Abstract
Food allergy prevalence has steadily increased worldwide over the past decades and immunotherapeutic treatment strategies are gaining attention. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) exhibit similar immune regulatory properties to bone marrow-derived MSCs. hUC-MCSs can be prepared with fewer ethical constraints and are potential candidates for allergic disorder therapies. The current study aimed to investigate potential antiallergic properties of hUC-MSCs in mice with ovalbumin (OVA)-induced food allergy. Administration of hUC-MSCs cells intraperitoneally combined with oral gavage of the culture medium significantly alleviated OVA-induced diarrhea symptoms. Additionally, this treatment significantly decreased IgE levels and the percentage of T helper 2 cells in the blood, which were increased in mice with OVA-induced food allergy. The mRNA levels of the inflammatory cytokines interleukin-4 and tumor necrosis factor-α, and inflammatory cell infiltration in mouse colons were significantly decreased in hUC-MSCs-treated animals compared with mice with OVA-induced food allergy. Goblet cells were detected in colons of allergy-induced mice and their numbers were reduced following treatment with hUC-MSCs. In addition, treatment with hUC-MSCs reestablished the gut flora. The results revealed that hUC-MSCs may have a potential application in food allergy therapy.
Keywords: food allergy, mesenchymal stem cell, colon, T helper 2 cell, immunotherapy, IgE
Introduction
Food allergy is a pathological immune response triggered by the exposure to allergenic foods and it results in clinical symptoms, including gastrointestinal disorders (1). Allergenic reactions may be triggered by dairy and other food products, including milk, peanuts, nuts, shellfish and eggs (2). This broad allergenic food spectrum suggests a high prevalence of food allergies worldwide (3). A retrospective study demonstrated that ≤6.7% of children in the United States have allergies to different food (4). A similar or increased food allergy prevalence in other countries has been reported in recent years (5). A previous study in China declared that the prevalence of food allergy in schoolchildren of Guangzhou and Shaoguan was 4 and 3.5% respectively, in 2015 (6). Such prevalence results in an increased number of food allergy anaphylaxis-associated hospital admissions and high costs to healthcare systems worldwide (5,7,8). The elucidation of the mechanisms and the development of preventive methods and novel therapies for patients with food allergies are of great value.
Sensitization to food antigens can result in inflammation and clinical symptoms similar to various common food allergies, which can be mechanistically classified into IgE-mediated, non-IgE-mediated and mixed-food allergies (9,10). Generally, the immune system in mammals distinguishes pathogenic antigens from harmless environmental antigens and maintains a state of tolerance to common food antigens (11). Oral tolerance is an active process that depends on diverse immune cell collaborations, including resident CD103+ dendritic cells (DCs) and regulatory T cells (Tregs) in the mucosa (12–14). Epithelial damage or inflammation in the gut allows an increased antigen entry and promotes epithelial cells to secrete inflammatory cytokines, including interleukin (IL)-33, IL-25 and thymic stromal lymphopoietin (15). These cytokines reprogram the properties of mucosal DCs and Tregs and induce the immune system towards a T helper 2 (Th2) cell response (16). The data from mouse models and patients with allergies demonstrated that food allergy is associated with a Th2 dominant response (17,18). Therefore, Th2 cells mediate the immune response and Th2-derived cytokine signaling pathways are potential targets in food allergy treatment.
Mesenchymal stromal cells (MSCs) are multipotent stem cells with self-renewing abilities, a differentiation potential and they were identified by Friedenstein et al in 1968 (19). In the last decade, accumulating data have suggested that MSCs have distinct immune properties and these cells have gained considerable attention as candidates for therapy in immune-associated diseases (20–22). MSCs express the major histocompatibility complex (MHC) class I molecule, but not MHC class II or co-stimulatory molecules, including CD80 and CD86 (23). This expression enables MSCs to avoid allogeneic rejection, which is mediated by alloreactive T and natural killer cells (24). MSCs have been applied as immunomodulators for autoimmune diseases and transplantation rejection (23,25). In inflammation, MSCs can suppress T cell-mediated responsiveness through the concerted action of chemokines and nitric oxide, and can promote regulatory cell differentiation (26,27). MSCs further regulate adaptive immunity by reprogramming the maturation and phenotype of DCs (28,29). Therefore, MSCs regulate the effects of various immune cells through multiple mechanisms, which include immunomodulatory soluble factor secretion and membrane-membrane direct contact (20).
Bone marrow (BM)-derived MSCs were the first MSCs to be identified and are best characterized. However, the special handling of BM-MSCs limits their clinical application due to a low frequency of cells and the invasive isolation procedure (30). Therefore, other tissues are now used to isolate MSCs, including adipose tissue and the umbilical cord (22,31). Human umbilical cord (hUC)-derived MSCs exhibit an immunosuppressive capability, which is similar to BM-MSCs, with regards to T cell activation and proliferation (32). Increasing numbers of experimental and clinic studies suggest that hUC-MSCs further exhibit therapeutic effects in autoimmune diseases, including diabetes, Crohn's disease and systemic lupus erythematosus (33–35). As an alternative source of MSCs, preparation from the hUC exhibits fewer ethical constraints and increased maneuverability compared with human BM (30). Furthermore, hUC-MSCs can be prepared in large quantities for therapeutic application in the clinic (30).
In the current study, the therapeutic effects of hUC-MSCs in food allergy treatment were investigated using an ovalbumin (OVA)-induced mouse model. Following treatment with hUC-MSCs, the main clinical symptoms and enteropathy in the allergy model significantly improved. Simultaneously, the levels of Th2 cells and IgE in the blood, and IL-4 mRNA levels in the colon were significantly decreased. The current experiments demonstrated a potential therapeutic function of hUC-MSCs in the treatment of food allergies.
Materials and methods
hUC-MSC culture
hUC-MSCs were isolated as previously described (36). Fresh human umbilical cords were obtained from 6 patients (age, 23–38 years) in the Fourth Affiliated Hospital of Jiangsu University (Jiangsu, China) from March 2016 to May 2017. Maternal blood samples were previously screened and patients were screened and found negative for infectious disease markers, including HIV I & II, HBV, HCV and syphilis. Umbilical cord samples were cut into 1–2 mm3 pieces and were floated on Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1; L310KJ; Shanghai Basalmedia Technologies Co., Ltd., Shanghai, China) with 10% fetal bovine serum (FBS; Biowest, Nuaillé, France), 100 U/ml penicillin and streptomycin at 37°C with 5% CO2. Medium was replaced every 3 days. When the adherent cells reached 80–90% confluence, cultures were trypsinized and transferred into a new flask for further expansion. The phenotypes of hUC-MSCs were analyzed via flow cytometric analysis. Following trypsinization and washing with PBS solution twice, the single cell suspensions were blocked with 2% rabbit serum (Stemcell Technologies, Vancouver, BC, Canada) at room temperature for 30 min. Samples were then labeled with different fluorescent antibodies (1:200), from Thermo Fisher Scientific, Inc. (Waltham, MA, USA) at 4°C for 30 min. Cells were further washed with PBS in triplicate, re-suspended with PBS (1 mM EDTA) and analyzed with a FACS Calibur flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). All data were analyzed using FlowJo (version 8.7; Tree Star, Inc., Ashland, OR, USA). The following fluorescent antibodies (Thermo Fisher Scientific, Inc.) were utilized: Anti-human CD73-Fluorescein isothiocyanate (FITC; cat. no. 11-0739-41), anti-human CD90-FITC (cat. no. 11-0909-41), anti-human CD105-phycoerythrin (PE; cat. no. 12-1057-41), anti-human CD34-FITC (cat. no. 11-0349-41), anti-human CD45-FITC (cat. no. 11-0459-41), anti-human-CD14-FITC (cat. no. 11-0149-42), anti-human-CD19-PE (cat. no. 50-102-58) and anti-human HLA-DR-FITC (cat. no. 11-9952-41). The protocol for human tissue collection was approved by the Ethical Committee of Jiangsu University and informed consent for umbilical cord tissue collection was provided by the patients.
hUC-MSC differentiation in vitro
Differentiation studies were performed as described previously with few modifications (37). Briefly, for adipogenic differentiation, cells were cultured at 37°C with 5% CO2 in DMEM/F12 (1:1) complete medium supplemented with 0.5 µM isobutylmethylxanthine, 1 µM dexamethasone, 10 µM insulin and 60 µM indomethacin (all Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 14 days. For osteogenic differentiation, cells were cultured at 37°C for 28 days with 5% CO2 in the complete medium with 0.1 µΜ dexamethasone, 10 mM β-glycerol phosphate and 50 µΜ ascorbate (all Sigma-Aldrich; Merck KGaA) for 28 days. Oil Red O and Alizarin Red staining was performed to visualize the adipogenic and osteogenic differentiations, respectively. Differentiated adipogenic cells were stained on day 14 with Oil Red O (ORO) staining kit (Solarbio Technology Co., Ltd, Beijing, China) according to the manufacturer's protocol. Cells were fixed with ORO fixative buffer for 10–15 min at room temperature, freshly prepared ORO staining solution was (mixed previously in the kit with distilled water in a 3:2 ratio) and dipped for 15 min at room temperature. The staining solution was then removed and cells were rinsed with 60% isopropyl alcohol for 20–30 sec. Samples were rinsed with PBS and stained with Mayer hematoxylin for 2 min at room temperature. ORO buffer was then added for 1 min. Cells were then washed with distilled water and dried. To perform Alizarin Red staining on day 28, differentiated osteogenic cells were stained using an Alizarin Red staining kit (Solarbio Technology Co., Ltd, Beijing, china) according to the manufacturer's protocol. Cells were fixed with 95% ethanol for 10–15 min at room temperature. The fixative solution was added for alizarin red S staining and dipped for 20 min. Following washing, cells were then dried. All stained cells were observed under microscope (Nikon Corporation, Tokyo, Japan) and photographs were obtained.
Food allergy model
A total of 20 mice Female BALB/c mice (H-2d; age, 6–8 weeks; weight, 25±2 g) were obtained from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China) and divided into four groups. Mice were housed in microisolator units under a 12 h light/dark cycle at a temperature of 24–26°C and a humidity of 30–50%, and with ad libitum access to food and water. To induce food allergy, Mice (n=5 per group, a total of 15 mice in three OVA challenged groups) were intraperitoneally (i.p.) injected with 50 µg OVA (Grade V; A-5503; Sigma-Aldrich; Merck KGaA) in 1 mg alum adjuvant on days 0 and 14 as previously described (38). From day 28, the mice were challenged intragastrically with 50 mg of OVA in 250 µl PBS every other day for 12 days (6 administrations). As immune regulatory functions of MSCs depend on the cell-cell contact and the secreted soluble factors in the medium, mice were treated with MSCs and MSC conditioned medium (39,40). Mice in the different groups (n=5 per group) were respectively challenged with 500 µl of PBS, OVA, medium/OVA and MSC supernatant/OVA (MSC/OVA group) by oral gavage on day 28, 30, 32, 34, 36 and 38. Subsequently, all mice were deprived of food for 3 h to limit antigen degradation in the stomach. In addition, mice in the MSC/OVA group were i.p. injected with 5×105 hUC-MSCs in 200 µl PBS every other day from days 21–38. Symptoms of allergic diarrhea were assessed 1 h following OVA oral challenge (day 28, 30, 32, 34, 36 and 38) and severity was classified as follows: Normal, soft and loose stool, mild and severe diarrhea, and fluid stool. Mice with profuse liquid stool (mild or severe diarrhea, or fluid stool) were recorded as having diarrhea (41). On day 38, all mice were sacrificed and their colons and mesenteric lymph nodes were collected. The amounts of solid faeces within colon specimens were observed and lymph nodes were weighed. All animal protocols have been approved by the Animal Care and Use Committee of Jiangsu University.
Concanavalin (Con) A-induced CD4+ T cell proliferation
To detect the immunosupression of hUC-MSC cells or MSC cell culture supernatant on CD4+ T cell activation and proliferation, 6-week old BALB/c mice were sacrificed via CO2 exposure at a flow rate of 3 l per min and spleens were collected to prepare cell suspensions for experiment as previously described (42). Spleens were collected, grinded and passed through strainers in PBS to prepare cell suspensions. Red blood cells in these splenocyte suspensions were removed using ACK lysis buffer. Cells were subsequently mixed with 5 µM carboxyfluorescein succinimidyl ester (Sigma-Aldrich; Merck KGaA) and incubated at 37°C for 10 min. Following incubation in 1640 medium (PAA Laboratories; GE Healthcare Life Sciences, Little Chalfont, UK) containing 10% FBS at room temperature for 10 min, cells were washed with PBS (3X) and plated at 2×106/well in 48-well plates with 5 µg/ml Con A (Sigma-Aldrich; Merck KGaA). To assess the direct immunosuppressive effects of MSC supernatants or hUC-MSC cells on ConA activated T cells, half of the medium was replaced with hUC-MSC culture supernatant or hUC-MSC cells (2×105/well) in wells following splenocyte seeding, which were then cultured at 37°C in 5% CO2 for 72 h. Cells were collected and stained with fluorescein-labeled anti-mouse CD4 (1:200; cat. no. 17-0041-81; eBioscience; Thermo Fisher Scientific, Inc.) at 4°C for 30 min. 7-aminoactinomycin D (7-AAD) was then added prior to analysis (cat. no. 00-6993-50; eBioscience; Thermo Fisher Scientific, Inc.). The proliferation of CD4+ cells was analyzed by flow cytometry. All the data were analyzed using FlowJo (version 8.7; FlowJo LLC, Ashland, OR, USA).
Intracellular cytokine staining
On day 38, mice were sacrificed and mesenteric lymph nodes were collected and mononuclear cells were prepared by gently pressing the tissues through a strainer (40 µm; BD biosciences) in cold PBS containing 1% FBS. Cell suspensions were washed twice with cold PBS. On day 38, prior to sacrifice, murine blood (300 µl) was collected from the orbital sinus to use for intracellular cytokine staining, or to prepare serum by storing samples at room temperature for 1 h following centrifugation at 1,500 × g for 15 min at 4°C. For intracellular staining, red blood cells (RBCs) in samples were removed using RBC lysis buffer (cat. no. 00-4333-57, eBioscience; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Cells were resuspended in the presence of a Cell Stimulation Cocktail plus protein transport inhibitors (eBioscience; Thermo Fisher Scientific, Inc.) at 37°C in 5% CO2 for 4 h. Then, cells were incubated with anti-mouse CD4-allophycocyanin (eBioscience; Thermo Fisher Scientific, Inc.) at 4°C for 20 min. Following washing with cold PBS (2X), cells were fixed with IC Fixation buffer (eBioscience; Thermo Fisher Scientific, Inc.) at 4°C for 30 min. Cells were washed twice more with permeabilization buffer (eBioscience; Thermo Fisher Scientific, Inc.) and incubated with anti-mouse interleukin (IL)-4-phycoerythrin (eBioscience; Thermo Fisher Scientific, Inc.) for 45 min at 4°C. Cells were washed twice with PBS and suspended in PBS-bovine serum albumin (1%; Sigma-Aldrich; Merck KGaA) for the flow cytometry analysis as described above.
ELISA analysis
Total IgE serum levels were measured using an ELISA kit (cat. no. 88-50460-22, eBioscience; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols (eBioscience; Thermo Fisher Scientific, Inc.). Briefly, 96-well plates were coated overnight at 4°C with 100 µl/well capture antibody (provided by the kit). Following two washes with wash buffer, plates were blocked with 250 µl blocking buffer at room temperature for 2 h. Plates were washed twice with wash buffer and 100 µl serum sample (1:50) was added to each well. Plates were further incubated at room temperature for 2 h. Next, 100 µl/well biotinylated detection antibody (obtained from the kit) was added and incubated at room temperature for 1 h. Following 4 washes, 100 µl/well streptavidin-horseradish peroxidase was added and incubated at room temperature for 30 min. Wells were further washed 4 times, 100 µl substrate solution was added per well and samples were incubated for 30 min at room temperature. Finally, stop solution was added and OD values were measured at 450 nm.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
For PCR analysis, the transverse colon was excised following the last OVA challenge on day 38 and flushed with ice-cold PBS. Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C for further experiments. Tissue was homogenized and total RNA was extracted with RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. The cDNA was synthesized using a PrimeScript RT Master mix (Takara Biotechnology Co., Ltd.) for 15 min at 37°C and then 15 sec at 85°C according to the manufacturer's protocol. qPCR amplification of the target cDNA was performed using the SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. Thermocycling conditions were as follows: 30 sec at 95°C, followed by 40 cycles of 5 sec at 95°C and 20 sec at 58°C. Alterations in expression of target genes in treated vs. untreated samples were observed; mRNA levels were quantified using the 2−∆∆Cq method (43). Levels were normalized to β-actin (internal control) and the PBS group with three replicates was used as calibrator. Primer sequences are as follows: IL-4 forward, 5′-GGTCTCAACCCCCAGCTAGT-3′ and reverse, 5′-GCCGATGATCTCTCTCAAGTGAT-3′; tumor necrosis factor (TNF)-α forward, 5′-GAACTGGCAGAAGAGGCACT-3′ and reverse, 5′-GGTCTGGGCCATAGAACTGA-3′; and β-actin forward, 5′-TGGCGCTTTTGACTCAGGAT-3′ and reverse, 5′-GGGATGTTTGCTCCAACCAA-3′.
Histological staining
Transverse colons were collected as aforementioned in PCR analysis. Following snap-freezing, tissues were fixed with 4% paraformaldehyde solution for 48 h at 4°C. Following ethanol treatment for dehydration (70% ethanol for 120 min, 80% ethanol for 120 min, 90% ethanol for 60 min, 95% ethanol for 40 min, 100% ethanol for 40 min, 100% ethanol for 40 min) at room temperature, colons were then embedded in paraffin (first wax, 58°C for 1 h; second wax, 58°C for 1 h) and cut into 4-µm-thick sections. Subsequently, sections were stained with hematoxylin and eosin at room temperature using the following procedure: Samples were washed twice with Xylene for 10 min, then twice with 100% ethanol for 5 min, 95% ethanol for 2 min, 70% ethanol for 2 min and rinsed with water. Hematoxylin solution was then added for 8 min, rinsed with water and added to 1% acid ethanol for 30 sec. Following a further rinse with water, 0.2% ammonia water was added for 1 min and samples were rinsed. Samples were dipped 10 times in 95% ethanol, stained with eosin-phloxine solution for 45 sec, added to 95% ethanol and 100% ethanol 5 min each and finally washed with xylene twice for 5 min. For the periodic acid-Schiff (PAS) staining, sections of the colons were stained with the Glycogen PAS Staining kit (Yeasen Biotechnology, Shanghai, China) (www.yeasen.com) according to the manufacturer's protocol. Briefly, paraffin-embedded slices were deparaffinized with xylene twice for 10 min, hydrated with 100% ethanol twice for 5 min, 95 and 70% ethanol for 2 min, washed with distilled water and oxidized at room temperature in periodic acid solution for 10 min. All deparaffinization and hydration steps were performed at room temperature. Subsequently, sections were placed in Schiff reagent for 10 min and counterstained with Mayer's hematoxylin stain for 20–30 sec at room temperature. All stained sections were sealed overnight at room temperature using neutral resin and digital photographs were taken using an automatic digital slide scanner under a microscope (ECLIPSE Ti, Nikon corporation; magnification, ×100, ×400, ×1,000).
Sequencing of 16S ribosomal (r)RNA in the stool
The sequencing of 16S ribosomal (r)RNA was performed by Zhenjiang Tianyi Biotechnology Co., Ltd. (Zhenjiang, China). Each kit was used according to the manufacter's protocol. Stool in the colon was collected on day 38 under sterile conditions and genomic DNA was extracted and purified using the QIAamp DNA Stool Mini kit and the QIAquick PCR Purification kit, respectively (Qiagen GmbH, Hilden, Germany). The PCR amplification of the target V4-V5 regions of the bacterial 16S rRNA genes was firstly performed using the primers 515F (5′-GTGCCGCCGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) and 2× Taq Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China). The thermocycling conditions were as follows: 3 min at 95°C, followed by 35 cycles of 30 sec at 95°C, 45 sec at 55°C and 60 sec at 72°C, and 5 min at 72°C. DNA products were used for further PCR amplification using a 2X Taq Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) and the following primers (44,45): 515F forward primer, barcoded [5′-AATGATACGGCGACCACCGAGATCTACACGCT (5′ Illumina adapter) XXX XXX XXX XXX (barcode) TATGGTAATT (pad) GT (linker) GTGYCAGCMGCCGCGGTAA-3′ (515F forward primer)] and 806R reverse primer [5′-CAAGCAGAAGACGGCATACGAGAT (3′ Illumina adapter) AGTCAGCCAG (pad) CC (linker) GGACTACNVGGGTWTCTAAT-3′ (806R reverse primer)]. Barcodes in the 515F forward primer enables the usage of various reverse primer constructs to obtain longer amplicons. Additional degeneracy (such as Y in 515F and N in 806R) was used to add bias against environmental archaea Crenarachaeota/Thaumarchaeota and the aquatic bacteria SAR11 (44,45). The Thermocycling conditions for the second PCR assay were as follows: 3 min at 95°C, followed by 35 cycles of 45 sec at 95°C, 60 sec at 55°C and 90 sec at 72°C, and then 10 min at 72°C. Amplicons were then purified using the Agencourt AMPure XP kit (Beckman Coulter, Inc., Brea, CA, USA) and quantified using a Qubit 3.0 fluorometer (Thermo Fisher Scientific, Inc.). DNA samples were further submitted to the Illumina sequencing platform (www.illumina.com) for analysis. The raw reads were filtered for noise deletion and then analyzed using the open source software package, Quantitative Insights into Microbial Ecology (version 1; http://qiime.org/). Operational taxonomy units (OTUs) of 16S rRNA were identified based on an open reference OTU pick using UCLUST (version 4.1.93; http://qiime.org/), which clustered at a threshold of 97% pair-wise identities and were classified taxonomically using the Ribosomal Database Project classifier 2.3 (ttps://rdp.cme.msu.edu/) (46).
Statistical analysis
The experiments were repeated at least three times and data are presented as the mean ± standard deviation. All statistical comparisons were made using one-way analysis of variance followed by the Newman-Keuls multiple comparison post-hoc tests using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of hUC-MSCs
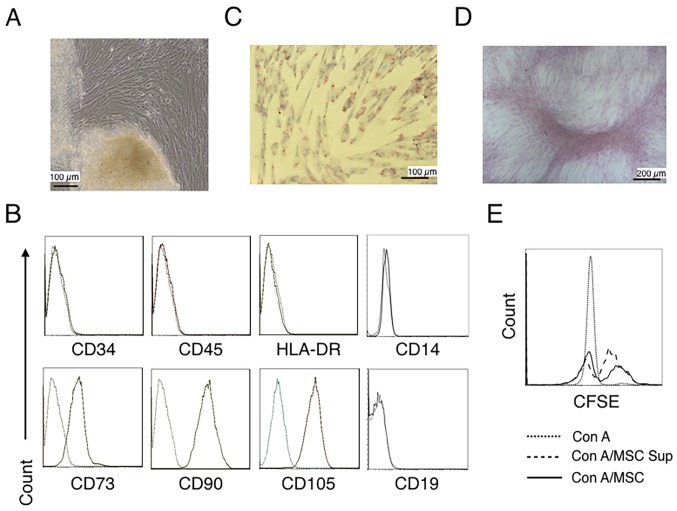
Human umbilical cord tissues were cultured for >15 days and spindle-shaped fibroblastic cells were observed to grow to confluence (Fig. 1A). hUC-MSCs were identified using a phenotype analysis and the capability of adipogenic and osteogenic differentiation (36,47). The phenotype of the cells was determined by flow cytometry. Fig. 1B demonstrates that cells were positive for CD73, CD90 and CD105, and negative for CD34 (hematopoietic stem cell), CD45 (leukocyte), CD14 (myeloid cell), CD19 (B cell) and HLA-DR (DC, macrophage and B cell) (47–49). When the cells were cultured in adipogenic medium to analyze adipocyte differentiation, a portion of the cells contained lipid droplets and tested positive for Oil-Red-O staining (Fig. 1C). The differentiation of cells in osteogenic medium revealed a portion of cells with positive Alizarin Red staining (Fig. 1D). Fig. 1E further revealed that the hUC-MSCs alone and the hUC-MSC-cultured supernatant inhibited the proliferation of CD4+ T cells. These results suggested that the cells cultured from umbilical cord tissues present with MSCs features.
Figure 1.
Characterization of hUC-MSCs. (A) Morphological observations of hUC-MSCs. Umbilical cord tissues were cultured for >15 days and long spindle-shaded fibroblastic cells were observed around the tissue using Zeiss light microscopy (scale bar, 100 µm). (B) Phenotyping of hUC-MSCs. hUC-MSCs were stained with a fluorescein-labeled antibodies (CD34, CD45, CD73, CD90, CD105, CD14, CD19 and HLA-DR) and analyzed with a flow cytometer. (C) Adipogenic and (D) osteogenic differentiation of hUC-MSCs. hUC-MSCs were cultured in adipogenic and osteogenic medium, respectively. Lipid droplets in the adipocytes are presented with Oil Red O staining (scale bar, 100 µm). hUC-MSCs-derived osteoblasts were detected with Alizarin Red staining (scale bar, 200 µm). (E) hUC-MSCs inhibit the proliferation of CFSE-labeled CD4+ T cells, which were activated by Con A stimulation. Experiments were repeated three times and representative graphs and images are presented. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; MSC Sup, culture supernatant of hUC-MSCs; Con A, concanavalin A; CFSE, carboxyfluorescein succinimidyl ester.
hUC-MSCs alleviate OVA-induced food allergy symptoms
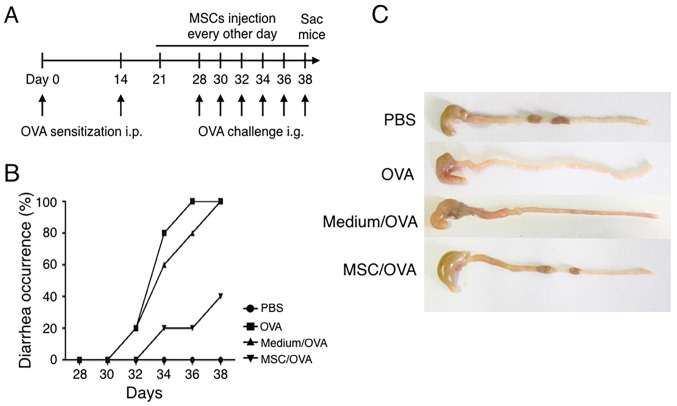
To explore effects of hUC-MSCs on food allergy, a mouse model was prepared by OVA i.p. sensitization and oral challenge (Fig. 2A). In the MSC/OVA group, hUC-MSCs were i.p. injected every other day from day 21 to day 38. These mice were also intragastrically administered with MSC culture supernatant and simultaneously challenged with OVA antigen on 6 alternating days. To account for medium effects, an equal volume of medium was administered by oral gavage in conjunction with OVA (medium/OVA group). The data in Fig. 2B demonstrated that OVA challenge induced diarrhea on day 30, following 3 days of treatment, in the PBS and medium/OVA groups. Following sacrifice on day 38, compared with mice in the PBS and medium/OVA, an increased amount of solid faeces was observed in the gut of the MSC/OVA mice (Fig. 2C). These results demonstrated that hUC-MSC treatment attenuated the allergic diarrhea scores of OVA-induced allergy in mice.
Figure 2.
Treatment with hUC-MSCs alleviates symptoms of OVA-induced food allergy in mice. (A) Timeline of the experimental food allergy procedure. Female BALB/c mice were divided into PBS, OVA, Medium/OVA and MSC/OVA groups (all n=5). (B) Diarrhea scores evaluated on the OVA oral challenge day (day 28 to 38). (C) Representative photographs of the caecum and colon of the mice. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; OVA, ovalbumin; SAC, sacrifice; Medium/OVA, group challenged with OVA and administered Dulbecco's modified Eagle's medium/nutrient mixture F12; MSC/OVA, group challenged with OVA, intraperitoneal injection of hUC-MSCs and oral gavage of MSC culture supernatant.
hUC-MSCs inhibit Th2 cells and IgE production in mice
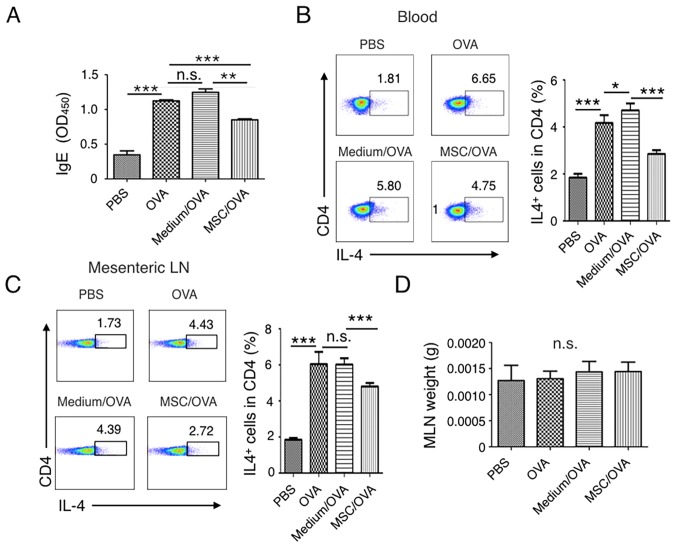
IgE-mediated degranulation of mast cells or basophils results in the rapid manifestation of symptoms of food allergy (50). The current study detected the levels of IgE in the peripheral blood of mice. The results indicated that IgE levels were increased in the PBS and medium/OVA groups compared with the MSC/OVA mice (Fig. 3A). A Th2-type immune response, stimulated by food allergens, contributes to the initiation and development of allergies and associates with enteropathy (18). The percentage of IL-4+ Th2 cells in the peripheral blood and mesenteric lymph nodes were further determined. Fig. 3B suggests that OVA challenge markedly increased the percentage of IL-4+ Th2 cells in CD4+ T cells in the blood. Treatment with hUC-MSCs inhibited the frequency of IL-4+ Th2 cells. Comparable IL-4+ Th2 cell results were detected in the mesenteric lymph nodes (Fig. 3C). No difference in the weight of the lymph nodes was determined between the groups (Fig. 3D).
Figure 3.
Treatment with hUC-MSCs inhibits the frequency of Th2 cells and IgE levels in the periphery. Mice were divided into PBS, OVA, Medium/OVA and MSC/OVA treatment groups. (A) Total IgE levels determined in the different groups. IgE levels in the serum were detected by ELISA and OD450 values are presented. The percentage of IL-4+ Th2 cells in (B) blood and (C) MLNs were analyzed using a flow cytometer by gating the CD4+ cells. Quantitative analysis on the percentage of Th2 cells in (B) and (C) was also performed. Numbers in the graphs represent the percentage of CD4+IL-4+ Th2 cells. (D) Weight of the MLNs of mice in the different treatment groups, representative of four independent experiments. *P<0.05; **P<0.01; ***P<0.001. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; Th2, T helper 2 cell; IL, interleukin; OD, optical density; MLN, mesenteric lymph node; OVA, ovalbumin; n.s., not significant; Medium/OVA, group challenged with OVA and administered Dulbecco's modified Eagle's medium/nutrient mixture F12; MSC/OVA, group challenged with OVA, intraperitoneal injection of hUC-MSCs and oral gavage of MSC culture supernatant.
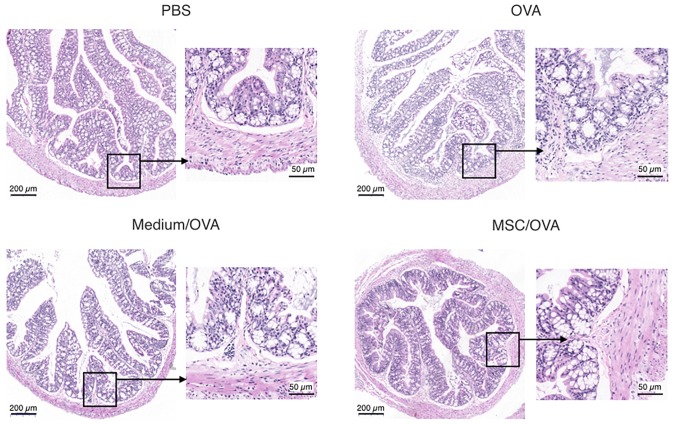
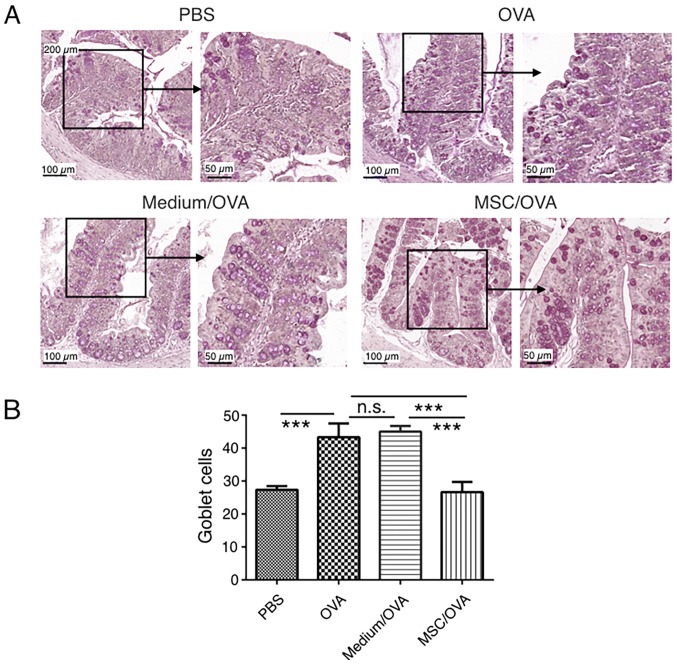
Treatment with hUC-MSCs reduces inflammation and the numbers of goblet cells in the colons of mice with food allergy
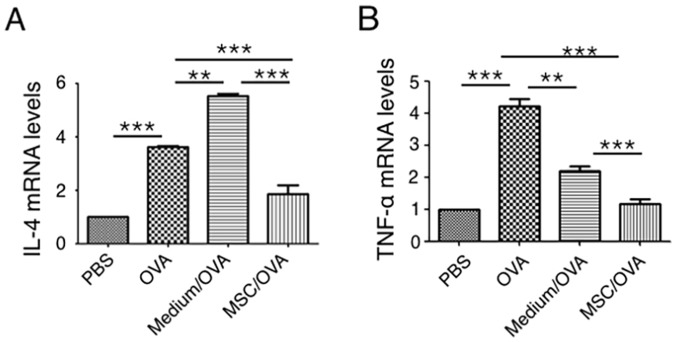
Cytokine profiles in mouse colons were analyzed using RT-qPCR. The data in Fig. 4 indicates that OVA challenge significantly upregulates IL-4 and TNF-α mRNA levels in the colons of the OVA group compared with the PBS control. hUC-MSC treatment (MSC/OVA) significantly reversed the OVA-induced higher mRNA levels of IL-4 and TNF-α in the OVA and the medium/OVA groups. The data indicated that the inflammation in the colon was suppressed by hUC-MSCs. This was further confirmed by histological data of the colons (Fig. 5). Fewer infiltrating inflammatory cells were detected in the lamina propria of the intestinal mucosa in the MSC/OVA group compared with the OVA and medium/OVA groups (Fig. 5). By using PAS staining, more goblet cells were detected in the OVA and medium/OVA group than in the PBS control group (Fig. 6). If treated with hUC-MSCs, the number of goblet cells was markedly decreased in the colon compared with other OVA-challenged groups (Fig. 6). Collectively the data suggested that treatment with hUC-MSCs inhibited the OVA-induced inflammation and decreased the numbers of goblet cells in the colon.
Figure 4.
hUC-MSCs reduce mRNA levels of inflammatory cytokines in the colon. Mice were divided into PBS, OVA, Medium/OVA and MSC/OVA treatment groups. Distal colons were collected following sacrifice on day 38. mRNA levels of (A) IL-4 and (B) TNF-α in the colon tissues were quantified using reverse transcription-quantitative polymerase chain reaction. **P<0.01; ***P<0.001. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; IL, interleukin; OVA, ovalbumin; TNF, tumor necrosis factor; Medium/OVA, group challenged with OVA and administered Dulbecco's modified Eagle's medium/nutrient mixture F12; MSC/OVA, group challenged with OVA, intraperitoneal injection of hUC-MSCs and oral gavage of MSC culture supernatant.
Figure 5.
Histology of the colon in allergic mice. Mice were divided into PBS, OVA, Medium/OVA and MSC/OVA treatment groups. Representative histological photomicrographs of the colons stained with hematoxylin and eosin and observed under a microscope. Scale bar, 200 µm or 50 µm. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; OVA, ovalbumin; Medium/OVA, group challenged with OVA and administered Dulbecco's modified Eagle's medium/nutrient mixture F12; MSC/OVA, group challenged with OVA, intraperitoneal injection of hUC-MSCs and oral gavage of MSC culture supernatant.
Figure 6.
Goblet cell staining in the colon. Mice were divided into PBS, OVA, Medium/OVA and MSC/OVA treatment groups. (A) Representative images of the goblet cells in the colon, stained using periodic acid-Schiff stain. Scale bar, 100 and 50 µm. (B) Quantification of the goblet cells in the fields under the microscope. hUC-MSC, human umbilical cord-derived mesenchymal stem cell; OVA, ovalbumin; Medium/OVA, group challenged with OVA and administered Dulbecco's modified Eagle's medium/nutrient mixture F12; MSC/OVAOVA/MSC, group challenged with OVA, intraperitoneal injection of hUC-MSCs and oral gavage of MSC culture supernatant. ***P<0.001. ns, not significant.
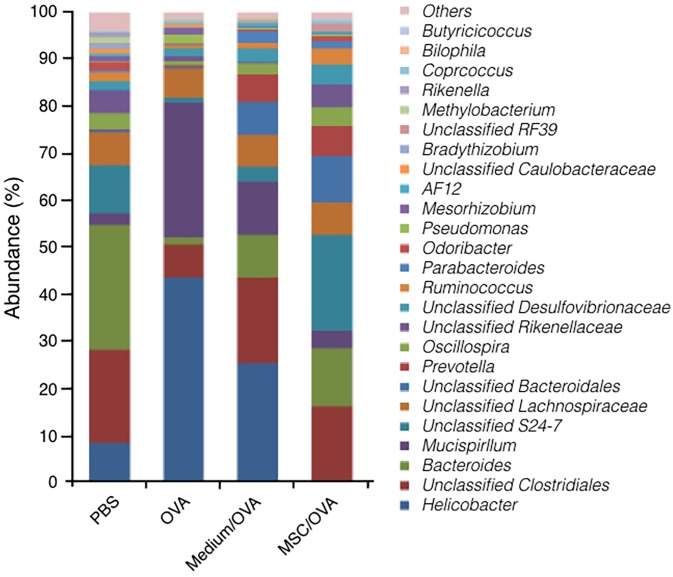
Treatment with hUC-MSCs recovers the flora populations in the gut
Recently, accumulating evidence suggested that the gut microbiota influences the development of allergic manifestations (51). Effects of treatment with hUC-MSCs on gut microbiota populations were explored using 16S rRNA gene sequencing. The data in Fig. 7 suggest that the population and relative abundance of the microbiota in gut significantly changed following OVA challenge. Following OVA challenge, the relative abundance of the genus Helicobacter and Mucispirillum significantly increased. At the same time, the relative abundance of the genus Bacteroides, S24-7 and Lachnospiraceae, which is known as butyrate-producing bacteria, significantly decreased (52). Furthermore, treatment with hUC-MSCs recovered the population and abundance of commensal bacteria in the gut, particularly for S24-7 and Lachnospiraceae. The results indicated that treatment with hUC-MSCs may partially alleviate food allergy symptoms by maintaining the population of gut microbiota.
Figure 7.
Sequencing of the 16S rRNA gene in gut microflora. The composition and relative abundance of the microflora in the colon were detected using the Illumina MiSeq System. rRNA, ribosomal RNA.
Discussion
Due to their high potential expansion capacity ex vivo, multi-lineage differentiation potential and immune suppression functions, MSCs have emerged as attractive therapeutic tools in transplantation, tissue regeneration and autoimmune diseases (25). In the past decades, MSCs were prepared from different tissues, including adipose tissue, bone marrow, tonsils and umbilical cords, for clinical and experimental disease therapies (21,49). These experimental therapies include allergic diseases, particularly experimental asthma, allergic rhinitis and allergic contact dermatitis (25,26,53). Compared with other tissues, the umbilical cord is an extra-embryonic tissue and is easily obtained in large quantities ex vivo (54). Therefore, the umbilical cord provides a novel source of MSCs for clinical therapy. Many different isolation and expansion procedures have been explored to efficiently prepare hUC-MSCs ex vivo, including enzymatic digestion and tissue explant culture (33,34,55). In the current study, explants of umbilical cords were cultured directly to avoid effects of enzymatic digestion on the biological properties of the MSCs. Spindle-shaped fibroblastic cells were observed to migrate from the tissue and adhered to the culture dish, as previous reported (48,54). Biological properties, including the transcriptomic profile of hUC-MSCs were not compared with BM-MSCs. However, cells met the basic criteria of multipotent MSCs, which are defined by the International Society for Cellular Therapy (48). Using flow cytometry analysis, it was observed that cells were positive for CD73, CD90 and CD105, and negative for CD34, CD45, CD14 and HLA-DR. Additionally, these fibroblastoid shaped cells differentiated into adipocytes and osteoblasts in conditional differentiation medium and exhibited an immune-suppressive function. Although, the immune suppression and its associated mechanisms for various MSCs and MSC-conditioned medium in autoimmune diseases have been widely explored (34,35,40,56), effects on food allergy and on the gut microbiota population have not been reported. In the current study, using hUC-MSC-conditioned medium by oral gavage and hUC-MSCs by direct injection, it was observed that treatment with hUC-MSCs alleviated allergy symptoms and recovered the population of the gut flora. Unfortunately, because no MSC supernatant/OVA control was utilized in the current study, the exact role of MSC culture supernatant on allergy was unlcear.
Food allergy in an adverse immunity to common food and is associated with significant morbidity, particularly if accidental ingestion is not prevented or adequately treated (1). Previously, a number of immunotherapeutic strategies have been investigated for the treatment and prevention of this disease, including allergen desensitization, anti-IgE antibodies injection for IgE blocking and other non-antigen specific therapies (57). In the current study, it was observed that the treatment with hUC-MSCs and MSC-conditioned medium significantly alleviated clinical symptoms of food allergy, which was further confirmed by histological data of the colon. As van Halteren et al (50) first described in 1997, this OVA intragastric challenge allergy model is food allergen-specific, IgE-dependent and relevant to Th2 cytokines. Following OVA challenge by oral gavage six times, IgE levels and Th2 cell percentages in the blood in OVA and Medium/OVA groups were significantly increased in the mice compared with the PBS group. Levels of IgE and percentage of Th2 cells in the blood were significantly decreased in the mice treated with hUC-SCs compared with other OVA-challenged groups. Protein levels of Th2 cytokines, including IL-4, were not determined in the blood. However, IL-4 mRNA levels in the colon were increased in OVA-challenged mice compared with the PBS-treated control. Treatment with hUC-MSCs decreased these OVA-induced effects. Published works declared that different mechanisms are involved in the immune regulation of IL-4 expression mediated by various MSCs, including TGF-β secretion and Treg cell differentiation (21,53,58). Although, levels of TGF-β and Treg cells in the blood were not detected here, the previous studies indicate that these immune-suppression mechanisms mediated by direct injection of MSCs may also serve potential roles in the results of the current study.
Goblet cells are columnar epithelial cells in the gastrointestinal, respiratory and reproductive tract. The basic function of goblet cells is to secrete gel-forming mucins in order to protect mucous membranes, including mucin-2 in the intestine and mucin-5AC in the airway (59). Generally, allergen-induced asthma promotes goblet cell metaplasia to secrete more mucins protecting the mucosa (60). Mechanistically, published data suggested that histamine and inflammatory cytokines, including IL-13 and IL-7, contribute to this goblet cell hyperplasia in the respiratory tract (61,62). In the intestinal tract, nutrition and probiotics regulate the number of goblet cells (63). These goblet-like shaped cells further act to preferentially deliver antigens to CD103+ DCs and do contribution to oral tolerance in the gut (64). Similar to the goblet cell metaplasia in asthma, an increased number of these cells in the intestinal tract indicates its protection in food allergy (65).
Recently, by sequencing the bacterial 16S rRNA gene to study the composition of the intestinal microbiota and the microbial metabolism, evidence from experimental models and clinical investigations suggested that a disturbed gut microbiota is associated with the development of allergic manifestations (51,66). The decreased gut microbiota richness, the decreased Bacteroidaceae/Enterobacteriaceae ratio or a lower relative abundance of Lachnospiraceae are associated with sensitization to allergens in the gut or airway (67). This was further observed and confirmed in the current study. Accumulating data demonstrated that butyrate-producing bacteria use fiber to produce short fatty acids, including butyrate, in order to maintain gut homeostasis and immune tolerance by promoting differentiation of Treg and resident CD103+ DCs (68,69). DC- or regulatory T cell-meditated tolerance can be reprogrammed by inflammation-associated cytokines to alter food-allergic immunity, which is mediated by Th2 cells or type 2 innate lymphoid cells (48,54). In an asthma model, MSCs were reported to alleviate clinical symptoms through multiple mechanisms, including promoting Treg or inhibiting Th1 cell differentiation, and modifying the phenotype of resident DCs and macrophages (50,53,57,58). However, the exact functions of the treatment with hUC-MSCs in the current study on the population and abundance of the gut microbiota requires further investigation. It is well known that a specific diet may promote particular bacterial strains to grow through altering the microbiota metabolism and drive the development of the pathological flora microenvironment in the gut (42,70). Additionally, published studies revealed that treatment with induced pluripotent stem cell-derived MSCs and adipose-derived MSCs partially restored the microbiome in mice with colitis (71). Similar to these reports, OVA by oral gavage in the current study successfully modified the richness and composition of the gut microbiota, which is associated with allergies in the gut. Treatment with MSC culture supernatant by oral gavage partially recovered the gut microbiota. The nutritional ingredients from the MSC supernatant in the treatment group may contribute to this restoration of the gut flora. In addition, it has been demonstrated that immunomodulators, including immunoglobulin and mammalian target of rapamycin inhibitors, affect the immune regulation through directly altering the phenotype of immune effector cells and by modifying the gut flora (72–74). Supported by these studies, it is suggested that hUC-MSCs as strong immune regulators may alter the gut flora. Collectively, though the exact mechanism is yet to be elucidated, these results indicate that treatment with hUC-MSCs may regulate the immune functions partially through modifying the bacterial richness and composition in the gut flora, particularly by increasing the abundance of butyrate-producing bacteria, including S24-7, Lachnospiraceae and Bacteroidaceae.
In conclusion, treatment with hUC-MSCs alleviated an IgE-dependent food allergy by modifying the immune balance, which included decreasing the IgE levels and the number of Th2 cells. Furthermore, treatment with hUC-MSCs exhibited restorative effects on goblet cells and commensal bacteria in the gut. Therefore, the present study suggested that hUC-MSCs affected the regulation on the intestinal immune system and the modification of the gut microenvironment. hUC-MSCs may have a potential clinical application in food allergy therapy.
Acknowledgements
The authors would like to thank the members of the Shao and Zhou Lab (School of Medicine, Jiangsu University) for helpful discussions and technical support.
Funding
The current study was funded by grants from the National Natural Science Foundation of China (grant nos. 31570879 and 81871234) and the Key Research and Development Program of Jiangsu Province, China (grant no. BE2017696).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
NY performed experiments and analyzed the data. JX, CZ and YW looked after the animals and performed flow cytometry. FG, CL and WZ performed cell culturing and ELISA. TX, XZ and QS performed tissue collections, histology and participated in discussions. SX designed the project, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All the protocols were reviewed and approved by the Ethics Committee of Jiangsu University and Animal Care and Use Committee of Jiangsu University. Patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yue D, Ciccolini A, Avilla E, Waserman S. Food allergy and anaphylaxis. J Asthma Allergy. 2018;11:111–120. doi: 10.2147/JAA.S162456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2010. J Allergy Clin Immunol. 2011;127:326–335. doi: 10.1016/j.jaci.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Parrish CP, Kim H. Food-induced anaphylaxis: An update. Curr Allergy Asthma Rep. 2018;18:41. doi: 10.1007/s11882-018-0795-5. [DOI] [PubMed] [Google Scholar]
- 4.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koplin JJ, Mills EN, Allen KJ. Epidemiology of food allergy and food-induced anaphylaxis: Is there really a western world epidemic? Curr Opin Allergy Clin Immunol. 2015;15:409–416. doi: 10.1097/ACI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Zheng W, Yung E, Zhong N, Wong GW, Li J. Frequency of food group consumption and risk of allergic disease and sensitization in schoolchildren in urban and rural china. Clin Exp Allergy. 2015;45:1823–1832. doi: 10.1111/cea.12532. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RJ, Dear KB, Tang ML. Time trends in Australian hospital anaphylaxis admissions in 1998–1999 to 2011–2012. J Allergy Clin Immunol. 2015;136:367–375. doi: 10.1016/j.jaci.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, Pumphrey R, Boyle RJ. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol. 2015;135:956–963. doi: 10.1016/j.jaci.2014.10.021. e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(Suppl 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spergel JM. Nonimmunoglobulin e-mediated immune reactions to foods. Allergy Asthma Clin Immunol. 2006;2:78–85. doi: 10.2310/7480.2006.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Sanchez P, Martin-Villa JM. Gut immune system and oral tolerance. Br J Nutr. 2013;109(Suppl 2):S3–S11. doi: 10.1017/S0007114512005223. [DOI] [PubMed] [Google Scholar]
- 12.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8:265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 14.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O'Riordan G, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YH. Developing food allergy: A potential immunologic pathway linking skin barrier to gut. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.9497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivas Noval M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–1072. doi: 10.1172/JCI200316142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima-Adachi H, Ebihara A, Kikuchi A, Ishida T, Sasaki K, Hirano K, Watanabe H, Asai K, Takahashi Y, Kanamori Y, et al. Food antigen causes TH2-dependent enteropathy followed by tissue repair in T-cell receptor transgenic mice. J Allergy Clin Immunol. 2006;117:1125–1132. doi: 10.1016/j.jaci.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 21.Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, Yu HS, Roh HJ. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014;2014:436476. doi: 10.1155/2014/436476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Aguiar CF, Castoldi A, Andrade-Oliveira V, Ignacio A, da Cunha FF, Felizardo RJF, Bassi ÊJ, Câmara NOS, de Almeida DC. Mesenchymal stromal cells modulate gut inflammation in experimental colitis. Inflammopharmacology. 2018;26:251–260. doi: 10.1007/s10787-017-0404-6. [DOI] [PubMed] [Google Scholar]
- 23.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, Takaori-Kondo A, Ichinohe T, Maekawa T. Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive t cell populations. Stem Cells. 2018;36:434–445. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 25.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 26.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Ghannam S, Pene J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 28.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 29.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: An overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293–1306. doi: 10.1517/14712598.2015.1051528. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 33.Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, Seo MS, Hong IS, Choi SW, Seo KW, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145(1392–1403):e1391–e1398. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 36.Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 38.Paula-Silva J, Santiago AF, Oliveira RP, Rosa ML, Carvalho CR, Amaral JF, Faria AM. Effect of a protein-free diet in the development of food allergy and oral tolerance in BALB/c mice. Br J Nutr. 2015;113:935–943. doi: 10.1017/S0007114515000173. [DOI] [PubMed] [Google Scholar]
- 39.Kay AG, Long G, Tyler G, Stefan A, Broadfoot SJ, Piccinini AM, Middleton J, Kehoe O. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep. 2017;7:18019. doi: 10.1038/s41598-017-18144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pouya S, Heidari M, Baghaei K, Aghdaei Asadzadeh H, Moradi A, Namaki S, Zali MR, Hashemi SM. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86–94. doi: 10.1016/j.intimp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Nagata Y, Yamamoto T, Hayashi M, Hayashi S, Kadowaki M. Improvement of therapeutic efficacy of oral immunotherapy in combination with regulatory T cell-inducer kakkonto in a murine food allergy model. PLoS One. 2017;12:e0170577. doi: 10.1371/journal.pone.0170577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, Xiao T, Yan N, An H, Zhou X, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. 2016;40:1–10. doi: 10.1016/j.intimp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Parada AE, Needham DM, Fuhrman JA. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18:1403–1414. doi: 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- 45.Metcalf JL, Xu ZZ, Weiss S, Lax S, Van Treuren W, Hyde ER, Song SJ, Amir A, Larsen P, Sangwan N, et al. Microbial community assembly and metabolic function during mammalian corpse decomposition. Science. 2016;351:158–162. doi: 10.1126/science.aad2646. [DOI] [PubMed] [Google Scholar]
- 46.Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal stem or stromal cells: Toward a better understanding of their biology? Transfus Med Hemother. 2010;37:75–83. doi: 10.1159/000290897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 49.Gazit Z, Pelled G, Sheyn D, Kimelman N, Gazit D. Mesenchymal Stem Cells. In: Lanza R, Atala A, editors. Essentials of Stem Cell Biology (third Edition) Academic Press; Boston: 2014. pp. 255–266. [DOI] [Google Scholar]
- 50.van Halteren AG, van der Cammen MJ, Biewenga J, Savelkoul HF, Kraal G. IgE and mast cell response on intestinal allergen exposure: A murine model to study the onset of food allergy. J Allergy Clin Immunol. 1997;99:94–99. doi: 10.1016/S0091-6749(97)81049-4. [DOI] [PubMed] [Google Scholar]
- 51.Sjodin Simonyte K, Vidman L, Ryden P, West CE. Emerging evidence of the role of gut microbiota in the development of allergic diseases. Curr Opin Allergy Clin Immunol. 2016;16:390–395. doi: 10.1097/ACI.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 52.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majore I, Moretti P, Stahl F, Hass R, Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011;7:17–31. doi: 10.1007/s12015-010-9165-y. [DOI] [PubMed] [Google Scholar]
- 55.Phillips CD, Wongsaisri P, Htut T, Grossman T. Purified umbilical cord derived mesenchymal stem cell treatment in a case of systemic lupus erythematosus. Clin Transl Med. 2017;6:31. doi: 10.1186/s40169-017-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Robinson AM, Sakkal S, Park A, Jovanovska V, Payne N, Carbone SE, Miller S, Bornstein JC, Bernard C, Boyd R, Nurgali K. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1115–G1129. doi: 10.1152/ajpgi.00174.2014. [DOI] [PubMed] [Google Scholar]
- 57.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–573. doi: 10.1016/j.jaci.2010.12.1098. quiz 574–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyagawa I, Nakayamada S, Nakano K, Yamagata K, Sakata K, Yamaoka K, Tanaka Y. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J Immunol. 2017;199:1616–1625. doi: 10.4049/jimmunol.1600230. [DOI] [PubMed] [Google Scholar]
- 59.Rubin BK. Secretion properties, clearance, and therapy in airway disease. Transl Respir Med. 2014;2:6. doi: 10.1186/2213-0802-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 61.Xia W, Bai J, Wu X, Wei Y, Feng S, Li L, Zhang J, Xiong G, Fan Y, Shi J, Li H. Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One. 2014;9:e98915. doi: 10.1371/journal.pone.0098915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8:384–392. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dock-Nascimento DB, Junqueira K, Aguilar-Nascimento JE. Rapid restoration of colonic goblet cells induced by a hydrolyzed diet containing probiotics in experimental malnutrition. Acta Cir Bras. 2007;1(Suppl 22):S72–S76. doi: 10.1590/S0102-86502007000700014. [DOI] [PubMed] [Google Scholar]
- 64.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaki K, Yoshino S. Remission of food allergy by the Janus kinase inhibitor ruxolitinib in mice. Int Immunopharmacol. 2014;18:217–224. doi: 10.1016/j.intimp.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 66.Clooney AG, Fouhy F, Sleator RD, O'Driscoll A, Stanton C, Cotter PD, Claesson MJ. Comparing apples and oranges?: Next generation sequencing and its impact on microbiome analysis. PLoS One. 2016;11:e0148028. doi: 10.1371/journal.pone.0148028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 68.Yamashiro Y. Gut microbiota in health and disease. Ann Nutr Metab. 2017;71:242–246. doi: 10.1159/000481627. [DOI] [PubMed] [Google Scholar]
- 69.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 70.Bibbo S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, Cammarota G. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016;20:4742–4749. [PubMed] [Google Scholar]
- 71.Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, Dow S. Mesenchymal stem cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl Med. 2018;7:456–467. doi: 10.1002/sctm.17-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, Bergman M, Orihuela CJ, Galvan V, Padrón Á, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henderson AL, Brand MW, Darling RJ, Maas KJ, Detzel CJ, Hostetter J, Wannemuehler MJ, Weaver EM. Attenuation of colitis by serum-derived bovine immunoglobulin/protein isolate in a defined microbiota mouse model. Dig Dis Sci. 2015;60:3293–3303. doi: 10.1007/s10620-015-3726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carbonnel F, Soularue E, Coutzac C, Chaput N, Mateus C, Lepage P, Robert C. Inflammatory bowel disease and cancer response due to anti-CTLA-4: Is it in the flora? Semin Immunopathol. 2017;39:327–331. doi: 10.1007/s00281-016-0613-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.