Abstract
Sensory neuron membrane proteins (SNMPs) play an important role in insect chemoreception; however, the SNMPs for Bactrocera minax (Enderlein) (Diptera: Tephritidae), an economically important pest of citrus, remain uncharacterized. Here, we report on the molecular characterization of SNMPs (BminSNMP1 and BminSNMP2) from adult B. minax. The open-reading frames of BminSNMP1 and BminSNMP2 were 1,608 and 1,647 nucleotides, encoding proteins of 535 and 557 amino acid residues, respectively. Phylogenetic analysis showed that the two BminSNMPs belonged to two distinct subgroups, indicating the possibility of their contrasting function in insect chemoreception. Real-time PCR results showed that BminSNMP1 was expressed primarily in the antennae of males and females, where levels of expression were similar at different developmental stages of females, but lower in 1- and 5-d-old males than in 15- and 20-d-old males. In both sexes, BminSNMP2 was expressed at high levels in antennae and in nonolfactory tissues, especially in legs, where levels were higher than in other nonolfactory tissues. We found highest levels of expression of BminSNMP2 in antennae of both sexes in 30-d-old adults, while in legs of both sexes, highest levels of expression were detected in 1- and 30-d-old adults. We discuss the possible physiological functions of BminSNMPs based on our findings.
Keywords: Bactrocera minax, sensory neuron membrane protein, molecular identification, spatiotemporal expression profile, real-time PCR
Olfaction is an essential sensory system involved in survival and reproduction of many insect species, because it is used to sense chemical cues to seek mates and host plants, and avoid predators (Zhou 2010, Leal 2013). Insects have evolved sophisticated olfactory sensilla that are generally located on the antennae and feature multipores on their cuticular wall (Schneider 1964, Steinbrecht 1997). Olfactory-associated proteins within a sensillum are used in olfaction sensation (Suh et al. 2014), and when hydrophobic odorants, such as pheromones or plant volatiles, enter the sensillum lymph via cuticular pores, two binding protein families (odorant binding proteins, OBPs, and chemosensory protein, CSP) will bind to the odorants and shuttle them across the aqueous lymph to two receptor families (odorant receptors, ORs, and ionotropic receptors, IRs) expressed in the olfactory sensory neurons (OSNs), where signal transduction to the central nervous system occurs (Pelosi et al. 2006, Benton et al. 2009, Zhou 2010, Leal 2013). Odorant degrading enzymes (ODEs) then degrade the odorants to quickly end the signal transduction in the sensillum (Vogt 2003, Leal 2013). The sensory neuron membrane proteins (SNMPs) are an addition protein family in the OSNs that is also thought to be involved in olfaction.
SNMPs were first identified in Antheraea polyphemus (Cramer) (Lepidoptera: Saturniidae) on the pheromone-sensory neuron membrane, indicating their role in insect pheromone reception (Rogers et al. 1997, Benton et al. 2007). SNMPs are homologs of the mammalian CD36 gene family of fatty acid and cholesterol transporters that feature two transmembrane domains (Rogers et al. 1997, Nichols and Vogt 2008), and to date, two SNMP subfamilies have been characterized from several insect orders, including the Diptera, Lepidoptera, and Hymenoptera (Nichols and Vogt 2008, Liu et al. 2014).
While the SNMP DmelSNMP1 functions as a vital signaling component in sensing the pheromone cis-vaccenyl acetate (cVA) in Drosophila melanogaster (Meigen) (Diptera: Drosophilidae) (Benton et al. 2007, Jin et al. 2008) and Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) (Benton et al. 2007), it has also been shown that SNMPs may inhibit pheromone neuron activity in the absence of cVA (Jin et al. 2008, Li et al. 2014). Thus, it is conceivable that SNMPs may act as molecular targets that facilitate the design of pheromone-mediated manipulation of behavior. The SNMP genes SNMP1 and SNMP2 have been found to be highly enriched in the antennae of some insect species (Liu et al. 2013a, Liu et al. 2014, Zhang et al. 2015), reflecting their role in olfactory sensation; however, SNMPs are also expressed in nonolfactory tissue (Jin et al. 2008), such as in the leg and wing, in Aedes aegypti (Linnaeus) (Diptera: Culicidae) and D. melanogaster (Vogt et al. 2009). This expression of SNMP genes in a range of body tissues indicates the likelihood of their multifunctional roles in insects.
In this study, we identified two new SNMP orthologues (BminSNMP1 and BminSNMP2) from the citrus fruit fly, Bactrocera minax (Enderlein) (Diptera: Tephritidae), which is an economically important citrus pest in China, to elucidate the degree of similarity and spatiotemporal expression profiles of BminSNMPs within the species.
Materials and Methods
Insect Material
We used a laboratory colony of adult B. minax from the College of Agriculture at Yangtze University, China, where they were maintained as described by Du et al. (2018).
We collected antennae, heads without antennae, thoraces, abdomens, legs, and wings from 1- to 3-d-old adults of both sexes, and additional antenna and leg material was removed from 1-, 5-, 10-, 15-, 20-, and 30-d-old adult males and females. These time points represent different behavioral phases of B. minax: newly emerged adult (1 d), feeding on nonhost plants (5, 10, 15, and 20 d), and reproduction on host plant (30 d). Samples were stored at −80°C prior to analysis.
SNMP Gene Identification and Phylogenetic Analysis
We searched for potential SNMP genes in a head transcriptome database for adult B. minax using ‘sensory neuron membrane protein’ as keywords, and using BLAST. Candidate SNMP genes were confirmed from Blastx searches compared against the nonredundant GenBank database. DNAstar software 5.01 (Madison, WI) was used to predict open-reading frames (ORFs), and the TMHMM 2.0 server was used to predict transmembrane helices (http://www.cbs.dtu.dk/services/TMHMM-2.0/). Multiple alignments of amino acid sequences were created by Clustal X software (version 1.83), and we used neighbor joining to create phylogenetic trees from 1,000 bootstrap replicates using MEGA 4.0 software (Tamura et al. 2007).
Spatiotemporal SNMP Gene Expression Analysis
Total RNA was extracted using a MiniBEST Universal RNA Extractin Kit (TaKaRa, Shiga, Japan), and single-strand cDNAs were synthesized using a PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa). We used tubulin and GAPDH genes as endogenous controls to normalize expression of BminSNMP1 and BminSNMP2, respectively. The gene-specific primers used to clone BminSNMP1, BminSNMP2, tubulin, and GAPDH are shown in Table 1, and were expected to amplify 181, 182, 198, and 136 bp fragments, respectively. PCR products were further confirmed using custom sequences (Genscript Biotechnologies, Nanjing, China).
Table 1.
Gene-specific primers used to clone BminSNMP1, BminSNMP2, tubulin, and GAPDH
| Primer | Strand | Sequence (5ʹ–3ʹ) |
|---|---|---|
| BminSNMP1 | Sense | TGTCGGCGATGCTGTTTG |
| Antisence | ACCTCGTCGGGATTAGTG | |
| BminSNMP2 | Sense | GCGTGCTCGAAATACAAA |
| Antisence | CTCCACCGAACGACAAAT | |
| GAPDH | Sense | GCAAACTGTGGCGTGATG |
| Antisence | GGTGTTGGGACACGGAAT | |
| tubulin | Sense | CTGAACGCTGACTTACGC |
| Antisence | GAGATAACGTCCGTGTCG |
Real-time PCR was performed using a Bio-Rad CFX connect Real-Time System (Applied Biosystems), with SYBR Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa) as DNA-binding fluorescence dye in a 25 μl reaction system that contained 12.5 μl of SYBR Premix Ex Tap II (Tli RnaseH Plus), 1 μl of each primer (10 μM), 2 μl of sample cDNA, and 8.5 μl of nuclease-free water. The thermal cycling conditions were 95°C for 30 s; 40 cycles of 95°C for 5 s, 57°C for 30 s, and 72°C for 30 s. A dissociation curve was used to determine primer specificity. All test samples were performed in three technical replicates and three biological replicates, except for the antennal samples of 15- and 30-d-old males and females that comprised three technical replicates of two biological replicates. Quantification of relative levels of expression used the 2-ΔΔCT method (Livak and Schmittgen 2001), and all data were normalized to the corresponding endogenous gene levels from the same samples. Temporal and body part differences in expression were analyzed using one-way analysis of variance followed by Tukey’s HSD test in SPSS 17.0 (SPSS Inc., Chicago, IL).
Amplification efficiencies of the target and reference genes using the comparative 2-ΔΔCT method should be approximately equal, so to verify this, we analyzed variation in ΔCT (CT, Target − CT, Reference) from three replicates of four serial 10-fold dilutions of amplified cDNA. Mean CT was calculated for SNMPs, tubulin, and GAPDH to determine ΔCT, and we plotted log cDNA dilution against ΔCT. We found that the amplification efficiencies of SNMP1 and tubulin, and SNMP2 and GAPDH were approximately equal (Supp Table 1 and Fig. 1 [online only]).
Results
Identification of SNMPs
Searches of the head transcriptome of B. minax identified SNMP1 and SNMP2 as candidate genes, which were named as BminSNMP1 and BminSNMP2, respectively. The ORFs of BminSNMP1 and BminSNMP2 consisted of 1,608 and 1,647 nucleotides, respectively. BminSNMP1 is a 535-amino acid protein, with a molecular weight of 60,559 Da and isoelectric point of 6.00, while BminSNMP2 is a 557-amino acid protein, with a molecular weight of 63,036 Da and isoelectric point of 8.84. BminSNMP1 and BminSNMP2 were predicted to have two transmembrane regions at the C- and N-terminals, and a large extracellular loop (Figs. 1 and 2 and Supp Fig. 2 [online only]). Six conserved cysteine residues were seen within the BminSNMP loop region and counterparts from other dipteran insects (Fig. 3).
Fig. 1.
Nucleotide and deduced amino acid sequences of SNMP1 from B. minax. The stop codon is marked with an asterisk, and the two putative transmembrane domains are underlined.
Fig. 2.
Nucleotide and deduced amino acid sequences of SNMP2 from B. minax. The stop codon is marked with an asterisk, and the two putative transmembrane domains are underlined.
Fig. 3.
Alignment of SNMPs from dipteran insects. The six conserved cysteines are highlight in gray, and the two transmembrane domains in BminSNMP1 and BminSNMP2 are boxed. Abbreviated species names and GenBank accession numbers of amino acid sequences are described in Fig. 4.
SNMP Phylogeny
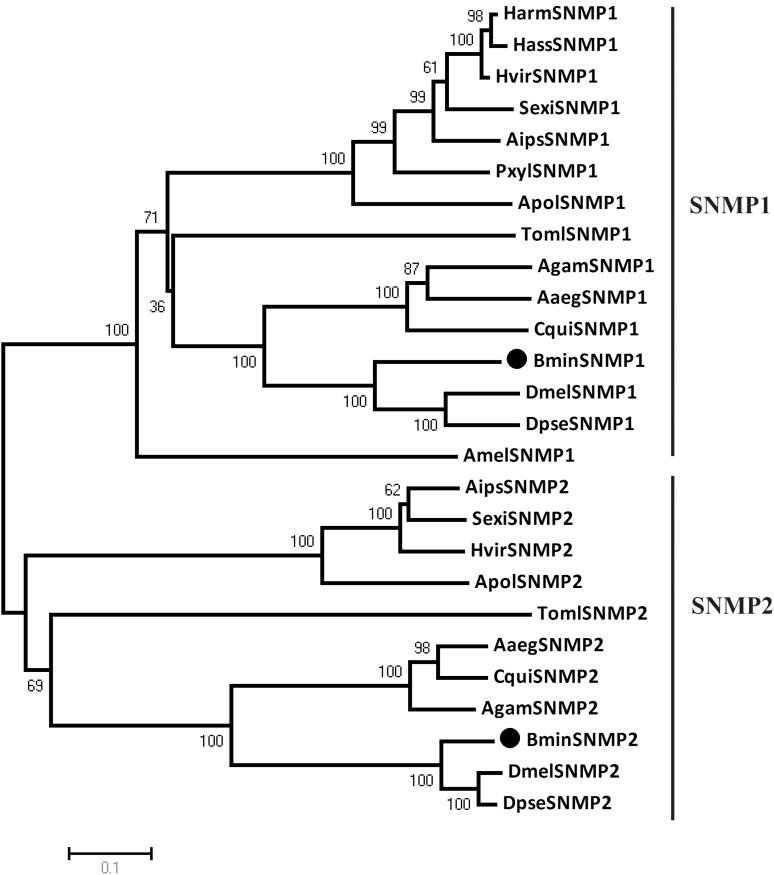
BminSNMP1 shared low sequence identity with BminSNMP2 (22.45%) and high identity with DmelSNMP1 (64.43%), DpseSNMP1 (63.10%), AgamSNMP1 (45.45%), CquiSNMP1 (43.04%), and AaegSNMP1 (42.98%), whereas BminSNMP2 showed high levels of similarity to DmelSNMP2 (85.13%), DpseSNMP2 (81.10%), AgamSNMP2 (45.34%), CquiSNMP2 (43.25%), and AaegSNMP2 (43.08%). SNMP sequences from dipteran, lepidopteran, hymenopteran, and coleopteran insects were summarized in a neighbor-joining tree (Fig. 4) that showed clustering of SNMPs into two clear subgroups (SNMP1 and SNMP2). In each of the two subgroups, all SNMPs from the same order always cluster together forming a clade. BminSNMP1 was closely clustered with the Drosophilidae SNMP1 proteins in SNMP1, and BminSNMP2 was closely clustered with the Drosophilidae SNMP2 proteins in SNMP2.
Fig. 4.
Phlylogenetic analysis of insect SNMPs. The tree is constructed by MEGA4.0 using Neihbor-joining method based on 1,000 bootstrap replicates. Boostrap values above 50 are shown. Those marked with black points refer to two B. minax SNMPs (BminSNMP1 and BminSNMP2). HarmSNMP1: Helicoverpa armigera (AAO15604.1); HassSNMP1: Helicoverpa assulta (ACC61201.1); HvirSNMP1: Heliothis virescens (Q9U1G3.1); SexiSNMP1: Spodoptera exigua (AGN52676.1); AipsSNMP1: Agrotis ipsilon (AGF87119.1); PxylSNMP1: Plutella xylostella (ADK66278.1); ApolSNMP1: Antheraea polyphemus (AAC47540.1); TomlSNMP1: Tenebrio molitor (AJO62245.1); TomlSNMP2: T. molitor (AJO62246.1); AgamSNMP1: Anopheles gambiae (Q7QC49.3); AgamSNMP2: Anopheles gambiae (Q7Q6R1.5); AaegSNMP1: Aedes aegypti (Q17A88.2); AaegSNMP2: Aedes aegypti (C3U0S3.3); CquiSNMP1: Culex quinquefasciatus (EDS40329.1); CquiSNMP2: C. quinquefasciatus, (AEK32389.1); DmelSNMP1: Drosophila melanogaster (AAF55863.2); DmelSNMP2: D. melanogaster (E1JI63.1); DpseSNMP1: D. pseudoobscura (XP_001359654); DpseSNMP2: D. pseudoobscura (XP_001352528); AmelSNMP1: Apis mellifera (P86905.1); AipsSNMP2: Agrotis ipsilon (AGF87120.1); SexiSNMP2: S. exigua (AGN52677.1); HvirSNMP2: Heliothis virescens (B2RFN2.1); and, ApolSNMP2: Antheraea polyphemus (CAP19029).
Spatiotemporal SNMP Gene Expression
There were higher levels of expression of BminSNMP1 in the antennae of both sexes than in the other body parts, where levels were low (Fig. 5A). There were no differences in level of expression of BminSNMP1 in the different developmental stages of adult females; however, there were differences in males, where levels were lower in 1- and 5-d-old adults than in 15- and 20-d-old adults (Fig. 5B).
Fig. 5.
Spatiotemporal expression pattern of the BminSNMP1. (A) Tissue expression profiles of BminSNMP1, calibrated by expression quantity of BminSNMP1 in male thorax. (B) Expression levels of BminSNMP1 in antennae from different developmental stages, calibrated by expression quantity of BminSNMP1 in 1-d-old adults. Error bars are SEMs, and different upper and lower case letters indicate differences in relative levels of expression at P < 0.05 (Tukey’s HSD test) among males and females, respectively.
Transcript levels of BminSNMP2 were significantly higher in male antenna and leg material than in other material, while in females, level of expression was highest in leg material (Fig. 6A). In the antennae of both sexes, we found that transcript levels of BminSNMP2 were relatively low in 1-, 5-, 10-, 15-, and 20-d-old adults, but significantly increased in 30-d-old adults (Fig. 6B). In the legs of both sexes, transcript levels of BminSNMP2 were significantly higher in 1- and 30-d-old adults than at the other developmental stages, except for 20-d-old males (Fig. 6C).
Fig. 6.
Spatiotemporal expression pattern of the BminSNMP2. (A) Tissue expression profiles of BminSNMP2, calibrated by expression quantity of BminSNMP1 in male thorax. (B) Expression levels of BminSNMP2 in antennae from different developmental stages, calibrated by expression quantity of BminSNMP1 in 5-d-old adults. (C) Expression levels of BminSNMP2 in legs from different developmental stages, calibrated by expression quantity of BminSNMP2 in 10-d-old adults. Error bars are SEMs, and different upper and lower case letters indicate differences in relative levels of expression at P < 0.05 (Tukey’s HSD test) among males and females, respectively.
Discussion
We identified two genes encoding BminSNMP1 and BminSNMP2 from a B. minax head transcriptome database. These two BminSNMPs bore all the hallmarks of SNMPs, including two transmembrane domains and six conserved cysteine residues that form a disulfide bridge to stabilize their 3D structures (Rasmussen et al. 1998). Our identification of two BminSNMPs adds new members to the insect SNMP family, and will facilitate future research into their functional and evolutionary relationships in the relevant taxa.
Phylogenetic analysis that indicated the selected SNMPs were divided into two clear subgroups (SNMP1 and SNMP2) was consistent with previous reports (Nichols and Vogt 2008, Liu et al. 2014, Zhang et al. 2015) and was supported by the similarity analyses of the sequences that showed general low similarity of amino acids between SNMP1 and SNMP2, but high similarity between homologous proteins. These results support the hypothesis that gene duplication events contributed to the two distinct SNMP subgroups (Vogt et al. 2009), and we suggest that duplication may have appeared at least prior to divergence of the holometabolous groups (Diptera, Lepidoptera, Hymenoptera, and Coleopteran) >300 Ma (Wiegmann et al. 2009). Our hypothesis should be tested by cloning SNMPs from a greater number of holometabolous groups to construct a more robust phylogenetic tree. We found that all SNMPs from the same order always cluster together forming a clade in each of the two subgroups, indicating that insect SNMPs may have undergone functional divergence over a long period of evolution. And we found that the BminSNMPs were phylogenetically closest to Drosophilidae SNMPs, indicating similar functional roles in these two dipteran families (Tephritidae and Drosophilidae).
The prevailing model for SNMP1 in previous studies is for its exclusive or primary expression in insect antennae (Liu et al. 2013a, 2014; Zhang et al. 2015); our data support this, because BminSNMP1 was highly expressed in male and female B. minax antennae (Fig. 5A). Such high levels of expression in the antennae indicate detection of odor in insects. For example, studies on D. melanogaster indicated that SNMP1 is necessary for the detection of the pheromone cVA (Benton et al. 2007, Jin et al. 2008), and it has been shown that SNMP1 is expressed in pheromone sensing sensilla (Rogers et al. 1997, Benton et al. 2007, Forstner et al. 2008, Zhang et al. 2015, Jiang et al. 2016).
In order to get more lines of evidence what the function of SNMP1 in B. minax may be, we analyzed expression levels of BminSNMP1 in antennae at different developmental stages. In many insect species, levels of olfactory gene expression are regulated during adult development (Vogt et al. 1993, Rogers et al. 1997, 2001; Gu et al. 2013) to meet the associated, but varied physiological demands (Soques et al. 2010). In Agrotis ipsilon (Rottemberg) (Lepidoptera: Noctuidae), expression of AipsSNMP1 was detected at relatively high levels from 1 d prior to adult emergence until 3 d post emergence, during a period of time when sex pheromones are produced (Gu et al. 2013). Similar expression profiles have been reported from other insects (Rogers et al. 1997, 2001), and support the hypothesis that SNMP1 is associated with detection of sex pheromones in insects. During the pre-oviposition stage, adult B. minax forage for food on nonhost plants in proximity to citrus orchard host plants to trigger sexual maturity; then, at about 30 d post emergence, mature adults shift from nonhost to host plants for mating and oviposition (Dong et al. 2014, Luo et al. 2016). Thus, if BminSNMP1 played a vital role in sex pheromone sensing, one would expect high levels of expression during the mating and oviposition period. However, we failed to detect higher levels of expression of BminSNMP1 in 30-d-old adults: rather, we observed gradually increased expression in 1- to 20-d-old male antennae, but a slight decrease at 30 d (Fig. 5B). In previous studies (Gu et al. 2013, Ronderos et al. 2014), these results indicated that BminSNMP1 may be involved in B. minax foraging behavior, and we suggest that insect SNMP1 may also have a functional role in sensing general odorants, such as host plant volatiles, in addition to pheromone detection.
We found that BminSNMP2 was expressed in all the body parts, similar to previous studies in Spodoptera litura (Fabr.) (Lepidoptera: Noctuidae) (Zhang et al. 2015), Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) (Liu et al. 2013a), Schistocerca gregaria (Jiang et al. 2016), Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) (Liu et al. 2014), and A. ipsilon (Gu et al. 2013). We detected relatively high levels of expression of BminSNMP2 in the antennae and legs of both sexes (Fig. 6A), where expression levels were similar in male antennae and legs, but higher in female legs than in antennae. These results contrast with those from previous studies that showed SNMP2 tended to be restricted to antennae (Liu et al. 2013a, 2014, Zhang et al. 2015). To investigate the functional roles of BminSNMP2 in antennae and legs, we analyzed its temporal expression patterns and found that transcript levels in antennae were similar in 1-, 5-, 10-, 15-, and 20-d-old adults, but higher in 30-d-old adults (Fig. 6B). Our results indicate that BminSNMP2 may be involved in detection of sex pheromones in B. minax, because sexually mature adults migrate from nonhost to host plants (citrus orchard) for copulation and oviposition at 30 d after emergence (Dong et al. 2014, Luo et al. 2016). It is likely that BminSNMP2 may play a more significant role in B. minax sex pheromone sensing than BminSNMP1, despite the highest levels of expression of BminSNMP1 in the antennae.
Previous reports of expression of SNMP2 in insect legs that suggest it may be involved in gustatory function (Jin et al. 2008, Liu et al. 2013b) are supported by high expression profiles of BminSNMP2 in leg material (Fig. 6A). The temporal expression profiles of BminSNMP2 in B. minax legs offer new insight into the function of SNMP2, because high levels of expression were recorded in 1- and 30-d-old adults (Fig. 6C). Newly emerged adult B. minax are vulnerable and seek shelter to begin the process of wing and body expansion (Denlinger and Zdárek 1994, Ran and Lv 2015), while after approximately 30 d, maturing adults begin to migrate from nonhost to host plants to establish territories for mating and oviposition (Dong et al. 2014, Luo et al. 2016). These changes in behavior show that physical and chemical sensing of substrates by legs becomes important. Many studies have shown that SNMPs in nonolfactory tissues are involved in gustatory sensation. In fact, the gustatory sensillum usually contains both chemosensory neurons and mechanosensory neurons in it (Isidoro et al. 1998). Therefore, we suggest that BminSNMP2 in legs may also be associated with tactile sensation in 1- and 30-d-old adult B. minax, and such nonolfactory functional roles of SNMPs should be the subject of future research.
Supplementary Material
Acknowledgments
We thank Zaiyuan Li and Yuekun Ma for their assistance in the insect rearing. We also thank Dr. Jianming Yan for her valuable suggestions on an earlier version of the manuscript. This research is supported by the National Natural Science Foundation of China (Grant no. 31501641).
References Cited
- Benton R., Vannice K. S., and Vosshall L. B.. 2007. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450: 289–293. [DOI] [PubMed] [Google Scholar]
- Benton R., Vannice K. S., Gomez-Diaz C., and Vosshall L. B.. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger D. L. 1994. Metamorphosis behavior of flies. Annu. Rev. Entomol. 39: 243–266. [DOI] [PubMed] [Google Scholar]
- Dong Y., Wan L., Pereira R., Desneux N., and Niu C.. 2014. Feeding and mating behaviour of Chinese citrus fly Bactrocera minax (Diptera, Tephritidae) in the field. J. Pest Sci. 87: 647–657. [Google Scholar]
- Du T. H., Hua D. K., He Z. Z., Wang F. L., and Gui L. Y.. 2018. Behavioral responses of adults Bactrocera minax (Diptera: Tephritidae) to volatile organic components from Castanea (Fagales: Fagaceae). J. Environ. Entomol. 40: 474–484. [Google Scholar]
- Forstner M., Gohl T., Gondesen I., Raming K., Breer H., and Krieger J.. 2008. Differential expression of SNMP-1 and SNMP-2 proteins in pheromone-sensitive hairs of moths. Chem. Senses. 33: 291–299. [DOI] [PubMed] [Google Scholar]
- Gu S. H., Yang R. N., Guo M. B., Wang G. R., Wu K. M., Guo Y. Y., Zhou J. J., and Zhang Y. J.. 2013. Molecular identification and differential expression of sensory neuron membrane proteins in the antennae of the black cutworm moth Agrotis ipsilon. J. Insect Physiol. 59: 430–443. [DOI] [PubMed] [Google Scholar]
- Isidoro N., Bartlet E., Ziesmann J., and Williams I. H.. 1998. Antennal contact chemosensilla in Psylliodes chrysocephala responding to cruciferous allelochemicals. Physiol. Entomol. 23: 131–138. [Google Scholar]
- Jiang X., Pregitzer P., Grosse-Wilde E., Breer H., and Krieger J.. 2016. Identification and characterization of two “sensory neuron membrane proteins” (SNMPs) of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). J. Insect Sci. 16: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Ha T. S., and Smith D. P.. 2008. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc. Natl Acad. Sci. USA 105: 10996–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal W. S. 2013. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58: 373–391. [DOI] [PubMed] [Google Scholar]
- Li Z., Ni J. D., Huang J., and Montell C.. 2014. Requirement for Drosophila SNMP1 for rapid activation and termination of pheromone-induced activity. Plos Genet. 10: e1004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang Y. R., Zhou W. W., Liang Q. M., Yuan X., Cheng J., Zhu Z. R., and Gong Z. J.. 2013a. Identification and characterization of two sensory neuron membrane proteins from Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Arch. Insect Biochem. Physiol. 82: 29–42. [DOI] [PubMed] [Google Scholar]
- Liu S., Qiao F., Liang Q. M., Huang Y. J., Zhou W. W., Gong Z. J., Chen J., and Zhu Z. R.. 2013b. Molecular characterization of two sensory neuron membrane proteins from Chilo suppressalis (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 106: 378–384. [Google Scholar]
- Liu C., Zhang J., Liu Y., Wang G., and Dong S.. 2014. Expression of SNMP1 and SNMP2 genes in antennal sensilla of Spodoptera exigua (Hübner). Arch. Insect Biochem. Physiol. 85: 114–126. [DOI] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Luo J., Gui L.Y., Gilles B., and Hua D. K.. 2016. Study on the application of insect harmonic radar in the behavior of Chinese citrus fly. J. Environ. Entomol. 38: 514–521. [Google Scholar]
- Nichols Z., and Vogt R. G.. 2008. The SNMP/CD36 gene family in Diptera, Hymenoptera and Coleoptera: Drosophila melanogaster, D. pseudoobscura, Anopheles gambiae, Aedes aegypti, Apis mellifera, and Tribolium castaneum. Insect Biochem. Mol. Biol. 38: 398–415. [DOI] [PubMed] [Google Scholar]
- Pelosi P., Zhou J. J., Ban L. P., and Calvello M.. 2006. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 63: 1658–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran F., and Lv L. L.. 2015. Emergence and feeding behavior of Bactrocera mixax in Chongqing. Chn. Hortic. Abstr. 4: 31–34. [Google Scholar]
- Rasmussen J. T., Berglund L., Rasmussen M. S., and Petersen T. E.. 1998. Assignment of disulfide bridges in bovine CD36. Eur. J. Biochem. 257: 488–494. [DOI] [PubMed] [Google Scholar]
- Rogers M. E., Krieger J., and Vogt R. G.. 2001. Antennal SNMPs (sensory neuron membrane proteins) of Lepidoptera define a unique family of invertebrate CD36-like proteins. J. Neurobiol. 49: 47–61. [DOI] [PubMed] [Google Scholar]
- Rogers M. E., Sun M., Lerner M. R., and Vogt R. G.. 1997. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J. Biol. Chem. 272: 14792–14799. [DOI] [PubMed] [Google Scholar]
- Ronderos D. S., Lin C. C., Potter C. J., and Smith D. P.. 2014. Farnesol-detecting olfactory neurons in Drosophila. J. Neurosci. 34: 3959–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. 1964. Insect antennae. Annu. Rev. Entomol. 9: 103–122. [Google Scholar]
- Soques S., Vásquez G. M., Grozinger C. M., and Gould F.. 2010. Age and mating status do not affect transcript levels of odorant receptor genes in male antennae of Heliothis virescens and Heliothis subflexa. J. Chem. Ecol. 36: 1226–1233. [DOI] [PubMed] [Google Scholar]
- Steinbrecht R. A. 1997. Pore structures in insect olfactory sensilla: a review of data and concepts. Int. J. Insect Morphol. Embryol. 26: 229–245. [Google Scholar]
- Suh E., Bohbot J., and Zwiebel L. J.. 2014. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 6: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., and Kumar S.. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- Vogt R. 2003. Biochemical diversity of odor detection: OBPs, ODEs and SNMPs, pp. 391–445. InBlomquist G. J. and R. G. Vogt (eds.), Insect pheromone biochemistry and molecular biology. Academic, San Diego, CA. [Google Scholar]
- Vogt R. G., Rybczynski R., Cruz M., and Lerner M. R.. 1993. Ecdysteroid regulation of olfactory protein expression in the developing antenna of the tobacco hawk moth, Manduca sexta. J. Neurobiol. 24: 581–597. [DOI] [PubMed] [Google Scholar]
- Vogt R. G., Miller N. E., Litvack R., Fandino R. A., Sparks J., Staples J., Friedman R., and Dickens J. C.. 2009. The insect SNMP gene family. Insect Biochem. Mol. Biol. 39: 448–456. [DOI] [PubMed] [Google Scholar]
- Wiegmann B. M., Jim J., and Trautwein M. D.. 2009. Holometabolous insects (holometabola), pp. 260–263. InHedges S. B. and S. Kumar (eds.), The timetree of life. Oxford University Press, New York. [Google Scholar]
- Zhang J., Liu Y., Walker W. B., Dong S. L., and Wang G. R.. 2015. Identification and localization of two sensory neuron membrane proteins from Spodoptera litura (Lepidoptera: Noctuidae). Insect Sci. 22: 399–408. [DOI] [PubMed] [Google Scholar]
- Zhou J. J. 2010. Odorant-binding proteins in insects. Vitam. Horm. 83: 241–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.