pain conditions.12,13 As a result, providers of nonpharmacological treatment for patients with musculoskeletal pain will be held accountable for demonstrating high value service can be improved through early identification and efficient provision of appropriate treatment for those susceptible to poor outcomes in real-world settings.48–50 Physical therapists are well positioned for this accountability challenge considering the magnitude of patients receiving their services for musculoskeletal pain.51,52 In outcomes registries, musculoskeletal pain assessment that includes OSPRO tools and other predictive measures can potentially be used to improve performance of predictive models by testing them in large datasets. Future studies using large datasets from outcome registries should consider including the OSPRO tools when developing predictive models. From a clinical practice perspective, there is an opportunity to enhance clinical decision making through treatment monitoring as initial assessment findings are likely to change early during an episode of care. The contribution of 4-week pain intensity change scores to prediction of persistent pain (OR = 1.37) was similar in magnitude to baseline pain intensity (OR = 1.75) in our full model. These findings suggest that to improve persistent pain outcome prediction by treatment monitoring pain intensity assessments should be structured to capture baseline status and a follow-up measure during an episode of care.
Strengths and Limitations
One strength of this study is that we conducted a pre-planned analysis from data generated form a cohort study that consisted of 9 clinical sites distributed across several different geographical regions in the United States.14 We intentionally used broad inclusion criteria to validate the predictive capacity of newly developed OSPRO-ROS and OSPRO-YF tools, alone and with other measures, across a wide spectrum of musculoskeletal pain conditions. Another strength of this study is that we used a multidimensional approach that included standardized review of system questions, a novel yellow flag tool (that incorporated positive coping aspects) and medical comorbidities for these analyses that is different from previous musculoskeletal pain studies. An additional strength of this study is that we used operational definitions for persistent pain at 12 months that distinguished between episodic and ongoing symptoms as the dependent variable in our analyses. Previous studies have used outcome measure thresholds to operationally define persistent pain, which may not account for the underlying construct of pain persistence.
This study also had several limitations. First, recruitment of participants relied on convenience sampling, which could have introduced selection bias. We did not require participating clinics to monitor people for enrollment in the study for pragmatic reasons, but our intentionally broad inclusion criteria resulted in similarities across many demographic and clinical variables between development24,25 and validation cohorts.14 Second, the 63.4% follow-up rate at 12 months was lower than anticipated; therefore selection bias could influence generalizability of results. Furthermore, there were several differences for those that completed followed-up compared to those that did not. Specifically, these predictive models may need to be adjusted for those who are younger, those who have lower income and education levels, those who are unemployed, and those with higher initial OSPRO-YF tool scores. Third, these findings should only be generalized to patients receiving physical therapy for musculoskeletal pain localized to the neck, low back, shoulder, or knee. We did not assess for multisite pain, which has been reported to account for approximately 20% to 30% of individuals experiencing musculoskeletal pain,41,53–55 or symptoms localized to other anatomical regions (eg, wrist or ankle). Fourth, we acknowledge that including both the Charlson Comorbidity Index and the Functional Comorbidity Index for full consideration of comorbidities may have affected the generalizability of findings to settings that only use 1 of these indexes. In addition, we used an operational definition for chronic low back pain9 and applied it to persistent musculoskeletal pain in other anatomical body regions at baseline. Although our definition was developed for low back pain,9 it accounted for pain duration and activity limitations, which is consistent with previous suggestions.10,11 Future studies would benefit from using standardized operational definitions for chronic pain at baseline and follow-up time points that account for persistence, severity, and high impact as a result of enduring participation restrictions, which is consistent with National Pain Strategy objectives.10
Conclusion
Comorbidities, pain intensity (baseline and 4-week changes), and OSPRO-ROS scores improved the prediction of persistent musculoskeletal pain at 12 months in this physical therapy cohort. These are potentially important findings because persistent pain has not been commonly evaluated in previous screening studies, but the prediction of this outcome is relevant in an era of front-line nonpharmacological pain management. However, these findings should be interpreted with caution, as there was a lower-than-anticipated follow-up rate at 12 months and selection bias could influence the generalizability of the results. Future research is needed before clinical recommendations for predicting persistent pain outcomes can be made.
Author Contributions
Concept/idea/research design: J.M. Beneciuk, S.Z. George, T.A. Lentz, S.S. Wu
Writing: J.M. Beneciuk, S.Z. George, Y. He, S.S. Wu
Data collection: J.M. Beneciuk, T.A. Lentz
Data analysis: J.M. Beneciuk, S.Z. George, Y. He, S.S. Wu
Project management: J.M. Beneciuk, S.Z. George, T.A. Lentz
Fund procurement: S.Z. George (PI), J.M Beneciuk, S.S. Wu
Consultation (including review of manuscript before submitting): J.M. Beneciuk, S.Z. George, S.S Wu, and T.A Lentz
Ethics Approval
This study was approved by the University of Florida Gainesville Health Science Center Institutional Review Board (IRB-01). All patients provided informed consent before participating in this study.
Funding
This study was funded by a 2013 Clinical Research Network grant from the Orthopaedic Section of the American Physical Therapy Association. J.M. Beneciuk received funding support from the National Institutes of Health Rehabilitation Research Career Development Program (K12-HD055929). T.A. Lentz received support from the Foundation for Physical Therapy with Promotion of Doctoral Studies (PODS) I and II Awards.
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet North Am Ed. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Atkinson C, Bhalla K et al. . The state of US health. 1990–2010. JAMA. 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Artus M, Campbell P, Mallen CD, Dunn KM, van der Windt DA. Generic prognostic factors for musculoskeletal pain in primary care: a systematic review. BMJ Open. 2017;7:e012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:403–425. [DOI] [PubMed] [Google Scholar]
- 5. Dowsey MM, Smith AJ, Choong PF. Latent class growth analysis predicts long term pain and function trajectories in total knee arthroplasty: a study of 689 patients. Osteoarthritis Cartilage. 2015;23:2141–2149. [DOI] [PubMed] [Google Scholar]
- 6. Kongsted A, Kent P, Axen I, Downie AS, Dunn KM. What have we learned from ten years of trajectory research in low back pain? BMC Musculoskelet Disord. 2016;17:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miedema HS, Feleus A, Bierma-Zeinstra SM, Hoekstra T, Burdorf A, Koes BW. Disability trajectories in patients with complaints of arm, neck, and shoulder (CANS) in primary care: prospective cohort study. Phys Ther. 2016;96:972–984. [DOI] [PubMed] [Google Scholar]
- 8. Walton DM, Eilon-Avigdor Y, Wonderham M, Wilk P. Exploring the clinical course of neck pain in physical therapy: a longitudinal study. Arch Phys Med Rehabil. 2014;95:303–308. [DOI] [PubMed] [Google Scholar]
- 9. Deyo RA, Dworkin SF, Amtmann D et al. . Report of the NIH Task Force on research standards for chronic low back pain. J Pain. 2014;15:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Department of Health and Human Services National pain strategy: a comprehensive population health strategy for pain. https://iprcc.nih.gov/National-Pain-Strategy/Overview 2016. Accessed June 21, 2017. [Google Scholar]
- 11. Blyth FM, Van Der Windt DA, Croft PR. Chronic Disabling Pain. Am J Prev Med. 2015;49:98–101. [DOI] [PubMed] [Google Scholar]
- 12. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514–530. [DOI] [PubMed] [Google Scholar]
- 13. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. George SZ, Beneciuk JM, Lentz TA, Wu SS. The Optimal Screening for Prediction of Referral and Outcome (OSPRO) in patients with musculoskeletal pain conditions: a longitudinal validation cohort from the USA. BMJ Open. 2017;7:e015188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George SZ, Zeppieri G Jr, Cere AL et al. . A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain (NCT00373867). Pain. 2008;140:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beneciuk JM, Bishop MD, Fritz JM et al. . The STarT Back Screening Tool and individual psychological measures: evaluation of prognostic capabilities for low back pain clinical outcomes in outpatient physical therapy settings. Phys Ther. 2013;93:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freburger JK, Holmes GM, Agans RP et al. . The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carey TS, Freburger JK, Holmes GM et al. . Race, care seeking, and utilization for chronic back and neck pain: population perspectives. J Pain. 2010;11:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolton JE. Accuracy of recall of usual pain intensity in back pain patients. Pain. 1999;83:533–539. [DOI] [PubMed] [Google Scholar]
- 20. Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30:1331–1334. [DOI] [PubMed] [Google Scholar]
- 21. Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 23. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. [DOI] [PubMed] [Google Scholar]
- 24. George SZ, Beneciuk JM, Bialosky JE et al. . Development of a review-of-systems screening tool for orthopaedic physical therapists: results from the Optimal Screening for Prediction of Referral and Outcome (OSPRO) cohort. J Orthop Sports Phys Ther. 2015;45:512–526. [DOI] [PubMed] [Google Scholar]
- 25. Lentz TA, Beneciuk JM, Bialosky JE et al. . Development of a yellow flag assessment tool for orthopaedic physical therapists: results from the Optimal Screening for Prediction of Referral and Outcome (OSPRO) cohort. J Orthop Sports Phys Ther. 2016;46:327–343. [DOI] [PubMed] [Google Scholar]
- 26. Bouwmeester W, Zuithoff NP, Mallett S et al. . Reporting and methods in clinical prediction research: a systematic review. PLoS Med. 2012;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Traeger A, Henschke N, Hubscher M et al. . Development and validation of a screening tool to predict the risk of chronic low back pain in patients presenting with acute low back pain: a study protocol. BMJ Open. 2015;5:e007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karran EL, McAuley JH, Traeger AC et al. . Can screening instruments accurately determine poor outcome risk in adults with recent onset low back pain? A systematic review and meta-analysis. BMC Med. 2017;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riley RD, Hayden JA, Steyerberg EW et al. . Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steyerberg EW, Moons KG, van der Windt DA et al. . Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604–b604. [DOI] [PubMed] [Google Scholar]
- 32. Hill JC, Dunn KM, Lewis M et al. . A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. [DOI] [PubMed] [Google Scholar]
- 33. Hockings RL, McAuley JH, Maher CG. A systematic review of the predictive ability of the Orebro Musculoskeletal Pain Questionnaire. Spine (Phila Pa 1976). 2008;33:E494–E500. [DOI] [PubMed] [Google Scholar]
- 34. George SZ, Coronado RA, Beneciuk JM, Valencia C, Werneke MW, Hart DL. Depressive symptoms, anatomical region, and clinical outcomes for patients seeking outpatient physical therapy for musculoskeletal pain. Phys Ther. 2011;91:358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. George SZ, Stryker SE. Fear-avoidance beliefs and clinical outcomes for patients seeking outpatient physical therapy for musculoskeletal pain conditions. J Orthop Sports Phys Ther. 2011;41:249–259. [DOI] [PubMed] [Google Scholar]
- 36. de Vos Andersen NB, Kent P, Hjort J, Christiansen DH. Clinical course and prognosis of musculoskeletal pain in patients referred for physiotherapy: does pain site matter? BMC Musculoskelet Disord. 2017;18:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wideman TH, Hill JC, Main CJ, Lewis M, Sullivan MJ, Hay EM. Comparing the responsiveness of a brief, multidimensional risk screening tool for back pain to its unidimensional reference standards: the whole is greater than the sum of its parts. Pain. 2012;153:2182–2191. [DOI] [PubMed] [Google Scholar]
- 38. Dagfinrud H, Storheim K, Magnussen LH et al. . The predictive validity of the Örebro Musculoskeletal Pain Questionnaire and the clinicians’ prognostic assessment following manual therapy treatment of patients with LBP and neck pain. Man Ther. 2013;18:124–129. [DOI] [PubMed] [Google Scholar]
- 39. Medeiros FC, Costa LOP, Added MAN, Salomão EC, Costa LDCM. Longitudinal monitoring of patients with chronic low back pain during physical therapy treatment using the STarT Back Screening Tool. J Orthop Sports Phys Ther. 2017;47:314–323. [DOI] [PubMed] [Google Scholar]
- 40. Peter WF, Dekker J, Tilbury C et al. . The association between comorbidities and pain, physical function and quality of life following hip and knee arthroplasty. Rheumatol Int. 2015;35:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hartvigsen J, Natvig B, Ferreira M. Is it all about a pain in the back? Best Pract Res Clin Rheumatol. 2013;27:613–623. [DOI] [PubMed] [Google Scholar]
- 42. Vogeli C, Shields AE, Lee TA et al. . Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kadam UT, Jordan K, Croft PR. Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Ann Rheum Dis. 2004;63:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Dijk GM, Veenhof C, Schellevis F et al. . Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord. 2008;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain. Spine (Phila Pa 1976). 2012;37:E668–E677. [DOI] [PubMed] [Google Scholar]
- 46. Tschudi-Madsen H, Kjeldsberg M, Natvig B et al. . A strong association between non-musculoskeletal symptoms and musculoskeletal pain symptoms: results from a population study. BMC Musculoskelet Disord. 2011;12:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maher CG, Grotle M. Evaluation of the predictive validity of the Orebro Musculoskeletal Pain Screening Questionnaire. Clin J Pain. 2009;25:666–670. [DOI] [PubMed] [Google Scholar]
- 48. Atlas SJ. Management of low back pain: getting from evidence-based recommendations to high-value care. Ann Intern Med. 2017;166:533–534. [DOI] [PubMed] [Google Scholar]
- 49. Delitto A. Pragmatic Clinical Trials: Implementation Opportunity, or Just Another Fad? Phys Ther. 2016;96:137–138. [DOI] [PubMed] [Google Scholar]
- 50. Lentz TA, Harman JS, Marlow NM, George SZ. Application of a value model for the prevention and management of chronic musculoskeletal pain by physical therapists. Phys Ther. 2017;97:354–364. [DOI] [PubMed] [Google Scholar]
- 51. Chevan J, Riddle DL, Reed SD. Out-of-pocket spending for ambulatory physical therapy services from 2008 to 2012: national panel survey. Phys Ther. 2015;95:1680–1691. [DOI] [PubMed] [Google Scholar]
- 52. Machlin SR, Chevan J, Yu WW, Zodet MW. Determinants of utilization and expenditures for episodes of ambulatory physical therapy among adults. Phys Ther. 2011;91:1018–1029. [DOI] [PubMed] [Google Scholar]
- 53. Henschke N, Ostelo RW, Terwee CB, van der Windt DA. Identifying generic predictors of outcome in patients presenting to primary care with nonspinal musculoskeletal pain. Arthritis Care Res. 2012;64:1217–1224. [DOI] [PubMed] [Google Scholar]
- 54. Hill JC, Thomas E, Hill S, Foster NE, van der Windt DA. Development and validation of the Keele Musculoskeletal Patient Reported Outcome Measure (MSK-PROM). PLoS One. 2015;10:e0124557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herin F, Vézina M, Thaon I, Soulat JM, Paris C, Group ESTEV. Predictive risk factors for chronic regional and multisite musculoskeletal pain: a 5-year prospective study in a working population. Pain. 2014;155:937–943. [DOI] [PubMed] [Google Scholar]


