Abstract
Recent studies have demonstrated that a number of environmental contaminants can act as metabolic disruptors and modulate metabolic function both in vitro and in vivo. 3T3-L1 mouse preadipocytes are commonly utilized to assess perturbations to adipogenesis, providing insight into environmental contaminants that may impact in vivo metabolic health. This study sought to assess whether various alkylphenol ethoxylates and alcohol ethoxylates (APEOs and AEOs, respectively), ubiquitous contaminants used in common household products, could disrupt metabolic health. 3T3-L1 cells were exposed to increasing concentrations of individual ethoxylated surfactants and base hydrophobes, and assessed for triglyceride accumulation (relative to a rosiglitazone-induced maximum response) and preadipocyte proliferation (relative to a differentiated vehicle control). We report herein that nonionic APEOs and AEOs promoted triglyceride accumulation and/or preadipocyte proliferation in 3T3-L1 cells at concentrations from 0.1 to 10 μM. Activity appeared to be an effect of the polyethoxylate chain length, as the alkylphenol/alcohol hydrophobes exhibited minimal or no adipogenic activity. In addition, nonylphenol ethoxylates (NPEO) of various ethoxylate chain lengths exhibited biphasic adipogenic activity, with increasing triglyceride accumulation and preadipocyte proliferation from NPEO (0, average ethoxylate number) through NPEO (4), and then decreasing activities from NPEO (4) through NPEO (20). Our results suggest potential metabolic impacts of these compounds at environmentally relevant concentrations, demonstrating a need to further assess molecular mechanisms and better characterize environmental concentrations of the specific AEOs and APEOs that are inducing the greatest degree of adipogenic activity herein.
Keywords: 3T3-L1, adipogenesis, alcohol ethoxylates, endocrine disrupting chemicals, ethoxylated surfactants
Nonionic surfactants are high production-volume chemicals that are widely used for a variety of industrial and residential purposes, including laundry detergents, hard-surface cleaners, pesticide mixtures, oils, textile and pulp production, paints, and other products (Maguire, 1999; Marcomini et al., 1988, 1989; Ying et al., 2002); 2 of the most important classes of these surfactants are alkylphenol ethoxylates (APEOs) and alcohol ethoxylates (AEOs). Nonylphenol ethoxylates (NPEOs) comprise about 80% of the total production volume of APEOs, with octylphenol ethoxylates (OPEOs) comprising the majority of the remaining 20% (Loos et al., 2007). Approximately two-thirds of AEOs are used in the production of household laundry detergents, cleaners, cosmetics, and agriculture (Talmage, 1994). High rates of efficiency and cost-effectiveness have resulted in global annual nonionic surfactant production of >13 million metric tons in 2008 (Reznik et al., 2010) and more than $33 billion in global revenues in 2014 (Ceresana, 2015). AEOs have widely been considered benign surfactants, with minimal toxicity for parent compounds and metabolites (Talmage, 1994). In contrast, APEO metabolite alkylphenol hydrophobes are highly lipophilic and toxic to aquatic organisms (Bandiera, 2006; Uguz et al., 2009; White et al., 1994). It has been reported that the primary toxicant mechanisms for APEOs and AEOs (such as estrogen agonist activity) decrease with increasing ethoxylate chain length (Talmage, 1994; White et al., 1994), providing further support that longer chain surfactants are of less concern.
Notably, a rich literature exists on the fate of APEO surfactants in water and sediments, as the degradation products, alkylphenols, are widely recognized as endocrine disrupting chemicals (EDCs; able to disrupt some aspect of hormone action The Endocrine Disruptor Exchange [TEDX] Exchange, 2017). Because of the known endocrine disrupting activity of the alkylphenols and the efficient (90%–95%) polyethoxylate chain-shortening during wastewater treatment (Ahel et al., 1994a; Giger et al., 1984, 1987), the majority of research has focused on the alkylphenol hydrophobes rather than the APEOs. However, removal is dependent on both biodegradation (influenced by temperature, oxygen content, etc.) and physicochemical processes such as adsorption, which can contribute to greater releases of long-chain APEOs into receiving streams (Ahel et al., 1994a; Mann and Reid, 1971; Manzano et al., 1999). As such, APEOs at μg/l concentrations have been widely reported in wastewater (Marcomini et al., 1987), effluent (Vega-Morales et al., 2010), rivers and streams (Ahel et al., 1994b; Ferguson et al., 2000; Loos et al., 2007), groundwater (Ahel et al., 1995), and at ng/l concentrations in drinking water (Clark et al., 2006), demonstrating retention, particularly of the shorter-chained ethoxylates. These compounds also readily partition into sediments (Ferguson et al., 2001), where their degradation rates are slower than in the water column (Ferguson et al., 2003; Ying et al., 2002). Several commonly used AEOs have been reported in surface water at μg/l concentrations (Talmage, 1994), and several laboratories have recently reported AEOs in hydraulic fracturing flowback water and effluent from wastewater treatment plants receiving wastewater from unconventional oil and gas production (Getzinger et al., 2015; Lester et al., 2015; Thurman et al., 2014). Getzinger reported ng/l to μg/l concentrations of AEOs (C6–C10 alkyl chain length and 2–12 ethoxymer chain number) in wastewater effluent discharged into a receiving stream after treatment. These studies indicate that APEOs and AEOs may be released into the environment at significant levels and may represent an underappreciated environmental contaminant, capable of accumulating in various biota (Bennie, 1999; Cailleaud et al., 2007; Dodder et al., 2014; Sabik et al., 2003; Schmitz-Afonso et al., 2003; Ying et al., 2002) and resulting in various adverse health impacts (Balch and Metcalfe, 2006; Nichols et al., 2001; Oliveira-Filho et al., 2009; Servos, 1999; Zoller, 2006). Research in our laboratory has recently demonstrated the presence of a variety of ethoxylated surfactants in household dust samples at high frequencies of detection (Ferguson et al., 2015). Given the high volume use of APEOs and AEOs in detergents, cleaners, paint, etc., it seems reasonable to assume that these chemicals migrate out and sorb to dust particles in the home. This suggests an additional source of chronic human exposure, from a matrix less prone to degradation than the aquatic environment.
Some EDCs have been demonstrated to directly modulate metabolic health, notably via weight gain in animal models and/or triglyceride accumulation in vitro (Heindel et al., 2015; Janesick and Blumberg, 2016). Importantly, several studies have raised concern of potential metabolic and endocrine disruption by anionic and nonionic surfactants. Recent research reported activation of peroxisome proliferator activated receptor gamma (PPARγ; a nuclear receptor signaling pathway critical for adipogenesis) by several constituent chemicals of the Corexit 9500 oil dispersant mixture, including Tween 80, a nonionic surfactant, and dioctyl sodium sulfosuccinate (DOSS), an anionic surfactant; DOSS was subsequently identified as a probable obesogen via in vitro and in vivo testing (Temkin et al., 2016). Further research by this group demonstrated adipogenic activity for the nonionic surfactants Tween 80 and Span 80 (Bowers et al., 2016). To our knowledge, the potential of APEOs and AEOs to promote adipogenesis is unknown, though several alkylphenol hydrophobes (nonylphenol, octylphenol) have been demonstrated to be metabolic disruptors in vitro and/or in vivo (Hao et al., 2012; Masuno et al., 2003; Miyawaki et al., 2008). These results suggest a critical need to evaluate additional surfactants, particularly those that are common environmental contaminants.
The goals of this study were to assess the potential of APEOs and AEOs to exhibit adipogenic activity in 3T3-L1 cells, independent of the base hydrophobes. Mouse 3T3-L1 preadipocytes are routinely used to assess putative adipogenic activity; following an adipogenic chemical stimulus, these cells accumulate triglycerides and differentiate into what resembles a mature human white adipocyte (Green and Kehinde, 1975; Green and Meuth, 1974) and have been used to assess metabolic disruption of environmental contaminants for more than forty years (Heindel et al., 2015; Janesick and Blumberg, 2016). A range of ethoxylated surfactants and their base hydrophobes were assessed for their abilities to promote triglyceride accumulation and/or preadipocyte proliferation. In addition, as degradation of these compounds relies on reduction of homolog chain lengths (Ahel et al., 1994a; Giger et al., 1984, 1987; Itrich and Federle, 2004), we also assessed a series of NPEOs of varying ethoxylate chain lengths to determine relative contributions to adipogenic response. We further assessed any active chemicals in PPARγ and thyroid receptor beta (TRβ) reporter gene assays, the 2 major adipogenic pathways we’ve reported in these cells previously (Kassotis et al., 2017b). We hypothesized that decreasing ethoxylate chain length and hydrophobe alkyl chain lengths would increase adipogenic activity, independent of minimal or null activity base hydrophobes, and that this effect would be mediated via activation of PPARγ and/or inhibition of TRβ.
MATERIALS AND METHODS
Chemicals
Chemicals used in this study are described in detail within Table 1, including CAS numbers, supplier, catalog, and lot numbers, and basic physicochemical properties. Stock solutions were prepared in 100% DMSO (Sigma cat no. D2650) using the molecular weight or average molecular weight (ethoxylated surfactants) and stored at −20 °C.
Table 1.
Alkylphenol and AEO and Base Hydrophobe Characteristics
| Chemical | Acronym | CAS No. | Manufacturer | Catalog No. | Lot No. | Avg MW | Density | Molecular Formula |
|---|---|---|---|---|---|---|---|---|
| Alcohol/alkylphenol Hydrophobes | ||||||||
| undecyl alcohol | N/A | 112-42-5 | Sigma | U1001 | MKBV3652V | 172.3 | 0.83 | C11H24O |
| lauryl alcohol | N/A | 112-53-8 | Sigma | W261718 | STBF6553V | 186.3 | N/A | C12H26O |
| tridecyl alcohol | N/A | 112-70-9 | Sigma | T57630 | BCBR7435V | 200.4 | 0.822 | C13H28O |
| 4-octylphenol | OPEO(0) | 1806-26-4 | Sigma | 44-2850 | LC21077V | 206 | N/A | C14H22O |
| 4-n-nonylphenol | N/A | 104-40-5 | Alfa Aesar | A15609 | L01813 | 220.4 | N/A | C15H24O |
| 4-nonylphenol | NPEO(0) | 84852-15-3 | Acros Organics | 416240010 | A0216749 | 220.4 | 0.94 | C15H24O |
| cetyl alcohol | N/A | 36653-82-4 | Sigma | 68824 | BCBQ9625V | 242.4 | N/A | C16H34O |
| Ethoxylated surfactants | ||||||||
| tomadol 1-9 (9) | Tomadol | N/A | Tomah Products | Tomadol 1-9 | N/A | 569 | 1.01 | C11H24O(C2H5O)9 |
| lauryl alcohol ethoxylate (4) | LAEO | N/A | Chem Service | S-313 | 229-66B | 362.5 | 0.95 | C12H26O(C2H5O)4 |
| tridecyl alcohol ethoxylate (9) | TdAEO | N/A | Chem Service | S-329 | 55-198B | 598 | 0.98 | C13H28O(C2H5O)9 |
| octylphenol ethoxylate (3) | OPEO | N/A | Chem Service | S-338 | 337-17C | 250.4 | 1.01 | C14H22O(C2H5O)3 |
| nonylphenol ethoxylate (1-2) | NPEO(1-2) | N/A | Chem Service | S-346 | 270-35A | 294 | 1.01 | C15H24O(C2H5O)1-2 |
| nonylphenol ethoxylate (4) | NPEO(4) | N/A | Chem Service | S-347 | 348-75A | 396 | 1.02 | C15H24O(C2H5O)4 |
| nonylphenol ethoxylate (6) | NPEO(6) | N/A | Chem Service | S-348 | 195-130C | 484 | 1.04 | C15H24O(C2H5O)6 |
| nonylphenol ethoxylate (9-10) | NPEO(9-10) | N/A | Chem Service | S-350 | 267-60C | 602.8 | 1.06 | C15H24O(C2H5O)9-10 |
| nonylphenol ethoxylate (20) | NPEO(20) | N/A | Chem Service | S-354 | 127-80C | 1101 | 1.13 | C15H24O(C2H5O)20 |
| cetyl alcohol ethoxylate (10) | CetAEO | N/A | Chem Service | S-316 | 238-24A | 1123.5 | N/A | C16H34O(C2H5O)10 |
Chemical identification, ordering information, and basic physicochemical properties for each of the APEO and AEOs and base hydrophobes examined in this study. Molecular formulae contain base carbon number as well as average ethoxylate chain number.
Cell care
3T3-L1 cells were obtained from Zenbio, Inc. at passage 8 (cat no. SP-L1-F, lot no. 3T3062104; Research Triangle Park, North Carolina) and were maintained as described previously in Dulbecco’s Modified Eagle Medium-High Glucose (DMEM-HG; Gibco cat no. 11995, Gaithersburg, MD) supplemented with 10% bovine calf serum and 1% penicillin and streptomycin (Gibco cat no. 15140, Gaithersburg, MD) (Kassotis et al., 2017b). Cells were maintained in a subconfluent state until differentiation and each thaw of frozen cells was utilized within 6 passages, with no significant changes in control chemical response observed in that time.
HEK 293 T and 293 H cells utilized for the GeneBlazer TRβ and PPARγ assays (Invitrogen cat no. K1686 and K1094, Carlsbad, CA), respectively, were purchased from Invitrogen and cultured according to manufacturer’s directions (Invitrogen, 2010a ,b). Briefly, both cell lines were cultured in DMEM-HG supplemented with 10% dialyzed fetal bovine serum (Invitrogen cat no. 26400-036), 1% nonessential amino acids (Invitrogen no. 11140, Carlsbad, CA), 1% sodium pyruvate (Invitrogen no. 11360, Carlsbad, CA), 1% penicillin-streptomycin (Invitrogen no. 15140, Carlsbad, CA), 25 mM HEPES (Invitrogen no. 15630, Carlsbad, CA), 80 µg/ml hygromycin (Invitrogen no. 10687, Carlsbad, CA), and 2% glutamax (Invitrogen no. 35050, Carlsbad, CA); TRβ cells were further supplemented with 100 µg/ml Zeocin (Invitrogen no. R250-01, Carlsbad, CA) and PPARγ cells with 500 µg/ml Geneticin (Invitrogen no. 10131, Carlsbad, CA). Each thaw of PPARγ cells was maintained in a subconfluent state and utilized within ten passages. TRβ cells were frozen within 3 passages after receipt and seeded directly into assay plates from frozen stocks.
3T3-L1 differentiation and outcome measurements
3T3-L1 Cells were induced to differentiate as described previously in Kassotis et al. (2017a,b). Briefly, cells were seeded in preadipocyte media into 96-well tissue culture plates (Greiner cat no. 655090, Monroe, NC) at approximately 30 000 cells per well. Once confluent, cells were cultured for an additional 48 h to promote growth arrest and allow initiation of clonal expansion. Media was then replaced with test chemicals and/or controls using a 0.1% DMSO vehicle in differentiation media (DMEM-HG with 10% fetal bovine serum, 1% penicillin/streptomycin, 1.0 μg/ml human insulin, and 0.5 mM 3-isobutyl-1-methylxanthine, IBMX). After 48 h, media was replaced with test chemicals diluted in adipocyte maintenance media (differentiation media without IBMX) and these treatments were refreshed every 2–3 days. For particular experiments, cells were induced as described earlier with the addition of 20 nM dexamethasone to the differentiation media to further enhance the extent of differentiation, as described previously in Kassotis et al. (2017b).
Plates were assayed for triglyceride accumulation and DNA content after 10 days of differentiation as described previously in Kassotis et al. (2017a,b). Briefly, media was removed and cells rinsed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco cat no. 14040, Gaithersburg, MD) before replacing with 200 μl of a dye mixture (19.5 ml DPBS, 1 drop/ml NucBlue Live ReadyProbes Reagent (Thermo cat no. R37605, Waltham, MA) and 500 μl AdipoRed (Lonza cat no. PT-7009, Walkersville, MD) per plate). Plates were incubated at room temperature for approximately 40 min, protected from light, and read using a Molecular Devices SpectraMax M5 microplate spectrofluorimeter: excitation 485 nm/emission 572 nm for AdipoRed, 360/460 for NucBlue.
Percent activities (efficacies) across the tested dose response range (1 nM to 10 μM) were calculated relative to the maximal rosiglitazone-induced fold induction over intra-assay differentiated vehicle controls (0.1% DMSO), after correcting for background fluorescence by subtracting raw fluorescence units determined from cell-free wells. DNA content was calculated as percent change from vehicle controls for each chemical at each concentration, and was then used to normalize total triglyceride values to obtain triglyceride content per cell. Potencies were determined using EC20/EC50 values (concentrations of each chemical that exhibit 20%/50% of their own maximal activity) as determined using GraphPad Prism 6.0.
3T3-L1 cell viability testing
Cell Viability was assessed using the CellTiter-Glo (Promega cat no. G7572, Durham, NC) and CytoTox-ONE (Promega cat no. G7890, Durham, NC) assays, according to modified manufacturer’s instructions. Briefly, before removing media from plates for lipid and DNA measurements, 25 μL of media from wells was aliquotted into separate 96-well plates and mixed with 25 μl CytoTox-ONE reagent. Plates were protected from light and incubated for 10 min, and then fluorescence was measured at 560/590 nm. Following the lipid and DNA readings, 170 μl of DPBS mixture was removed from all wells and remaining 30 μl was mixed with 30 μl of CellTiter-Glo reagent. Plates were incubated for 10 min prior to reading luminescence. Cell viability was calculated as follows: CytoTox-ONE viability was assessed as a percent viability loss relative to the maximal lysed control response, and CellTiter-Glo viability was assessed as a percent change from vehicle controls. Significant viability loss was considered for deviations of >15%.
TRβ and PPARγ GeneBLAzer reporter assays
TRβ antagonism and PPARγ agonism were assessed according to manufacturer’s instructions (Invitrogen, 2010a,b) and as described previously in Fang et al. (2015). TRβ assays utilized a modified protocol where frozen cells were thawed and directly plated for each assay, rather than maintained in culture. Cells were seeded at 10 000 and 30 000 cells per well (TRβ and PPARγ assays, respectively) into duplicate 384-well black clear-bottom plates (Corning cat no. 3764, Corning, NY for TRβ; Corning cat no. 354663 for PPARγ, Corning, NY) and allowed to settle for 2–4 h. Cells were then induced with test chemical and positive/negative controls (0.1 nm–1 μM rosiglitazone for PPARγ, 0.3 nM triiodothyronine (T3) for TRβ assay), using a 0.1% DMSO vehicle. Plates were incubated for approximately 18 and 16 h, respectively, and then replicate plates were assayed for receptor activity and cell viability. Receptor activity was assessed using the LiveBLAzer-FRET B/G Loading Kit (Invitrogen cat no. K1095, Carlsbad, CA), according to manufacturer’s instructions (Invitrogen, 2010a,b), and fluorescence was measured at 409/460 and 409/530 nm on a Molecular Devices SpectraMax M5 microplate spectrofluorimeter. Percent activity was assessed as a background corrected fold induction of test chemical response relative to maximal rosiglitazone-induced response for PPARγ, or as percent inhibition of 0.3 nM T3 (EC80) for TRβ.
Cell viability was assessed as described previously in Fang et al. (2015), adding 0.025 mg/ml resazurin solution to each well, incubating at 37 °C for 2 h, and then measuring fluorescence at 560/590 nm using the SpectraMax spectrofluorimeter. Cell viability loss of >15% was used as a cutoff for distinguishing cytotoxicity.
PPARγ coactivator recruitment assay
PPARγ agonism, as measured through the GeneBLAzer Reporter Assay, experienced technical difficulties; despite response ratios that indicated robust receptor activation, fluorescence decreased in both channels in conflict with the reported assay mechanisms. To further assess potential PPARγ contribution, we utilized the Invitrogen SelectScreen service to test each surfactant using the LanthaScreen time-resolved fluorescence resonance energy transfer (TR-FRET) PPARγ Coactivator Assay (Invitrogen cat no. PV4548, Carlsbad, CA). Samples were shipped in amber glass vials to Invitrogen for analysis, and assayed as described by the manufacturer (Invitrogen, 2010c). Briefly, PPARγ ligand binding domain was added to replicate dose responses of test compounds and positive control (GW1929) in duplicate, followed by the addition of a mixture containing: fluorescein-coactivator peptide (TRAP220/DRIP-2) and terbium antiGST antibody. Samples were incubated at room temperature for 1 h, and then the TR-FRET ratio of 520:495 emissions was used to determine percent activity relative to the maximal positive control response.
Statistical analysis
Data are presented as means ± SEM from 4 technical replicates of 3 or 4 independent biological replicates (2 for co-activator recruitment assays). Linear mixed models were used to analyze the results from the adipogenesis and PPARγ biological replicate assays, and incorporated random effects to account for dependence among quadruplicate technical replicates. Post-test comparison between treatment groups was performed between groups using least-square means to determine 95% CIs and the Tukey-Kramer multiple comparison test with differences considered statistically significant at p < .05 to determine differences between treatment groups and from vehicle control. Cell proliferation results were log transformed for normal distributions and adjusted means back-transformed for presentation. Proc GLIMMIX in SAS 9.4 (SAS Inc.) was used for this analysis. EC20/50 values were estimated using curves generated from raw fluorescence data using a 4-parameter variable-slope Hill model in GraphPad Prism 7.0. TRβ results were analyzed using a 1-way ANOVA and Dunnett’s posthoc test relative to the T3 EC80 control, using GraphPad Prism 7.0.
RESULTS
Six ethoxylated nonionic surfactants and respective alcohol/alkylphenol hydrophobes were assessed for adipogenic activity utilizing 3T3-L1 cells (Table 1). Adipogenic activity was examined with respect to both varying alkyl chain length (different carbon backbones of base alkylphenols/alcohols) and ethoxylate chain length (4 additional NPEOs with varying ethoxylate chain lengths). Cells were differentiated for 10 days and assessed for preadipocyte proliferation (DNA content, relative to vehicle control), triglyceride accumulation (total per well and per cell, normalized to DNA content; both relative to maximal rosiglitazone response), and cell viability (ATP production and lactate dehydrogenase [LDH] release). Rosiglitazone induced a typical, robust response for triglyceride accumulation and preadipocyte proliferation (Supplementary Figs. 1A–C), no impact on ATP production independent of increased cell number (Supplementary Figure 1D), and a large release of LDH (Supplementary Figure 1E), as our laboratory and others have described previously in Kassotis et al. (2017a,b), Staiger and Loffler (1998), and Zeng et al. (2012).
Adipogenic Activity of Varying Alkyl Chain Length Ethoxylated Surfactants
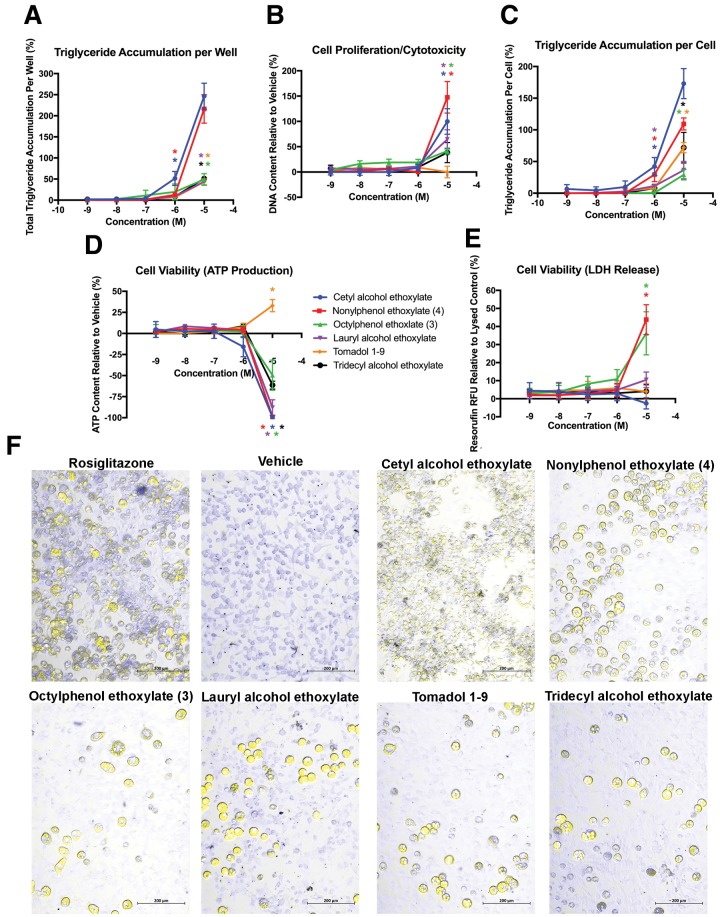
Six ethoxylated nonionic surfactants were selected to span alkyl chain lengths of 11–16, along with base hydrophobes (Table 1), and 3T3-L1 cells exposed to these chemicals were induced to differentiate as described previously (Kassotis et al., 2017a,b). All 6 ethoyxlated surfactants induced triglyceride accumulation (Figs. 1A and 1C), with cetyl AEO (CetAEO) and NPEO inducing supramaximal triglyceride accumulation at 215%–250% relative to the maximal rosiglitazone-induced response. In total 4 of the 6 compounds exhibited an increase in cell proliferation at 10 μM, ranging from approximately 40% increased DNA content for OPEO up to 150% for NPEO, relative to the vehicle control (Figure 1B). Tomadol 1-9 was the only compound that did not exhibit increased DNA content, and was also the only compound that did not exhibit inhibited ATP production; the remaining 5 compounds induced from 50% to 100% inhibition of ATP relative to the vehicle control (Figure 1D). Despite this, only the APEOs, OPEO and NPEO, induced an accompanying increased LDH release (Figure 1E). Finally, while NPEO, OPEO, LAEO, Tomadol, and tridecyl AEO-induced classical and similar triglyceride accumulation morphologies to rosiglitazone, though to varying extents, CetAEO induced a phenotypically distinct pattern composed of much more numerous but smaller intracellular lipid droplets (Figure 1F). Interestingly, despite this morphological difference in lipid accumulation, CetAEO exhibited similar levels of accumulation to NPEO and twice that exhibited by rosiglitazone, both of which exhibited the phenotypically normal triglyceride accumulation pattern.
Figure 1.
Various alkyl chain length alkylphenol and alcohol surfactants induce adipogenic activity. 3T3-L1 cells were induced to differentiate as described in Methods and assessed for degree of adipocyte differentiation (Nile Red staining of intracellular lipids) and preadipocyte proliferation (Hoechst nuclear DNA staining) after 10 days of differentiation while exposed to various ethoxylated surfactants from 1 nM to 10 μM in concentration. Percent raw triglyceride accumulation per well relative to maximal intra-assay response for rosiglitazone (A), increase (preadipocyte proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B), percent normalized triglyceride accumulation per cell relative to maximal intra-assay rosiglitazone response (normalized to DNA content) (C), increase or decrease in cell viability (ATP content) relative to vehicle control (D), and increase in lactate dehydrogenate release (cell viability measure) relative to lysed control (E). Data presented as mean ± SEM from 3 independent experiments. Representative image of differentiated cells induced in a 24-well plate following 10 days of exposure to 1 μM rosiglitazone, 0.1% DMSO, or 10 μM CetAEO, NPEO, OPEO, lauryl AEO, Tomadol 1-9, or tridecyl AEO (F), using a Zeiss Axio Observer microscope and Photometrics CoolSNAP ES2 CCD camera and ZEN Pro 2.3. Imaging merged a phase contrast field with yellow fluorescent protein (Nile Red lipid staining) and DAPI (NucBlue DNA content) filters to visualize the cells. Bars are provided for scale. * indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p < .05, as per linear mixed model in SAS 9.4.
Modulating the activation of the glucocorticoid receptor has been demonstrated previously to vary the extent of triglyceride accumulation and allow for the detection of weaker or more active adipogenic chemicals (Sargis et al., 2010). As such, to increase sensitivity of adipogenic activity to delineate this from the observed cytotoxicity or inhibited viability, we altered our differentiation media to include a physiologically relevant dose of dexamethasone (20 nM, as described previously in Kassotis et al. [2017b]), and otherwise differentiated cells as above. The addition of dexamethasone increased the potency of triglyceride accumulation across our test chemicals; this resulted in significant adipogenic responses at concentrations lower than previously observed and successful separation of adipogenic activity from apparent cytotoxicity/inhibited cell viability (Supplementary Figs. 2A and 2C). The extent of triglyceride accumulation was reduced for all chemicals except OPEO, for which it was enhanced, suggesting a greater than additive response with dexamethasone. Significant proliferation was no longer observed for CetAEO or OPEO, with CetAEO instead exhibiting significant cytotoxicity at 10 μM, and Tomadol still exhibiting no change in DNA content (Supplementary Figure 2B). This succeeded in distinguishing the triglyceride accumulation from the cell viability loss, with reduced ATP only observed at 10 μM for certain chemicals (Supplementary Figure 2D). In contrast, more of the chemicals exhibited an increase in LDH release (Supplementary Figure 2E), perhaps due to dexamethasone promoting further differentiation and subsequent increased leptin production.
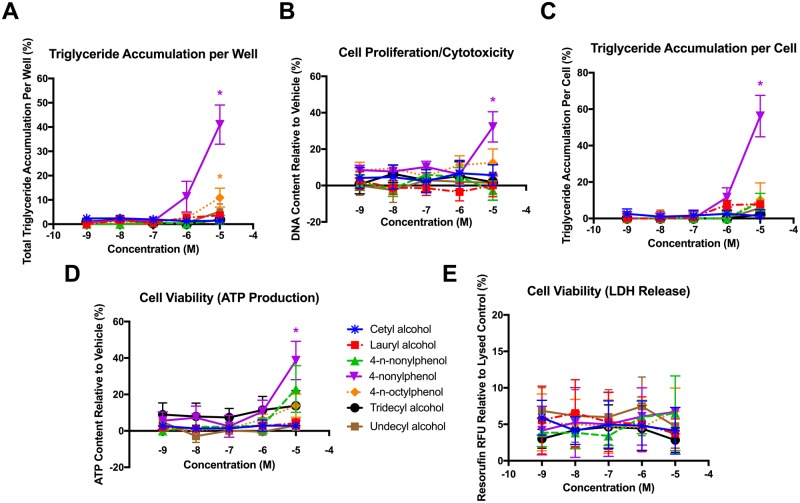
In order to determine whether these activities were associated with the ethoxylate chains or the base hydrophobes, we further assessed the base compounds for adipogenic activity. Only 2 of the hydrophobes, 4-nonylphenol (NPEO [0]) and 4-n-octylphenol (OPEO [0]), exhibited significant triglyceride accumulation at 10 μM (Figure 2A), though this occurred at much lower maximal responses than for the ethoxylated versions (approximately 40% triglyceride accumulation for 4-nonylphenol relative to 150% for NPEO [4], approximately 11% for 4-n-octylphenol relative to approximately 50% for OPEO [3]). Only 4-nonylphenol stimulated preadipocyte proliferation (Figure 2B), and after correcting for DNA content, 4-n-octylphenol no longer exhibited significant triglyceride accumulation (Figure 2C). Neither an inhibition of ATP content nor promotion of LDH release were found for any hydrophobe, verifying that there were no overt toxic effects on the cells for these compounds (Figs. 2D and 2E).
Figure 2.
Alkylphenol and alcohol hydrophobes induce limited or no adipogenic activity. 3T3-L1 cells were induced to differentiate as described in Methods and assessed for degree of adipocyte differentiation (Nile Red staining of intracellular lipids) and preadipocyte proliferation (Hoechst nuclear DNA staining) after 10 days of differentiation while exposed to various alkylphenol or alcohol hydrophobes from 1 nM to 10 μM in concentration. Percent raw triglyceride accumulation per well relative to maximal intra-assay response for rosiglitazone (A), increase (preadipocyte proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B), percent normalized triglyceride accumulation per cell relative to maximal intra-assay rosiglitazone response (normalized to DNA content) (C), increase or decrease in cell viability (ATP content) relative to vehicle control (D), and increase in lactate dehydrogenate release (cell viability measure) relative to lysed control (E). Data presented as mean ± SEM from 3 independent experiments. * indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p < .05, as per linear mixed model in SAS 9.4.
Adipogenic Activity of Varying Ethoxylate Chain Length NPEOs
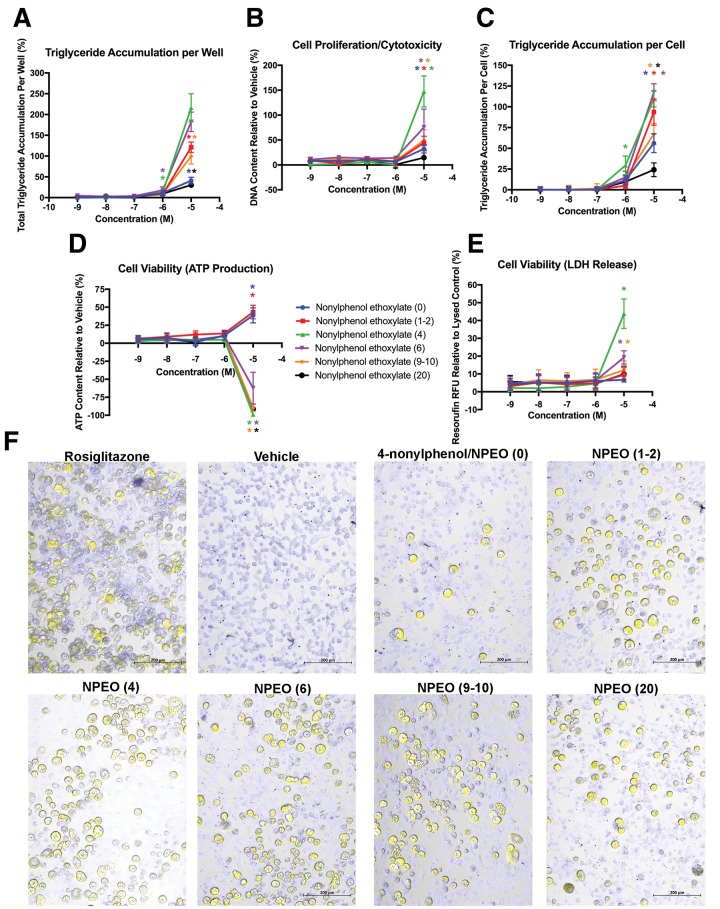
Four additional (five total) NPEOs were selected with varying ethoxylate chain lengths (Table 1), to further assess the contribution of ethoxylate chain length to the degree of adipogenic activity described earlier. All the NPEOs, as well as the 4-nonylphenol hydrophobe (NPEO [0]), induced significant triglyceride accumulation at 1 and/or 10 μM (Figs. 3A and 3C), and all but NPEO (20) induced significant cell proliferation at 10 μM (Figure 3B). The activity appeared to be related to the ethoxylate chain length, with the greatest triglyceride accumulation and cell proliferation observed for NPEO (4), the next highest for NPEO (6), and then progressively lower adipogenic activity with decreasing ethoxylate length for NPEO (1-2) to (0) and with increasing ethoxylate length for NPEO (9–10) to (20). NPEO (20) induced equivalent adipogenic activity to the 4-nonylphenol hydrophobe, suggesting that a shorter ethoxylate chain length is essential to modulating adipogenic activity. NPEO (4), (6), (9–10), and (20) induced a near complete inhibition of ATP content (Figure 3D), and NPEO (4), (6), and (9–10) promoted LDH release (Figure 3E), demonstrating that adipogenic activity was co-occurring with inhibition of cell viability. The shorter chain lengths did not appear to inhibit cell viability, with 4-nonylphenol and NPEO (1–2) neither inhibiting ATP nor increasing LDH. Imaging confirmed classical triglyceride accumulation morphologies that were similar to rosiglitazone, only varying by the extent of accumulation (Figure 3F).
Figure 3.
Various ethoxylate chain length NPEOs induce biphasic adipogenic activity. 3T3-L1 cells were induced to differentiate as described in “Materials and methods” section and assessed for degree of adipocyte differentiation (Nile Red staining of intracellular lipids) and preadipocyte proliferation (Hoechst nuclear DNA staining) after 10 days of differentiation while exposed to various ethoxylate length NPEOs from 1 nM to 10 μM in concentration. Percent raw triglyceride accumulation per well relative to maximal intra-assay response for rosiglitazone (A), increase (preadipocyte proliferation) or decrease (potential cytotoxicity) in DNA content relative to vehicle control (B), percent normalized triglyceride accumulation per cell relative to maximal intra-assay rosiglitazone response (normalized to DNA content) (C), increase or decrease in cell viability (ATP content) relative to vehicle control (D), and increase in lactate dehydrogenate release (cell viability measure) relative to lysed control (E). Data presented as mean ± SEM from 3 independent experiments. Representative image of differentiated cells induced in a 24-well plate following 10 days of exposure to 1 μM rosiglitazone, 0.1% DMSO, or 10 μM 4-nonylphenol, and NPEOs with an average of 1–2, 4, 6, 9–10, or 20 ethoxylate chains (F), using a Zeiss Axio Observer microscope and Photometrics CoolSNAP ES2 CCD camera and ZEN Pro 2.3. Imaging merged a phase contrast field with yellow fluorescent protein (Nile Red lipid staining) and DAPI (NucBlue DNA content) filters to visualize the cells. Bars are provided for scale. *indicates lowest concentration with significant increase in triglyceride over vehicle control or cell proliferation/cytotoxicity relative to vehicle control, p < .05, as per linear mixed model in SAS 9.4.
To attempt to better distinguish adipogenic activity from cytotoxicity or inhibited viability, we supplemented our differentiation media with dexamethasone as above and re-examined various ethoxylate length NPEOs. The addition of dexamethasone increased the adipogenic potency of most NPEOs, with significant triglyceride accumulation at 1 μM for not only NPEO (4) and (6) as before, but also NPEO (0), (9–10), and (20) (Supplementary Figs. 3A and 3C); however, it did not increase the potency of NPEO (1–2). Dexamethasone also significantly altered the cell proliferation profile, with NPEO (6) inducing a >400% increase in DNA content at 10 μM, and NPEO (4) and (9–10) inducing 125%–175% (Supplementary Figure 3B); however, NPEO (0–2) did not induce any significant change in DNA content, and NPEO (20) induced significant cytotoxicity at 10 μM. This result successfully distinguished triglyceride accumulation from cell viability loss, with inhibited ATP only at 10 μM for NPEO (4) and longer ethoxylate length compounds (Supplementary Figure 3D). In contrast, dexamethasone again resulted in a greater LDH release across most NPEOs (Supplementary Figure 3E).
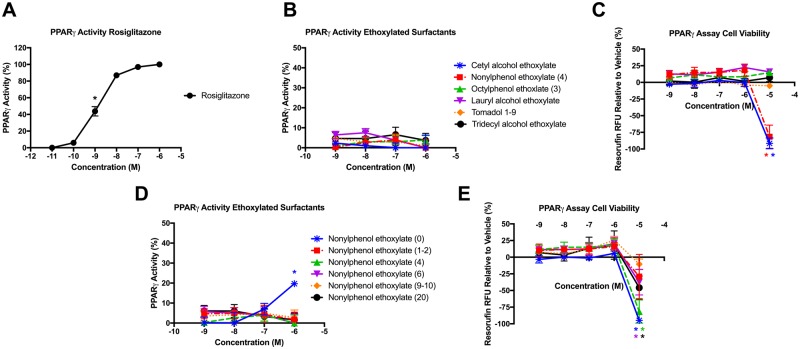
PPARγ Activity of Adipogenic Ethoxylated Surfactants
Each of the chemicals for which we observed adipogenic activity (each ethoxylated surfactant, as well as the 4-nonylphenol and 4-n-octylphenol bases) were further assessed for PPARγ activity using the GeneBLAzer PPARγ FRET assay relative to a rosiglitazone control (Figure 4A). None of the various alkyl length surfactants induced significant PPARγ activation at 1 μM (Figure 4B), and higher concentrations could not be assessed due to inhibition of cell viability and interference with assay chemistry (Figure 4C). In addition, none of the various ethoxylate length NPEOs induced significant PPARγ activation at 1 μM, except for the 4-nonylphenol hydrophobe, which induced approximately 20% activation relative to the rosiglitazone-induced maximum (Figure 4D). As before, nearly all NPEOs induced significant cell viability loss and interfered with assay chemistry at 10 μM (Figure 4E). To address the technical issues with the GeneBLAzer assay in a cell-free system and ensure that these surfactants were in fact occurring via PPARγ, we utilized the Invitrogen LanthaScreen TR-FRET PPARγ Coactivator Assay, relative to a GW1929 positive control (Supplementary Figure 4A). Neither the various alkyl length surfactants (Supplementary Figure 4B) nor the various ethoxylate length NPEOs (Supplementary Figure 4C) induced significant binding to PPARγ and recruitment of the coactivator, further confirming the adipogenic mechanism was not PPARγ-mediated.
Figure 4.
Ethoxylated surfactants do not act as agonists for PPARγ. Agonist activity for the PPARγ as measured via FRET reporter gene assay using the PPARγ GeneBLAzer Reporter Assay as described in the Methods. Percent agonist PPARγ activity for the rosiglitazone positive control relative to its maximum response (A), percent PPARγ activity for various alkyl chain length ethoxylated surfactants relative to the intra-assay rosiglitazone-induced maximum (B), inhibition or enhancement of cell viability relative to vehicle control for various alkyl chain length ethoxylated surfactants (C), percent PPARγ activity for various ethoxylate chain length NPEOs relative to the intra-assay rosiglitazone-induced maximum (D), and inhibition or enhancement of cell viability relative to vehicle control for various ethoxylate chain length NPEOs (E). Data presented as mean ± SEM from 3 independent experiments. * indicates lowest concentration with significant agonist activity relative to vehicle control, p < .05, as per linear mixed model in SAS 9.4.
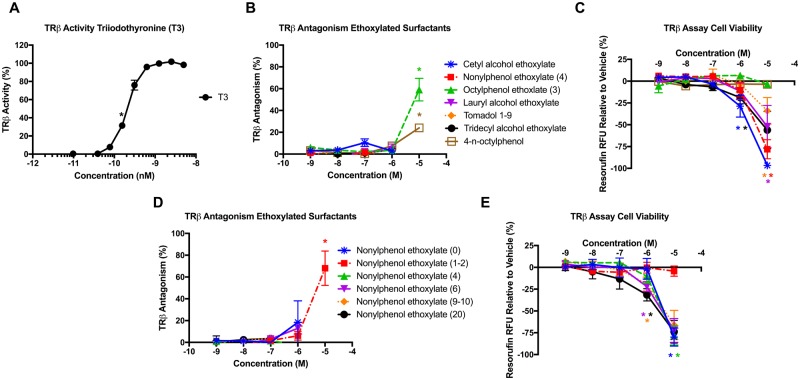
TRβ Activity of Adipogenic Ethoxylated Surfactants
Each of the adipogenic chemicals (each ethoxylated surfactant, as well as the alkylphenol hydrophobes) were further assessed for TRβ antagonism, another potential adipogenic mechanism (Kassotis et al., 2017b), using the GeneBLAzer TRβ FRET assay relative to a T3 control (Figure 5A). OPEO (3) and NPEO (1-2) were the only ethoxylated surfactants that induced significant TRβ inhibition (Figs. 5B and 5D), exhibiting approximately 59% and 68% inhibition at 10 μM, respectively, though the other compounds could not be assessed at concentrations >1 μM due to inhibition of cell viability (Figs. 5C and 5E). Interestingly, the octylphenol hydrophobe also induced approximately 24% TRβ inhibition at 10 μM (Figure 5B), suggesting that the base might contribute to some degree of the antagonism, though the ethoxylate chains appeared to increase this activity; notably, the 4-nonylphenol hydrophobe did not induce antagonism, suggesting that the activity exhibited by NPEO (1–2) was due to the presence of the ethoxylate chains, and not due to the base hydrophobe.
Figure 5.
Ethoxylated Surfactants May Promote Adipogenesis via Inhibition of TRβ. Antagonist activity for the TRβ as measured via FRET reporter gene assay using the TRβ GeneBLAzer Reporter Assay as described in the Methods. Percent agonist TRβ activity for the T3 positive control relative to its maximum response (A), percent TRβ antagonism of intra-assay EC80 T3 (concentration that exhibits 80% of maximum T3 response; 0.3 nM) for various alkyl chain length ethoxylated surfactants (B), inhibition or enhancement of cell viability relative to vehicle control for various alkyl chain length ethoxylated surfactants (C), percent TRβ antagonism relative to intra-assay EC80 T3 for various ethoxylate chain length NPEOs (D), and inhibition or enhancement of cell viability relative to vehicle control for various ethoxylate chain length NPEOs (E). Data presented as mean ± SEM from 3 independent experiments. * indicates lowest concentration with significant antagonist activity relative to EC80 T3, p < .05, as per 1-way ANOVA and Dunnett’s posthoc test in GraphPad Prism 7.0.
DISCUSSION
We report for the first time that nonionic alkylphenol and AEOs, ubiquitous environmental contaminants used in many common household products, promote triglyceride accumulation and/or preadipocyte proliferation in 3T3-L1 cells. This effect appeared to be associated with the length of the surfactants’ ethoxylate chains, as the respective alkylphenol and alcohol hydrophobes exhibited minimal or no adipogenic activity. In addition, NPEOs of various ethoxylate lengths exhibited biphasic adipogenic activity, with increasing triglyceride accumulation and preadipocyte proliferation from NPEO (0) through NPEO (4), and then decreasing activities with increasing length to NPEO (20); similar effects were observed in the cell viability results from the GeneBLAzer assays. This is in direct contrast to previous results reporting decreasing toxicity with increasing ethoxylate chain length (Talmage, 1994; White et al., 1994), which has suggested less environmental toxicity for longer chain ethoxylated surfactants. It’s possible that a short ethoxylate chain allows for greater lipophilicity, whereas the longer tails inhibit membrane permeability, limiting nuclear receptor activation. In addition, despite some similar toxicity concerns described in aquatic species and wildlife following exposure to AEOs, AEO-induced effects have generally occurred at higher concentrations than APEOs (reviewed in Talmage, 1994), suggesting that AEOs may be of less concern than APEOs and base hydrophobes. Despite this, we report a dramatic promotion of triglyceride accumulation and cell proliferation following exposure to AEOs in addition to the APEOs (ie, 250% triglyceride accumulation and doubling of DNA content for CetAEO), suggesting that these compounds may have similar potencies/magnitude of effects on metabolic disruption. AEOs such as CetAEO are high-production volume chemicals that are widely used in household detergents, cleaners, cosmetics, agriculture, and in pharmaceutical and plastic applications (Cowan-Ellsberry et al., 2014; Sanderson et al., 2013; Talmage, 1994); as such, these may represent a common environmental contaminant and potential concern for adverse human and animal health.
Two primary adipogenic mechanisms, agonism of PPARγ and antagonism of TRβ (Kassotis et al., 2017b), were queried using cell-based reporter and cell-free coregulator recruitment assay. No significant PPARγ activity was observed for any of the ethoxylated surfactants, though the 4-nonylphenol base induced significant agonist activity in the GeneBLAzer reporter assay. This suggests that these compounds promoted triglyceride accumulation and preadipocyte proliferation through a nonPPARγ-dependent mechanism. We have previously demonstrated a strong effect of TR inhibition on these adipogenic endpoints (Kassotis et al., 2017b), so we also assessed TRβ antagonism. Significant putative antagonism was noted for OPEO, 4-n-octylphenol, and NPEO (1–2) at concentrations that were also active in the 3T3-L1 adipogenesis assays. This suggests that TRβ could be a contributory mechanism of the adipogenic responses reported herein, though due to the inhibition of cell viability across the surfactants at similar and greater concentrations, we cannot rule out a toxicity-mediated inhibition of fluorescence rather than a specific TRβ-mediated decrease in beta-lactamase expression. Significant triglyceride accumulation was noted for NPEO (1–2) at concentrations below which any TRβ inhibition was measured. This could be due to varying cell lines exhibiting different sensitivities via different coregulatory protein environments (McKenna and O’Malley, 2002; Smith and O’Malley, 2004), among other factors (Cheng, 2000), or could reflect other contributory receptor pathways that have yet to be elucidated.
As noted above, we experienced issues with cell viability loss and interference with assay chemistry when using the GeneBLAzer PPARγ assay, and to a lesser degree using the TRβ assay. As a FRET-based assay, the proportion of blue fluorescence should increase when the activated beta-lactamase reporter cleaves the green-fluorescing substrate; response is then determined using a blue: green fluorescence ratio. At concentrations higher than 1 μM in the PPARγ assay, we instead observed dramatic reductions in both green and blue fluorescence as well as inhibited viability in our replicate cell viability plates. Despite the precipitous inhibition of fluorescence for both green and blue channels, the response ratio for many of the surfactants (CetAEO, NPEO 1–2, 4, and 6, OPEO, and 4-nonylphenol all exhibited this phenotype) resulted in putative 150%–300% activations relative to rosiglitazone, suggesting efficacious PPARγ activities. This phenomenon has been described previously as a systematic artifact by other researchers using the GeneBLAzer assays (Hsieh, 2016; Hsieh et al., 2015), and necessitates careful quality control to ensure these artifacts are not reported as activity. Although these same assay artifacts were not observed to the same extent in the TRβ assays, the apparent toxicity of the tested surfactants makes it difficult to tease apart true receptor inhibition from putative toxicity or other nonreceptor-mediated mechanisms for altered beta-lactamase activity, including receptor-independent reduction in reporter expression (Freyberger and Schmuck, 2005), compound aggregation-based inhibition (Thorne et al., 2010), pH-mediated transcriptional inhibition/enhancement (Hornung et al., 2017), cell stress-mediated nonspecific receptor activation, ie, cytotoxic signal burst (Judson et al., 2016), or other physicochemical properties that might interfere with assay chemistry (Inglese et al., 2007). However, the OPEO, 4-n-octylphenol hydrophobe, and NPEO (1–2) did not exhibit toxicity or inhibited cell viability in the TRβ assay, lending confidence that these may be specific effects and TRβ antagonism a potential mechanism for at least a portion of the observed adipogenic activity.
We also routinely observed an apparent disconnect between our cell proliferation/cytotoxicity screen (DNA content) and our cell viability screens (ATP content, LDH release) in the adipogenesis assays. Although DNA content assays are a well-recognized, robust measure of cell number via nuclear DNA staining, we suspected that metabolically disrupted cells could have compromised cell viability without experiencing gross toxicity. We’ve previously reported conflicting results between the DNA and ATP assays for certain semivolatile indoor contaminants (Kassotis et al., 2017a); several mitochondrial toxicants were able to inhibit ATP production while also promoting proliferation. To attempt to better delineate true cytotoxicity from cell viability for these chemicals, we added a LDH release assay, providing us 3 independent measures of cell health. These assays sometimes provided disparate results: our positive control rosiglitazone-induced cell proliferation (increased DNA content), and this mitogenic effect was mirrored via increased ATP content. However, a large LDH release was observed at these same concentrations, which would seemingly suggest impaired cell viability. However, a rosiglitazone-induced LDH release has been described previously in 3T3-L1 cells (Staiger and Loffler, 1998; Zeng et al., 2012), and may be due to 1) the increased leptin production following differentiation, as leptin has been reported to increase LDH activity (Oliveira et al., 2017), or 2) “leaky” membranes due to the accumulation of triglycerides that may allow for the release of LDH from still-viable cells (Staiger and Loffler, 1998). As such, if LDH release may be due to either inhibited cell viability or as a measure of typical triglyceride accumulation, it complicates the interpretation of results. Consistent with this hypothesis, addition of dexamethasone to the differentiation media resulted in a greater LDH release for most of the surfactants (Supplementary Figs. 1 and 2), consistent with a further degree of differentiation. These differences may also reflect the heterogeneous nature of 3T3-L1 cells; by the final day of differentiation, each culture typically contains fully differentiated adipocytes, adipocytes in varying degrees of differentiation, and preadipocytes that have failed to differentiate and may still be proliferating. These different populations may contribute to the altered responses observed.
We are cognizant of the inherent toxicity of ethoxylated surfactants, and the issues this causes with interpretation of cell-based assays. Surfactants have been well reported to modify the structure and permeability of membrane phospholipids, potentially creating issues in interpretation of cell-based cytotoxicity and/or cell viability assays (Cserhati, 1995). Interestingly, surfactants have also been reported to modulate the biological activity of various enzymes (Cserhati, 1995), creating additional concerns for the interpretation of these assays and interpretation of nuclear receptor activation or inhibition results. As such, we took a rigorous approach to reporting nuclear receptor disruption, not reporting any receptor activity values for concentrations where any suggestion of cytotoxicity or inhibited cell viability was observed. Although it appeared that impaired cell health was also observed at the highest concentrations in the adipogenesis assay (ie, ATP inhibition for all ethoxylated surfactants except Tomadol and NPEO [1–2]), we were able to improve the triglyceride accumulation sensitivity (significant accumulation at lower concentrations) and separate it from the putative toxicity by modulating the differentiation cocktail. As such, and due to the extended duration of the differentiation assay, we feel confident in our assessment of the adipogenic phenotype, although determining the mechanism is more difficult. Additional receptor screening in cell-based reporter assays may unveil disruption at lower concentrations for other nuclear receptors, which can be more clearly distinguished from cytotoxicity or assay interference. Cell-free receptor screening assays should be employed where possible, such as the coactivator recruitment assay for PPARγ used herein, though due to the known heterogeneity in coactivator recruitment by various ligands (Burris et al., 2013; McDonnell, 1999), we suggest that relative binding affinity assays be employed to ascertain simple binding determinations for these (and similar) chemicals.
We noted some distinct differences in the lipid accumulation phenotypes observed with different treatments of ethoxylated surfactants. Rosiglitazone induced a robust adipogenic phenotype, with increased DNA staining and numerous large lipid droplets, oftentimes displacing the nucleus. These general phenotypes were reproduced following exposure to nearly all the APEOs and AEOs, reinforcing the quantitative adipogenic phenotypes reported herein. However, CetAEO exhibited a notably distinct intracellular triglyceride phenotype, with a high degree of lipid staining, but in much smaller droplets. This phenotypical difference could be due to: different nuclear receptor mechanisms promoting the CetAEO-induced lipid accumulation relative to other ethoxylated surfactants, a varying degree of differentiation achieved by CetAEO-treated cells relative to other APEOs and AEOs (a larger proportion of cells induced to differentiate, although a lesser overall degree of differentiation for these cells), or perhaps a higher degree of toxicity for this treatment relative to others, altering the resultant morphology. Further research should utilize specific nuclear receptor ligands to determine potential molecular mechanism impacts on resultant morphology.
Notably, we observed effects on both preadipocyte proliferation and triglyceride accumulation at concentrations as low as 0.1 μM for AEOs and 1 μM for APEOs, or at equivalent water concentrations of approximately 0.1 and 0.4 ng/l for the most potent AEOs and APEOs, respectively. However, as noted earlier, these classes of compounds are routinely detected at μg/l concentrations (approximately 1–10 nM, for the most potent AEOs and APEOs) in wastewater and other surface water sources, and at ng/l (approximately 1–10 pM) concentrations in drinking water. They have also been detected in the indoor environment and appear to accumulate in indoor house dust, representing an additional source of chronic human exposure. This suggests that there could be metabolic impacts of these individual compounds at environmentally relevant concentrations, demonstrating a need to further assess molecular mechanisms and better characterize environmental concentrations. In addition, given the recent reports of high concentration of AEOs in unconventional oil and gas wastewater, the potential contribution of these chemicals to surface water via wastewater spills and effluent releases suggests another potential environmental exposure pathway. It’s also important to note that the primary degradation mechanism of these long-chain surfactants involves cleavage of the ethoxylate chains, which resulted in increased adipogenic responses, and could contribute to increased biological effects following wastewater treatment.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by a grant from the National Institute of Environmental Health Sciences (R01 ES016099).
Supplementary Material
REFERENCES
- Ahel M., Giger W., Koch M. (1994a). Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment - I. Occurrence and transformation in sewage treatment. Water Res. 28, 1131–1142. [Google Scholar]
- Ahel M., Giger W., Schaffner C. (1994b). Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment - II. Occurrence and transformation in rivers. Water Res. 28, 1143–1152. [Google Scholar]
- Ahel M., Schaffner C., Giger W. (1995). Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment - III. Occurrence and elimination of their persistent metabolites during infiltration of river water to groundwater. Water Res. 30, 37–46. [Google Scholar]
- Balch G., Metcalfe C. (2006). Developmental effects in Japanese medaka (Oryzias latipes) exposed to nonylphenol ethoxylates and their degradation products. Chemosphere 62, 1214–1223.http://dx.doi.org/10.1016/j.chemosphere.2005.02.100 [DOI] [PubMed] [Google Scholar]
- Bandiera S. M. (2006). Reproductive and endocrine effects of p-nonylphenol and methoxychlor: A review. Immunol. Endocr. Metab. Agents Med. Chem. 6, 12..http://dx.doi.org/10.2174/187152206775528833 [Google Scholar]
- Bennie D. T. (1999). Review of the environmental occurrence of alkylphenols and alkylphenol ethoxylates. Water Qual. Res. J. Canada 34, 79–122. [Google Scholar]
- Bowers R. R., Temkin A. M., Guillette L. J., Baatz J. E., Spyropoulos D. D. (2016). The commonly used nonionic surfactant Span 80 has RXRalpha transactivation activity, which likely increases the obesogenic potential of oil dispersants and food emulsifiers. General Comp. Endocrinol. 238, 61–68. [DOI] [PubMed] [Google Scholar]
- Burris T. P., Solt L. A., Wang Y., Crumbley C., Banerjee S., Griffett K., Lundasen T., Hughes T., Kojetin D. J. (2013). Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 65, 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailleaud K., Forget-Leray J., Souissi S., Lardy S., Augagneur S., Budzinski H. (2007). Seasonal variation of hydrophobic organic contaminant concentrations in the water-column of the Seine Estuary and their transfer to a planktonic species Eurytemora affinis (Calanoid, copepod). Part 2: Alkylphenol-polyethoxylates. Chemosphere 70, 281–287. [DOI] [PubMed] [Google Scholar]
- Ceresana. (2015). Market Study: Surfactants (2nd edition). Available at: http://www.ceresana.com/en/market-studies/chemicals/surfactants. Accessed 1 December 2016. [Google Scholar]
- Cheng S. Y. (2000). Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord. 1, 9–18.http://dx.doi.org/10.1023/A:1010052101214 [DOI] [PubMed] [Google Scholar]
- Clark L. B., Rosen R. T., Hartman T. G., Louis J. B., Suffet I. H., Lippincott R. L., Rosen J. D. (2006). Determination of alkylphenol ethoxylates and their acetic acid derivatives in drinking water by particle beam liquid chromatography/mass spectrometry. Int. J. Environ. Anal. Chem. 47, 167–180. [Google Scholar]
- Cowan-Ellsberry C., Belanger S., Dorn P., Dyer S., McAvoy D., Sanderson H., Versteeg D., Ferrer D., Stanton K. (2014). Environmental safety of the use of major surfactant classes in North America. Crit. Rev. Environ. Sci. Technol. 44, 1893–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserhati T. (1995). Alkyl ethoxylated and alkylphenol ethoxylated nonionic surfactants: Interaction with bioactive compounds and biological effects. Environ. Health Perspect. 103, 358–364.http://dx.doi.org/10.1289/ehp.95103358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodder N. G., Maruya K. A., Lee Ferguson P., Grace R., Klosterhaus S., La Guardia M. J., Lauenstein G. G., Ramirez J. (2014). Occurrence of contaminants of emerging concern in mussels (Mytilus spp.) along the California coast and the influence of land use, storm water discharge, and treated wastewater effluent. Marine Pollut. Bull. 81, 340–346. [DOI] [PubMed] [Google Scholar]
- Fang M., Webster T. F., Stapleton H. M. (2015). Activation of human peroxisome proliferator-activated nuclear receptors (PPARgamma1) by semi-volatile compounds (SVOCs) and chemical mixtures in indoor dust. Environ. Sci. Technol. 49, 10057–10064.http://dx.doi.org/10.1021/acs.est.5b01523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson P. L., Bopp R. F., Chillrud S. N., Aller R. C., Brownawell B. J. (2003). Biogeochemistry of nonylphenol ethoxylates in urban estuarine sediments. Environ. Sci. Technol. 37, 3499–3506. [DOI] [PubMed] [Google Scholar]
- Ferguson P. L., Iden C. R., Brownawell B. J. (2000). Analysis of alkylphenol ethoxylate metabolites in the aquatic environment using liquid chromatography - electrospray mass spectrometry. Anal. Chem. 72, 4322–4330.http://dx.doi.org/10.1021/ac000342n [DOI] [PubMed] [Google Scholar]
- Ferguson P. L., Iden C. R., Brownawell B. J. (2001). Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environ. Sci. Technol. 35, 2428–2435.http://dx.doi.org/10.1021/es001871b [DOI] [PubMed] [Google Scholar]
- Ferguson P. L., Vogler B., Stapleton H. M. Non-targeted analysis to assess human exposure to semi-volatile organic contaminants in the indoor environment, 31 May–4 June, 2015 2015, St. Louis, MO.
- Freyberger A., Schmuck G. (2005). Screening for estrogenicity and anti-estrogenicity: A critical evaluation of an MVLN cell-based transactivation assay. Toxicol. Lett. 155, 1–13.http://dx.doi.org/10.1016/j.toxlet.2004.06.014 [DOI] [PubMed] [Google Scholar]
- Getzinger G. J., O’Connor M. P., Hoelzer K., Drollette B. D., Karatum O., Deshusses M. A., Ferguson P. L., Elsner M., Plata D. L. (2015). Natural gas residual fluids: Sources, endpoints, and organic chemical composition after centralized waste treatment in Pennsylvania. Environ. Sci. Technol. 49, 8347–8355. [DOI] [PubMed] [Google Scholar]
- Giger W., Ahel M., Koch M., Laubscher H. U., Schaffner C., Schneider J. (1987). Behaviour of alkylphenol polyethoxylate surfactants and of nitrilotriacetate in sewage treatment. Water Sci. Technol. 19, 449–460. [Google Scholar]
- Giger W., Brunner P. H., Schaffner C. (1984). 4-nonylphenol in sewage sludge: Accumulation of toxic metabolites from nonionic surfactants. Science 225, 623–625.http://dx.doi.org/10.1126/science.6740328 [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. (1975). An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27.http://dx.doi.org/10.1016/0092-8674(75)90087-2 [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. (1974). An established pre-adipose cell line and its differentiation in culture. Cell 3, 127–133.http://dx.doi.org/10.1016/0092-8674(74)90116-0 [DOI] [PubMed] [Google Scholar]
- Hao C. J., Cheng X. J., Xia H. F., Ma X. (2012). The endocrine disruptor 4-nonylphenol promotes adipocyte differentiation and induces obesity in mice. Cell. Physiol. Biochem. 30, 382–394.http://dx.doi.org/10.1159/000339032 [DOI] [PubMed] [Google Scholar]
- Heindel J. J., vom Saal F. S., Blumberg B., Bovolin P., Calamandrei G., Ceresini G., Cohn B. A., Fabbri E., Gioiosa L., Kassotis C., et al. (2015). Parma consensus statement on metabolic disruptors. Environ. Health 14, 54.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung M. W., Tapper M. A., Denny J. S., Sheedy B. R., Erickson R., Sulerud T. J., Kolancyzk R. C., Schmieder P. K. (2017). Avoiding false positives and optimizing identification of true negatives in estrogen receptor binding and agonist/antagonist assays. Appl. In Vitro Toxicol. 3, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. H. (2016). Accounting artifacts in high-throughput toxicity assays. Methods Mol. Biol. 1473, 143–152. [DOI] [PubMed] [Google Scholar]
- Hsieh J. H., Sedykh A., Huang R., Xia M., Tice R. R. (2015). A data analysis pipeline accounting for artifacts in Tox21 quantitative high-throughput screening assays. J. Biomol. Screen 20, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J., Johnson R. L., Simeonov A., Xia M., Zheng W., Austin C. P., Auld D. S. (2007). High-throughput screening assays for the identification of chemical probes. Nat. Chem. Biol. 3, 466–479. [DOI] [PubMed] [Google Scholar]
- Invitrogen. (2010a). GeneBLAzer® PPAR gamma 293H DA and PPAR gamma-UAS-bla 293H Cell-based Assay Protocol Available at: https://tools.thermofisher.com/content/sfs/manuals/geneblazer_PPARgammaUASbla293H_man.pdf. Accessed December 27, 2016.
- Invitrogen. (2010b). GeneBLAzer® TR beta HEK 293T DA and TR beta-UAS-bla HEK 293T Cell-based Assay Available at: https://tools.thermofisher.com/content/sfs/manuals/geneblazer_TRbetaUASblaHEK293T_man.pdf. Accessed December 27, 2016.
- Invitrogen. (2010c). LanthaScreen® TR-FRET Peroxisome Proliferator Activated Receptor gamma Coactivator Assay. Available at: https://tools.thermofisher.com/content/sfs/manuals/lanthascreen_PPARgamma_competitivebinding_man.pdf. Accessed March 10, 2016.
- Itrich N. R., Federle T. W. (2004). Effect of ethoxylate number and alkyl chain length on the pathway and kinetics of linear alcohol ethoxylate biodegradation in activated sludge. Environ. Toxicol. Chem. 23, 2790–2798.http://dx.doi.org/10.1897/04-053.1 [DOI] [PubMed] [Google Scholar]
- Janesick A. S., Blumberg B. (2016). Obesogens: An emerging threat to public health. Am. J. Obstetr. Gynecol. 214, 559–565.http://dx.doi.org/10.1016/j.ajog.2016.01.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R., Houck K., Martin M., Richard A. M., Knudsen T. B., Shah I., Little S., Wambaugh J., Setzer R. W., Kothiya P., et al. (2016). Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol. Sci. 153, 409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C. D., Hoffman K., Stapleton H. M. (2017a). Characterization of adipogenic activity of semi-volatile indoor contaminants and house dust. Environ. Sci. Technol. 51, 8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis C. D., Masse L., Kim S., Schlezinger J. J., Webster T. F., Stapleton H. M. (2017b). Characterization of adipogenic chemicals in three different cell culture systems: Implications for reproducibility based on cell source and handling. Sci. Rep. 7, 42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester Y., Ferrer I., Thurman E. M., Sitterley K. A., Korak J. A., Aiken G., Linden K. G. (2015). Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Sci. Total Environ. 512–513, 637–644. [DOI] [PubMed] [Google Scholar]
- Loos R., Hanke G., Umlauf G., Eisenreich S. J. (2007). LC-MS-MS analysis and occurrence of octyl- and nonylphenol, their ethoxylates and their carboxylates in Belgian and Italian textile industry, waste water treatment plant effluents and surface waters. Chemosphere 66, 690–699.http://dx.doi.org/10.1016/j.chemosphere.2006.07.060 [DOI] [PubMed] [Google Scholar]
- Maguire R. J. (1999). Review of the persistence of nonylphenol and nonylphenol ethoxylates in aquatic environments. Water Qual. Res. J. Canada 34, 37–78. [Google Scholar]
- Mann A. H., Reid V. W. (1971). Biodegradation of synthetic detergents evaluation by community trials Part 2: Alcohol and alkylphenol ethoxylates. J. Am. Oil Chem. Soc. 48, 294–297.http://dx.doi.org/10.1007/BF02609284 [Google Scholar]
- Manzano M. A., Perales J. A., Sales D., Quiroga J. M. (1999). The effect of temperature on the biodegradation of a nonylphenol polyethoxylate in river water. Water Res. 33, 2593–2600. [DOI] [PubMed] [Google Scholar]
- Marcomini A., Capri S., Giger W. (1987). Determination of linear alkylbenzenesulphonates, alkylphenol polyethoxylates and nonylphenol in waste water by high-performance liquid chromatography after enrichment on octadecylsilica. J. Chromatogr. 403, 243–252.http://dx.doi.org/10.1016/S0021-9673(00)96358-1 [DOI] [PubMed] [Google Scholar]
- Marcomini A., Filipuzzi F., Giger W. (1988). Aromatic surfactants in laundry detergents and hard-surface cleaners: Linear alkylbenzenesulphonates and alkylphenol polyethoxylates. Chemosphere 17, 853–863.http://dx.doi.org/10.1016/0045-6535(88)90058-6 [Google Scholar]
- Marcomini A., Stelluto S., Pavoni B. (1989). Determination of linear alkylbenzenesulphonates and alkylphenol polyethoxylates in commercial products and marine waters by reversed- and normal-phase HPLC. Int. J. Environ. Anal. Chem. 35, 207–218.http://dx.doi.org/10.1080/03067318908028395 [Google Scholar]
- Masuno H., Okamoto S., Iwanami J., Honda K., Shiosaka T., Kidani T., Sakayama K., Yamamoto H. (2003). Effect of 4-nonylphenol on cell proliferation and adipocyte formation in cultures of fully differentiated 3T3-L1 cells. Toxicol. Sci. 75, 314–320. [DOI] [PubMed] [Google Scholar]
- McDonnell D. P. (1999). The Molecular Pharmacology of SERMs. Trends Endocrinol. Metab. 10, 301–311.http://dx.doi.org/10.1016/S1043-2760(99)00177-0 [DOI] [PubMed] [Google Scholar]
- McKenna N. J., O'Malley B. W. (2002). Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108, 465–474.http://dx.doi.org/10.1016/S0092-8674(02)00641-4 [DOI] [PubMed] [Google Scholar]
- Miyawaki J., Kamei S., Sakayama K., Yamamoto H., Masuno H. (2008). 4-tert-octylphenol regulates the differentiation of C3H10T1/2 cells into osteoblast and adipocyte lineages. Toxicol. Sci. 102, 82–88. [DOI] [PubMed] [Google Scholar]
- Nichols K. M., Snyder E. M., Snyder S. A., Pierens S. L., Miles-Richardson S. R., Giesy J. P. (2001). Effects of nonylphenol ethoxylate exposure on reproductive output and bioindicators of environmental estrogen exposure in fathead minnows Pimephales promelas. Environ. Toxicol. Chem. 20, 510–522. [DOI] [PubMed] [Google Scholar]
- Oliveira P. F., Sousa M., Silva B. M., Monteiro M. P., Alves M. G. (2017). Obesity, energy balance and spermatogenesis. Reproduction 153, R173–R185. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho E. C., Grisolia C. K., Paumgartten F. J. (2009). Trans-generation study of the effects of nonylphenol ethoxylate on the reproduction of the snail Biomphalaria tenagophila. Ecotoxicol. Environ. Saf. 72, 458–465. [DOI] [PubMed] [Google Scholar]
- Reznik G. O., Vishwanath P., Pynn M. A., Sitnik J. M., Todd J. J., Wu J., Jiang Y., Keenan B. G., Castle A. B., Haskell R. F. (2010). Use of sustainable chemistry to produce an acyl amino acid surfactant. Appl. Microbiol. Biotechnol. 86, 1387–1397. [DOI] [PubMed] [Google Scholar]
- Sabik H., Gagne F., Blaise C., Marcogliese D. J., Jeannot R. (2003). Occurrence of alkylphenol polyethoxylates in the St. Lawrence River and their bioconcentration by mussels (Elliptio complanata). Chemosphere 51, 349–356.http://dx.doi.org/10.1016/S0045-6535(02)00862-7 [DOI] [PubMed] [Google Scholar]
- Sanderson H., van Compernolle R., Dyer S. D., Price B. B., Nielsen A. M., Selby M., Ferrer D., Stanton K. (2013). Occurrence and risk screening of alcohol ethoxylate surfactants in three U.S. river sediments associated with wastewater treatment plants. Sci. Total Environ. 463-464, 600–610. [DOI] [PubMed] [Google Scholar]
- Sargis R. M., Johnson D. N., Choudhury R. A., Brady M. J. (2010). Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 18, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Afonso I., Loyo-Rosales J. E., de la Paz Aviles M., Rattner B. A., Rice C. P. (2003). Determination of alkylphenol and alkylphenolethoxylates in biota by liquid chromatography with detection by tandem mass spectrometry and fluorescence spectroscopy. J. Chromatogr. A 1010, 25–35. [DOI] [PubMed] [Google Scholar]
- Servos M. R. (1999). Review of the aquatic toxicity, estrogenic responses and bioaccumulation of alkylphenols and alkylphenol polyethoxylates. Water Qual. Res. J. Canada 34, 123–177. [Google Scholar]
- Smith C. L., O’Malley B. W. (2004). Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71. [DOI] [PubMed] [Google Scholar]
- Staiger H., Loffler G. (1998). The role of PDGF-dependent suppression of apoptosis in differentiating 3T3-L1 preadipocytes. Eur. J. Cell Biol. 77, 220–227.http://dx.doi.org/10.1016/S0171-9335(98)80110-6 [DOI] [PubMed] [Google Scholar]
- Talmage S. S. (1994). Environmental and human safety of major surfactants: Alcohol ethoxylates and alkylphenol ethoxylates. 374. Available at: http://www.aciscience.org/docs/13_Alcohol_Ethoxylates.pdf Accessed October 16, 2017.
- Temkin A. M., Bowers R. R., Magaletta M. E., Holshouser S., Maggi A., Ciana P., Guillette L. J., Bowden J. A., Kucklick J. R., Baatz J. E. (2016). Effects of crude oil/dispersant mixture and dispersant components on PPARgamma activity and: Identification of dioctyl sodium sulfosuccinate (DOSS; CAS #577-11-7) as a probable obesogen. Environ. Health Perspect. 124, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Endocrine Disruptor Exchange (TEDX) (2017). TEDX List of Potential Endocrine Disruptors. Available at: https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list. Accessed November 5, 2017. [Google Scholar]
- Thorne N., Auld D. S., Inglese J. (2010). Apparent activity in high-throughput screening: Origins of compound-dependent assay interference. Curr Opin Chem Biol 14, 315–324.http://dx.doi.org/10.1016/j.cbpa.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman E. M., Ferrer I., Blotevogel J., Borch T. (2014). Analysis of hydraulic fracturing flowback and produced waters using accurate mass: Identification of ethoxylated surfactants. Anal. Chem. 86, 9653–9661.http://dx.doi.org/10.1021/ac502163k [DOI] [PubMed] [Google Scholar]
- Uguz C., Varisli O., Agca C., Agca Y. (2009). Effects of nonylphenol on motility and subcellular elements of epididymal rat sperm. Reprod. Toxicol. 28, 542–549.http://dx.doi.org/10.1016/j.reprotox.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Vega-Morales T., Sosa-Ferrera Z., Santana-Rodriguez J. J. (2010). Determination of alkylphenol polyethoxylates, bisphenol-A, 17alpha-ethynylestradiol and 17beta-estradiol and its metabolites in sewage samples by SPE and LC/MS/MS. J. Hazard. Mater. 183, 701–711. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. (1994). Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 135, 175–182. [DOI] [PubMed] [Google Scholar]
- Ying G. G., Williams B., Kookana R. S. (2002). Environmental fate of alkylphenols and alkylphenol ethoxylates - A review. Environ. Int. 28, 215..http://dx.doi.org/10.1016/S0160-4120(02)00017-X [DOI] [PubMed] [Google Scholar]
- Zeng X. Y., Zhou X., Xu J., Chan S. M., Xue C. L., Molero J. C., Ye J. M. (2012). Screening for the efficacy on lipid accumulation in 3T3-L1 cells is an effective tool for the identification of new anti-diabetic compounds. Biochem. Pharmacol. 84, 830–837. [DOI] [PubMed] [Google Scholar]
- Zoller U. (2006). Estuarine and coastal zone marine pollution by the nonionic alkylphenol ethoxylates endocrine disrupters: Is there a potential ecotoxicological problem? Environm. Int. 32, 269–272.http://dx.doi.org/10.1016/j.envint.2005.08.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.