Abstract
In this study, a series of fourteen ring-substituted 8-hydroxyquinoline derivatives were prepared. The synthesis procedures are presented. The compounds were analyzed using RP-HPLC to determine lipophilicity. They were tested for their activity related to inhibition of photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. Primary in vitro screening of the synthesized compounds was also performed against four mycobacterial strains and against eight fungal strains. Several compounds showed biological activity comparable with or higher than the standards isoniazid or fluconazole. For all the compounds, the relationships between the lipophilicity and the chemical structure of the studied compounds are discussed.
Keywords: quinolines, lipophilicity, PET inhibition, spinach chloroplasts, in vitro antifungal activity, in vitro antimycobacterial activity
1. Introduction
A quinoline moiety is present in many classes of biologically-active compounds. A number of them have been used clinically as antifungal, antibacterial and antiprotozoic drugs [1,2], as well as antituberculotic agents [3,4,5]. Some quinoline-based compounds also show antineoplastic, antiasthmatic and antiplatelet activity [6,7,8,9,10,11]. A series of compounds derived from 8-hydroxyquinoline and styrylquinoline derivatives were recently synthesized as potential HIV-1 integrase inhibitors [12,13,14,15]. These compounds showed a significant similarity to some novel antifungal agents, namely homoallylamines. [16]. Our previous study dealing with 8-hydroxyquinoline and styrylquinoline derivatives showed that they could also possess strong antifungal activity [17,18]. According to the results reported recently, some new hydroxyquinoline derivatives also possess interesting herbicidal activities [17,19,20,21,22]. In addition, some of the investigated quinoline derivatives also showed antineoplastic activity [19,23].
Tuberculosis is a worldwide pandemic. About 1/3 of the world's population is infected with Mycobacterium tuberculosis, and every year almost 2 million people die as a result [24]. The Mycobacterium genus is composed of the M. tuberculosis complex and other species known as nontuberculous mycobacteria (NTM). In recent decades, the decrease in the prevalence of tuberculosis in developed countries has resulted in the increase in the proportion of diseases caused by NTM [25]. Among these species, the M. avium complex (MAC) has emerged as a major human pathogen, being a common cause of disseminated disease and death in patients with HIV/AIDS [26].
Chronic pulmonary disease is the most common clinical manifestation among the diseases caused by NTM, and the most common pathogens are the species belonging to the MAC, followed by M. kansasii. The clinical characteristics of NTM-related pulmonary disease are, in many cases, extremely similar to those of tuberculosis. Other clinical manifestations are caused principally by M. fortuitum, M. smegmatis and M. abscessus due to peritoneal infection as a result of catheterization, postsurgical infections, such as those following mammoplasty and heart transplant, as well as those following invasive procedures [27]. The above mentioned non-tuberculous strains are sometimes resistant to commonly used drugs (isoniazid, rifampicin, pyrazinamide) and other anti-tuberculous drugs [24], therefore systematic development of new effective compounds is necessary. Similarly, the discovery of new drugs for the treatment of systemic mycoses with novel modes of action due to the rapid growth of the immunocompromised patient population and development of resistance to the present azole therapies, and high toxicity of polyenes [28] is indispensable. It should be stressed that hydroxyquinoline and its derivatives were introduced as antifungal or antimycobacterial agents in clinical practice and novel compounds of this type are still investigated [3,4,5,29,30].
Over 50% of commercially available herbicides act by reversibly binding to photosystem II (PS II), a membrane-protein complex in the thylakoid membranes which catalyses the oxidation of water and the reduction of plastoquinone [31] and thereby inhibit photosynthesis [32,33,34]. Some organic compounds, e.g., substituted benzanilides [35] or substituted anilides of 2,6-disubstituted pyridine-4-thiocarboxamides [36] or pyrazine-2-carboxylic acids [37] were found to interact with tyrosine radicals TyrZ and TyrD which are situated in D1 and D2 proteins on the donor side of PS II and due to this interaction the photosynthetic electron transport is interrupted.
This is a follow-up paper to our previous articles [12,13,14,15,17,18,19,20,21,22,23] dealing with syntheses and biological activities of ring-substituted quinoline derivatives. On the basis of formerly described azanaphtalenes we tried to search for new modifications of quinoline moiety that can trigger interesting biological activity.
Primary in vitro screening of the synthesized compounds was performed against four mycobacterial strains and against eight fungal strains. The compounds were also tested for their photosynthesis-inhibiting activity (the inhibition of photosynthetic electron transport) in spinach chloroplasts (Spinacia oleracea L.). Relationships among the structure and in vitro antimicrobial activities or/and inhibitory activity related to inhibition of photosynthetic electron transport (PET) in spinach chloroplasts of the new compounds are discussed.
2. Results and Discussion
2.1. Chemistry
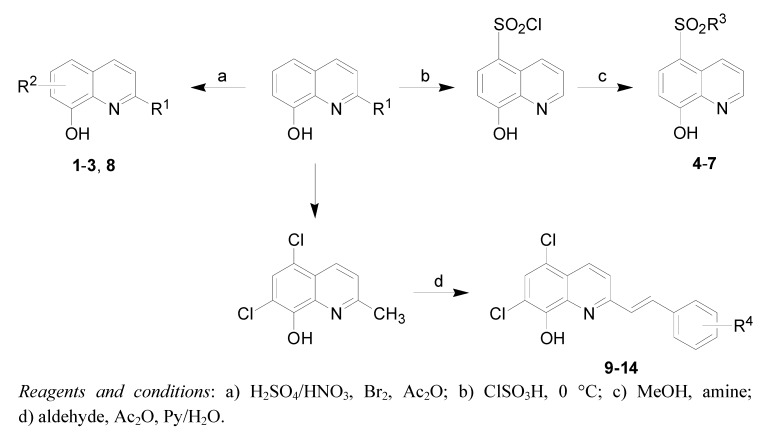
All studied compounds were prepared according to Scheme 1. Compounds 1-3 were obtained according to a previously described procedure [14]. Sulfonamides 4-7 were obtained from 8-hydroxyquinoline through chlorosulfonation and amination. Compound 8 was obtained from commercially available 8-hydroxy-2-aminoquinoline by acylation in acetic anhydride. Styryquinolines 9-14 were obtained from 5,7-dichloro-8-hydroxyquinaldine and the appropriate aldehyde in a two-step reaction in acetic anhydride followed by pyridine/water.
Scheme 1.
Synthesis of studied compounds.
2.2. Lipophilicity
Many low molecular weight drugs cross biological membranes through passive transport, which strongly depends on their lipophilicity. Lipophilicity is a property that has a major effect on absorption, distribution, metabolism, excretion, and toxicity (ADME/Tox) properties as well as pharmacological activity. Lipophilicity has been studied and applied as an important drug property for decades [38].
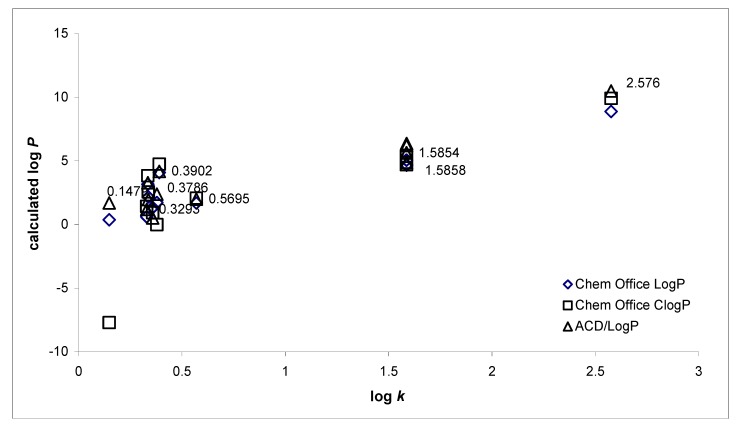
Hydrophobicities (log P/Clog P) of the compounds 1-14 were calculated using two commercially available programs (ChemDraw Ultra 10.0 and ACD/LogP) and also measured by means of the RP-HPLC determination of capacity factors k with subsequent calculation of log k. The procedure was performed under isocratic conditions with methanol as an organic modifier in the mobile phase using an end-capped non-polar C18 stationary RP column. The ChemDraw program did not resolve various lipophilicity values of individual positional isomers, that is, the same log P/Clog P data were calculated for 9/10 and 11/12. The results are shown in Table 1 and illustrated in Figure 1.
Table 1.
Comparison of the calculated lipophilicities (log P/Clog P) with the determined log k values.
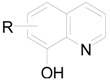 | ||||
| Comp. | R | log k | log P/Clog P | log P |
| ChemOffice | ACD/LogP | |||
| 1 | 5-NO2 | 0.5695 | 1.69 / 2.0836 | 2.00 ± 0.32 |
| 2 | 5-SO3H-7-NO2 | 0.1479 | 0.37 / -0.703 | 1.70 ± 0.88 |
| 3 | 5-SO3H-7-Br | 0.3786 | 1.72 / -0.004 | 2.39 ± 0.91 |
| 4 |
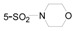
|
0.3293 | 0.61 / 1.43775 | 1.19 ± 0.81 |
| 5 | 5-SO2NHCH(CH3)2 | 0.3373 | 2.17 / 2.5697 | 1.97 ± 0.78 |
| 6 | 5-SO2NHC2H4Ph | 0.3349 | 3.17 / 3.83597 | 3.29 ± 0.79 |
| 7 | 5-SO2NHC4H8Ph | 0.3902 | 4.09 / 4.74397 | 4.18 ± 0.78 |
| 8 |
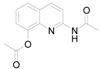
|
0.3583 | 1.33 / 0.93375 | 0.52 ± 0.73 |
| ||||
| Comp. | R | log k | log P/Clog P | log P |
| ChemOffice | ACD/LogP | |||
| 9 | 2-OH | 1.5854 | 5.07 / 5.36425 | 5.62 ± 0.35 |
| 10 | 4-OH | 1.5866 | 5.07 / 5.36425 | 6.37 ± 0.36 |
| 11 | 2,4-OH | 1.5858 | 4.68 / 4.69725 | 5.65 ± 0.37 |
| 12 | 3,5-OH | 1.5867 | 4.68 / 4.69725 | 6.20 ± 0.37 |
| 13 | 2-OH-5-OAc | 1.5864 | 4.66 / 4.89345 | 4.87 ± 0.36 |
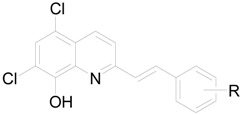
| 14 |
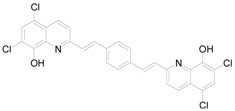
|
2.5760 | 8.88 / 9.92051 | 10.50 ± 0.39 |
Figure 1.
Comparison of the computed log P/Clog P values using two programs with the calculated log k values.
The results obtained with all the compounds show that the experimentally-determined lipophilicities (log k) of compounds 1-14 are lower than those indicated by the calculated log P/Clog P, as shown in Figure 1. The results indicate that experimentally-determined log k values correlate relatively poorly with the calculated values. The correlation factors R2 yielded 0.83 for ChemOffice log P; 0.86 for ACD/LogP and 0.55 for ChemOffice Clog P respectively.
These facts are probably caused by intramolecular interactions. As expected, compound 14 showed the highest lipophilicity, while compound 2 exhibited the lowest. The presence of the sulfonic acid moiety decreased the lipophilicity. Compound 6 showed less hydrophobicity compared with 5, contrary to the lipophilicity results calculated by software. This observation has been made previously [19]. Interesting results were obtained with subseries 9-13. Comparing the lipophilicity data log k of both OH-monosubstituted isomers 9 and 10, it can be stated that 4-hydroxy derivative 10 possessed higher hydrophobicity than 2-hydroxy isomer 9. This fact was predicted only by ACD/LogP. When OH-disubstituted compounds were compared, it was found that strong intramolecular interactions play a significant role. Hydrophobicity increased in the following order: 11 (2,4-OH) < 12 (3,5-OH). These results were also predicted by ACD/LogP.
It can be assumed, that log k values specify lipophilicity within the individual series of studied compounds. Calculated log P data (ChemDraw) of compounds 1-8 corresponded with experimentally-determined log k, while for compounds 9-14 there was better agreement between the calculated log P data (ACD/LogP) and experimentally-determined log k.
2.3. Biological activities
The compounds under investigation could be divided into two groups based on their chemical structure. Group 1 included compounds 1-8, and Group 2 compounds 9-14. The compounds showed a wide range of biological activities and structure-activity relationships observations are interesting. All the results are shown in Table 2 and Table 3.
Table 2.
IC80 values related to PET inhibition in spinach chloroplasts in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard and in vitro antifungal activity (IC80) of compounds 1-10, 12 and 14 compared with fluconazole (FLU) standard. (ND = not determined due to insufficient solubility of the compound).
| Comp. | PET reductionIC50 [ μmol/L] | MIC [µmol/L] | |||||||
| CAa | CTa | CKa | CGa | TBa | AFb | ACb | TMb | ||
| 24h | 24h | 24h | 24h | 24h | 24h | 24h | 72h | ||
| 48h | 48h | 48h | 48h | 48h | 48h | 48h | 120h | ||
| 1 | 78.0 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | ||
| 2 | ND | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | ||
| 3 | 33.5 | 0.77 | 1.95 | 3.90 | 1.95 | 3.90 | 0.77 | 7.80 | 1.95 |
| 1.95 | 3.90 | 3.90 | 3.90 | 3.90 | 1.95 | 7.80 | 1.95 | ||
| 4 | ND | 125 | 500 | 250 | 500 | 500 | 500 | 125 | 250 |
| 500 | 500 | 500 | 500 | 500 | 500 | 250 | 250 | ||
| 5 | 54.4 | 125 | 250 | 250 | 125 | 250 | 250 | 125 | 62.50 |
| 250 | 250 | 250 | 250 | 500 | 250 | 125 | 125 | ||
| 6 | 304 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | ||
| 7 | 616 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | ||
| 8 | 306 | 15.60 | 31.25 | 62.50 | 31.25 | 62.50 | 62.50 | 62.50 | 62.50 |
| 62.50 | 31.25 | 62.50 | 62.50 | 125 | 62.50 | 62.50 | 62.50 | ||
| 9 | ND | >125 | >125 | >125 | >125 | >125 | 125 | 62.50 | 62.50 |
| >125 | >125 | >125 | >125 | >125 | 125 | 125 | 62.50 | ||
| 10 | 660 | 125 | 125 | 125 | 125 | 125 | 125 | 31.25 | 15.62 |
| >125 | >125 | >125 | >125 | >125 | 125 | 62.50 | 62.50 | ||
| 12 | 341 | >125 | >125 | >125 | >125 | >125 | 125 | 62.50 | 15.62 |
| >125 | >125 | >125 | >125 | >125 | 125 | 62.50 | 15.62 | ||
| 14 | 334 | 0.98 | 1.95 | 0.98 | 0.49 | 3.90 | 3.90 | 15.62 | 3.90 |
| 3.90 | 7.81 | 1.95 | 1.95 | 15.62 | 15.62 | 31.25 | 7.81 | ||
| DCMU | 1.9 | – | – | – | – | – | – | – | – |
| FLU | – | 0.06 | 0.12 | 3.91 | 0.98 | 0.24 | >125 | >125 | 1.95 |
| 0.12 | >125 | 15.62 | 3.91 | 0.48 | >125 | >125 | 3.91 | ||
The MIC determination was performed according to the CLSI reference protocol: a M27-A2 for yeasts (IC80 value) and b M38-A for moulds (IC50 value).
Table 3.
Antimycobacterial activity MIC/IC90 [µg/mL] of compounds 9-14 in comparison with the standards, pyrazinamide (PZA) and isoniazid (INH).
| Comp. | MIC/IC90 [µg/mL] | |||
| M. smegmatis | M. absessus | M. kansasii | M. avium complex | |
| 9 | 100 | >300 | >300 | >300 |
| 10 | 150 | >300 | >300 | >300 |
| 11 | 100 | >100 | >100 | >100 |
| 12 | >100 | >300 | >300 | >300 |
| 13 | 100 | >300 | >300 | >300 |
| 14 | 40 | 130 | 40 | >300 |
| PZA | >100 | >100 | >100 | >100 |
| INH | 39 | >100 | <10 | <10 |
2.3.1. Inhibition of photosynthetic electron transport (PET) in spinach chloroplasts
Quinoline derivatives 1-10, 12 and 14 showed a wide range of activity related to inhibition of photosynthetic electron transport (PET) in spinach chloroplasts, see Table 2. Three compounds showed moderate inhibitory IC50 values: 33.5 µmol/L (3), 54.4 µmol/L (5) and 78.0 µmol/L (1) while the activity of all the other studied compounds was very low. PET inhibition by 2, 4 and 9 could not be determined due to precipitation of the compounds during the experiments.
The highest PET inhibition was shown by compounds 3, 5 and 1 with suitable aqueous solubility as well as lipophilicity. The sulfonic acid moiety contributed to enhanced aqueous solubility whereas a 7-bromo moiety (compound 3) increased the lipophilicity. Herbicidal effects of organic compounds with bromo or nitro substituents were observed previously [39]. Bulkiness and rigidity of the chain in the R substituent play a fundamental role in the series of sulfonamides 4-7, in addition to lipophilicity and water-solubility. The least lipophilic morpholine derivative 4 did not show any activity. On the other hand, phenylbutyl substitution in compound 7 is probably too bulky for it to reach the site of action in the photosynthetic electron transport chain and thus the inhibitory activity of this compound is low. Within the sulfonamide series the isopropyl substituent was the most advantageous from the aspect of effective PET inhibition and further lipophilicity increase caused reduction of inhibitory activity. On the other hand, great differences in the activity of compounds characterized with rather small differences in the lipophilicity (the log k values of compounds belonging to Group 1) indicate that the position of substituents and their electronic Hammett's parameters σ (especially electron-withdrawing effect) seem to be also important for biological activity. Lipophilicity of the discussed compounds is only a secondary parameter influencing PET-inhibiting activity. In general, Group 2 compounds exhibited only slight inhibition of PET and IC50 values determined for three compounds do not enable conclusions about structure-activity relationships.
2.3.2. In vitro antifungal susceptibility testing
Quinoline derivatives 1-10, 12 and 14 were tested for their in vitro antifungal activity. The results are shown in Table 2. Two compounds 3 and 14 showed very high antifungal activity, comparable or higher than the standard fluconazole.
Generally, Group 1 showed lower biological activity than Group 2, although compound 3 demonstrated high activity against all fungal strains. Compounds 1, 2, 4-7 did not show any antifungal activity. Compound 8 showed only moderate activity especially against Candida albicans ATCC 44859.
Unfortunately due to general low activity, no thorough structure-activity relationships (SAR) can be established. Nevertheless, an interesting relationship can be observed. According to the results presented in Table 1 and Table 2 it can be concluded that bulky substituents and low lipophilicity decreased antifungal activity. A bromo moiety (compound 3) seems to be very important for high antifungal effect, as was reported recently [17]. Contrary to all expectations [18], compounds with a nitro moiety (i.e., 1, 2) did not show any activity. It can be concluded that relatively minor differences in lipophilicity were correlated with major differences in the activity of compounds. This suggests that for biological activity, the position of substituents and their electronic Hammett's parameters σ seem to be important. Similar observations were made with the PET-inhibiting activity.
Compound 14 showed the highest antifungal activity within Group 2. The compounds 9-12 showed only a moderate activity especially against Absidia corymbifera 272 and Trichophyton mentagrophytes 445. Contrary to all expectations [18], 9 (2-OH substitution) possessed less activity than 10 and 12 (4-OH or 3,5-OH respectively). This fact is probably caused by low solubility of 9. Lipophilicity is probably an important parameter influencing the activity.
2.3.3. In vitro antimycobacterial evaluation
Six compounds within Group 2 9-14 were evaluated for their in vitro antimycobacterial activity against four mycobacterial strains. The results are shown in Table 3. Compound 12 (3,5-OH) did not exhibit any significant antimycobacterial activity and compounds 9-11, 13 showed only medium and/or moderate activities. Nevertheless, compound 14 had an interesting MIC especially against M. smegmatis, M. kansasii and M. absessus. This compound was more active than the standard pyrazinamide (PZA) and in case of M smegmatis, its activity was comparable with the standard isoniazid (INH).
Due to medium and/or moderate activity of the majority of evaluated compounds, it is difficult todetermine simple structure-activity relationships. According to Table 1 and Table 3, it can be concluded that lipophilicity (log k) and other physico-chemical parameters only influence secondary characteristics of the compounds.
3. Conclusions
A series of fourteen ring-substituted 8-hydroxyquinolines were prepared and characterized. Compounds 1,3-14 are original new structures. All the prepared quinoline derivatives were analyzed using a RP-HPLC method for the measurement of lipophilicity and their lipophilicity was determined. The prepared compounds were tested for their antifungal and antimycobacterial activity and for their activity related to the inhibition of photosynthetic electron transport (PET) in spinach chloroplasts (Spinacia oleracea L.). 7-Bromo-8-hydroxyquinoline-5-sulfonic acid (3) and 5,7-dichloro-2-(2-{4-[2-(5,7-dichloro-8-hydroxy-quinolin-2-yl)vinyl]phenyl}vinyl)quinolin-8-ol (14) possessed the highest in vitro antifungal activity and 3 and 8-hydroxyquinoline-5-sulfonic acid isobutylamide (5) showed the highest PET-inhibiting activity. Compound 14 showed the highest antimycobacterial activity. In general, compounds 3 and 14 showed the highest inhibitory effects within the series.
4. Experimental
4.1. General
All reagents were purchased from Aldrich. Kieselgel 60, 0.040-0.063 mm (Merck, Darmstadt, Germany) was used for column chromatography. TLC experiments were performed on alumina-backed silica gel 40 F254 plates (Merck, Darmstadt, Germany). The plates were illuminated under UV (254 nm) and evaluated in iodine vapour. The melting points were determined on Boetius PHMK 05 (VEB Kombinat Nagema, Radebeul, Germany) and are uncorrected. Elemental analyses (E.A.) were carried out on an automatic Perkin-Elmer 240 microanalyser (Boston, MA, USA) for C, H, N and are within 0.4% of theoretical values. The purity of the final compounds was checked by the HPLC separation module Waters Alliance 2695 XE (Waters Corp., Milford, MA, USA). The detection wavelength 210 nm was chosen. The peaks in the chromatogram of the solvent (blank) were deducted from the peaks in the chromatogram of the sample solution. The purity of individual compounds was determined from the area peaks in the chromatogram of the sample solution. UV spectra (λ, nm) were determined on a Waters Photodiode Array Detector 2996 (Waters Corp., Milford, MA, USA) in ca 6×10-4 mol methanolic solution and log ε (the logarithm of molar absorption coefficient ε) was calculated for the absolute maximum λmax of individual target compounds. All 1H-NMR spectra were recorded on a Bruker AM-500 (499.95 MHz for 1H), and 13C-NMR spectra were recorded on a Bruker AM-400 (100 MHz) instrument (Bruker BioSpin Corp., Germany). Chemicals shifts are reported in ppm (δ) with reference to internal Si(CH3)4, when diffused easily exchangeable signals are omitted.
4.2. Synthesis
5-Nitroquinolin-8-ol (1). Yield: 25% of a yellow crystalline compound; Mp. 179-180 °C; HPLC purity 98.09%; UV (nm), λmax/log ε: 398.4/3.67) [14].
8-Hydroxy-7-nitroquinoline-5-sulfonic acid (2). Yield: 54% of a bright yellow crystalline compound; Mp. 265 °C; HPLC purity 97.97%; UV (nm), λmax/log ε: 427.9/3.71) [40].
7-Bromo-8-hydroxyquinoline-5-sulfonic acid (3). Yield: 25% of a beige crystalline compound; Mp. 280 °C; HPLC purity 98.32%; UV (nm), λmax/log ε: 264.2/3.69) [14].
5-(Morpholine-4-sulfonyl)quinolin-8-ol (4). 8-Hydroxyquinoline-5-sulfonyl chloride (2.4 g, 0.01 mol) was dissolved in dry MeOH (30 mL) and heated to reflux. Then morpholine (1.75 mL, 0.02 mol) was added dropwise. After 2 h of heating the resulted mixture was concentrated in vacuo and the resulting brown oil put into dichloromethane (45 mL). The orange precipitate formed was filtered off and recrystallized from MeOH. Yield 73% of a light yellow crystalline compound; Mp. 172 °C; E.A. Calcd.: C 54.53%, H 5.23%, N 9.08%; found: C 54.26%, H 5.31%, N 9.27%; HPLC purity 97.89%; UV (nm), λmax/log ε: 264.0/3.65; 1H-NMR (DMSO-d6), δ: 2.52 (s, 1H), 3.03 (t, J = 4.6 Hz, 4H), 3.70 (t, J = 4.7 Hz, 4H), 6.95 (d, J = 7.9 Hz, 1H), 7.80 (d, J = 7.9 Hz, 1H), 9.05 (d, J = 8.4 Hz, 1H), 9.10 (d, J = 8.4 Hz, 2H).
8-Hydroxyquinoline-5-sulfonic acid isobutylamide (5). 8-Hydroxyquinoline-5-sulfonyl chloride (2.2 g, 0.01 mol) was dissolved in dry MeOH (30 mL) and heated to reflux. Then 2-butylamine (2.02 mL, 0.02 mol) was added dropwise. After 2 h of heating a yellow solid was precipitated from the reaction mixture. After cooling the solid was filtered and crystallized from MeOH. Yield 50% of a yellow crystalline compound; Mp. 151 °C; E.A. Calcd.: C 55.70%, H 5.75%, N 9.99%; found: C 55.67%, H 5.63%, N 9.84%; HPLC purity 97.29%; UV (nm), λmax/log ε: 265.2/3.64; 1H-NMR (DMSO-d6), δ: 0.85 (t, J = 7.4 Hz, 3H), 1.05 (d, J = 6.4 Hz, 1H), 1.50 (m, 2H), 2.50 (s, 1H), 2.95 (m, 1H), 6.95 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 7.9 Hz, 1H), 9.05 (d, J = 7.6 Hz, 1H), 9.10 (d, J = 7.3 Hz, 2H).
8-Hydroxyquinoline-5-sulfonic acid (2-phenylethyl)amide (6). Obtained in similar way to 4 from 8-hydroxyquinoline-5-sulfonyl chloride and phenylethylamine. Yield: 25% of a yellow crystalline compound; Mp. 104 °C; E.A. Calcd.: C 62.18%, H 4.91%, N 8.53%; found: C 62.32%, H 4.86%, N 8.71%. HPLC purity 97.37%; UV (nm), λmax/log ε: 265.5/3.68; 1H-NMR (DMSO-d6), δ: 1.38 (d, J = 6.7 Hz, 2H), 2.50 (t, J = 1.6 Hz, 1H), 2.66 (s, 1H), 6.90 (d, J = 7.8 Hz, 1H), 7.30-7.41 (m, 2H), 7.72 (d, J = 8.0 Hz, 2H), 8.96 (d, J = 8.8 Hz, 1H), 7.42-7.44 (m, 5H), 7.25 (d, J = 7.5 Hz, 1H).
8-Hydroxyquinoline-5-sulfonic acid (4-phenylbutyl)amide (7). Obtained in similar way to 4 from 8-hydroxyquinoline-5-sulfonyl chloride and 4-phenylbuthylamine. Yield: 55% of a yellow crystalline compound; Mp. 125 °C; E.A. Calcd: C 64.84%, H 5.99%, N 7.56%; found: C 65.02%, H 5.85%, N 7.69%; HPLC purity 98.60%; UV (nm), λmax/log ε: 265.3/3.68; 1H-NMR (DMSO-d6), δ: 1.44-1.58 (m, 2H), 2.49 (s, 1H), 2.67 (t, J = 6.8 Hz, 1H), 6.95 (d, J = 7.9 Hz, 1H), 2.56 (t, J = 7.2 Hz, 2H), 7.18 (d, J = 6.8 Hz, 1H), 7.24 (t, J = 7.0 Hz, 5H), 7.54-7.58 (m, 2H), 7.82 (d, J = 7.8 Hz, 1H), 9.06 (d, J = 8.5 Hz, 1H), 9.12 (d, J = 8.5 Hz, 2H).
2-Acetylaminoquinolin-8-yl acetate (8). 2-Amino-8-hydroxyquinoline (2.5 g, 0.01 mol) and acetic anhydride (4 g, 3 mL, 0.04 mol) was mixed and heated in the microwave reactor at the boiling point for 5 min, then evaporated until dryness. Crude product was recrystalized from EtOH and dried. Yield: 53% of a white crystalline compound; Mp. 120-126 °C; HPLC purity 99.69%; UV (nm), λmax/log ε: 258.3/3.69; E.A. Calcd: C 63.93%, H 4.95%, N 11.47%; found: C 63.82%, H 4.56%, N 11.25%; 1H-NMR (DMSO-d6), δ: 2.19 (s, 3H), 2.22 (s, 3H), 7.45 (m, 2H), 7.61 (d, J = 8.65Hz, 1H), 7.8 (t, J = 9.5Hz, 1H), 8.37 (d, J = 9.0Hz, 1H), 10.5 (s, 1H).
4.2.1. General method for synthesis of compounds 9-14.
To quinaldine (0.01 mol) in acetic anhydride (30 mL) an appropriate aldehyde (0.04 mol) was added and resulting mixture was heated under reflux for 24 h. Then, the liquid was evaporated in vacuo, pyridine (30 mL) and water (10 mL) were added and the mixture further heated for 3 h under reflux. Then mixture was evaporated to dryness and solid was taken up in dichloromethane and filtered. Crude product was recrystalized from EtOH and dried.
5,7-Dichloro-2-[2-(2-hydroxyphenyl)vinyl]quinolin-8-ol (9). Yield 87% of a beige crystalline compound; Mp. 141 °C; E.A. Calcd.: C 61.47%, H 3.34%, N 4.22%; found: C 61.87%, H 3.05%, N 4.12%; HPLC purity 98.10%; UV (nm), λmax/log ε: 309.9/3.61; 1H-NMR (DMSO-d6), δ: 6.88 (t, J = 7.4 Hz, 1H, phenyl), 6.95 (d, J = 8.1 Hz, 1H, phenyl), 7.20 (t, J = 7.7 Hz, 1H, phenyl), 7.57 (d, J = 16.3 Hz, 1H, vinyl), 7.61 (d, J = 7.7 Hz, 1H, phenyl), 7.68 (s, 1H, quinaldine), 7.93 (d, J = 8.8 Hz, 1H, quinaldine), 8.23 (d, J = 16.3 Hz, 1H, vinyl), 8.39 (d, J = 8.8 Hz, 1H, quinaldine), 10.08 (s, 1H, OH), 10.46 (bs, 1H, OH); 13C-NMR (DMSO-d6), δ: 116.01, 116.66, 119.63, 119.93, 122.19, 123.40, 123.94, 127.22, 127.84, 129.10, 130.56, 133.06, 133.55, 139.35, 149.11, 156.57, 156.74.
5,7-Dichloro-2-[2-(4-hydroxyphenyl)vinyl]quinolin-8-ol (10). Yield 86% of a beige crystalline compound; Mp. 198 °C; E.A. Calcd.: C 61.47%, H 3.34%, N 4.22%; found: C 61.54%, H 3.32%, N 4.28%; HPLC purity 98.52%; UV (nm), λmax/log ε: 309.9/3.58; 1H-NMR (DMSO-d6), δ: 6.85 (d, J = 7.2 Hz, 2H, phenyl), 7.28 (d, J = 16.0 Hz, 1H, vinyl), 7.56 (d, J = 7.3 Hz, 2H, phenyl), 7.69 (d, J = 1.7 Hz, 1H, quinaldine), 7.86 (dd, J = 1.2 Hz, J = 8.8 Hz, 1H, quinaldine), 8.18 (d, J = 16.1 Hz, 1H, vinyl), 8.40 (dd, J = 1.3 Hz, J = 8.8 Hz, 1H, quinaldine), 9.84 (s, 1H, OH), 10.30 (bs, 1H, OH); 13C-NMR (DMSO-d6), δ: 115.17, 115.73, 119.02, 122.07, 123.19, 123.41, 126.41, 127.14, 128.97, 132.97, 136.53, 138.66, 148.36, 155.67, 158.52.
5,7-Dichloro-2-[2-(2,4-dihydroxyphenyl)vinyl]quinolin-8-ol (11). Yield 83% of a beige crystalline compound; Mp. 102-1030 °C; HPLC purity 98.96%; UV (nm), λmax/log ε: 321.9/3.65 [41].
5,7-Dichloro-2-[2-(3,5-dihydroxyphenyl)vinyl]quinolin-8-ol (12). Yield 93% of a beige crystalline compound; Mp. 206 °C; HPLC purity 98.59%; UV (nm), λmax/log ε: 314.5/3.68 [42].
5,7-Dichloro-2-[2-(2-hydroxy-5-acetoxyphenyl)vinyl]quinolin-8-ol (13). Yield 93% of a beige crystalline compound; Mp. 205-206 °C; E.A. Calcd.: C 31.62%, H 1.59%, N 3.69%; found: C 33.00%, H 2.35%, N 3.72%; HPLC purity 98.58%; UV (nm), λmax/log ε: 345.1/3.63; 1H-NMR (CDCl3), δ: 2.34 (s, 3H, CH3), 7.22 (d, J = 16.4Hz, 1H, vinyl), 7.85 (d, J = 16.4 Hz, 1H, vinyl), 7.40 (s, 1H, quinaldine), 6.61 (d, J = 8.7 Hz, 1H, quinaldine), 8.15 (d, J = 8.7 Hz, 1H, quinaldine), 7.54 (d, J = 8.8 Hz, 1H, phenyl), 7.31 (d, J = 2.7 Hz, 1H, phenyl), 6.81 (dd, J = 2.7 Hz, J = 8.7 Hz, 1H, phenyl); 13C-NMR (CDCl3), δ: 25.58, 115.27, 117.31, 119.97, 120.64, 121.21, 122.78, 123.24, 124.79, 127.43, 128.00, 129.01, 130.43, 133.97, 138.81, 147.84, 144.99, 152.21, 155.71, 177.45.
5,7-Dichloro-2-(2-{4-[2-(5,7-dichloro-8-hydroxyquinolin-2-yl)vinyl]phenyl}vinyl)quinolin-8-ol (14). Yield 69% of a dark brown crystalline compound; Mp. 300 °C; HPLC purity 98.68%; UV (nm), λmax/log ε: 309.9/3.67 [42].
4.3. Lipophilicity determination by HPLC (capacity factor k/calculated log k)
A Waters Alliance 2695 XE HPLC separation module and Waters Photodiode Array Detector 2996 (Waters Corp., Milford, MA, USA) were used. A Symmetry® C18 5 μm, 4.6 × 250 mm, Part No. WAT054275, (Waters Corp., Milford, MA, USA) chromatographic column was used. The HPLC separation process was monitored by Millennium32® Chromatography Manager Software, Waters 2004 (Waters Corp., Milford, MA, USA). A mixture of MeOH p.a. (55.0%) and H2O-HPLC– Mili-Q Grade (45.0%) was used as a mobile phase. The total flow of the column was 0.9 mL/min, injection 30 μL, column temperature 30 °C and sample temperature 10 °C. The detection wavelength 210 nm was chosen. The KI methanolic solution was used for the dead time (tD) determination. Retention times (tR) were measured in minutes.
The capacity factors k were calculated using the Millennium32® Chromatography Manager Software according to formula k = (tR - tD)/tD, where tR is the retention time of the solute, whereas tD denotes the dead time obtained via an unretained analyte. Log k, calculated from the capacity factor k, is used as the lipophilicity index converted to log P scale. The log k values of the individual compounds are shown in Table 1.
4.4. Lipophilicity calculations
Log P, i.e. the logarithm of the partition coefficient for n-octanol/water, was calculated using the programs CS ChemOffice Ultra ver. 10.0 (CambridgeSoft, Cambridge, MA, USA) and ACD/LogP ver. 1.0 (Advanced Chemistry Development Inc., Toronto, Canada). Clog P values (the logarithm of n-octanol/water partition coefficient based on established chemical interactions) were generated by means of CS ChemOffice Ultra ver. 10.0 (CambridgeSoft, Cambridge, MA, USA) software. The results are shown in Table 1.
4.5. In vitro antifungal susceptibility testing
The broth microdilution test [43,44,45] was used for the assessment of in vitro antifungal activity of the synthesized compounds against Candida albicans ATCC 44859 (CA), Candida tropicalis 156 (CT), Candida krusei ATCC 6258 (CK), Candida glabrata 20/I (CG), Trichosporon beigelii 1188 (TB), Aspergillus fumigatus 231 (AF), Absidia corymbifera 272 (AC), and Trichophyton mentagrophytes 445 (TM). Fluconazole (FLU) was used as the standard of a clinically used antimycotic drug. The procedure was performed with twofold dilution of the compounds in RPMI 1640 (Sevapharma a.s., Prague, Czech Republic) buffered to pH 7.0 with 0.165 mol of 3-morpholino-propane-1-sulfonic acid (MOPS, Sigma, Germany). The final concentrations of the compounds ranged from 500 to 0.975 μmol/L. Drug–free controls were included. The MIC determination was performed according to the CLSI (formerly NCCLS) reference protocol M27-A2 for yeasts (IC80 value) and M38-A for moulds (IC50 value). IC80 and IC50 were defined as an 80% resp. 50% or greater reduction of growth in comparison with the control. The values of MICs were determined after 24 and 48 h of static incubation at 35 °C. For T. mentagrophytes, the final MICs were determined after 72 and 120 h of incubation. The results are summarized in Table 2.
4.6. Study of inhibition photosynthetic electron transport (PET) in spinach chloroplasts
Chloroplasts were prepared from spinach (Spinacia oleracea L.) according to Masarovicova and Kralova [46]. The inhibition of photosynthetic electron transport (PET) in spinach chloroplasts was determined spectrophotometrically (Genesys 6, Thermo Scientific, USA) using an artificial electron acceptor 2,6-dichlorophenol-indophenol (DCIPP) according to Kralova et al. [47] and the rate of photosynthetic electron transport was monitored as a photoreduction of DCPIP. The measurements were carried out in phosphate buffer (0.02 mol/L, pH 7.2) containing sucrose (0.4 mol/L), MgCl2 (0.005 mol/L) and NaCl (0.015 mol/L). The chlorophyll content was 30 mg/L in these experiments and the samples were irradiated (~100 W/m2) from 10 cm distance with a halogen lamp (250 W) using a 4 cm water filter to prevent warming of the samples (suspension temperature 22 °C). The studied compounds were dissolved in DMSO due to their limited water solubility. The applied DMSO concentration (up to 4%) did not affect the photochemical activity in spinach chloroplasts. The inhibitory efficiency of the studied compounds was expressed by IC50 values, i.e., by molar concentration of the compounds causing 50% decrease in the oxygen evolution rate relative to the untreated control. The comparable IC50 value for a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (Diurone®) was about 1.9 μmol/L [48]. The results are summarized in Table 2.
4.7. In vitro antimycobacterial evaluation
Clinical isolates of Mycobacterium avium complex CIT19/06, M. kansasii CIT11/06, M. absessus CIT21/06 and strain M. smegmatis MC2155 were grown in Middlebrook broth (MB), supplemented with OADC supplement (Oleic, Albumin, Dextrose, Catalase, Becton Dickinson, U.K.). Identification of these isolates was performed using biochemical and molecular protocols. At log phase growth, the 10 mL culture was centrifuged at 15,000 RPM for 20 minutes using a bench top centrifuge (Model CR 4-12 Jouan Inc U.K). Following the removal of the supernatant, the pellet was washed in fresh Middlebrook 7H9GC broth and re-suspended in 10 ml of fresh supplemented MB. The turbidity was adjusted to match McFarland standard No. 1 (3 × 108 cfu) with MB broth. A further 1:20 dilution of the culture was then performed in MB broth.
The antimicrobial susceptibility of all four mycobacteria was investigated in 96 well plate format. Here, 300 µL of sterile deionised water was added to all outer-perimeter wells of the plates to minimize evaporation of the medium in the test wells during incubation. 100 µL of each dilution was incubated with 100 µL of each of the mycobacterial species. Dilutions of each compound were prepared in duplicate. For all synthesized compounds, final concentrations ranged from 300 µg/mL to 10 µg/mL. All compounds were prepared in DMSO and subsequent dilutions were made in supplemented Middlebrook broth. The plates were sealed with parafilm and were incubated at 37 °C overnight in the case of M. smegmatis and M. absessus and for 5 days in the case of M. kansasii and M. avium complex. Following incubation, a 10% addition of alamarBlue (AbD Serotec) was mixed into each well and readings at 570 nm and 600 nm were taken, initially for background subtraction and subsequently after 24 hour re-incubation. The background subtraction is necessary with strongly coloured compounds which may interfere with the interpretation of any colour change. In non-interfering compounds, a blue colour in the well was interpreted as an absence of growth, and a pink colour was scored as growth. The MIC was initially defined as the lowest concentration which prevented a visual colour change from blue to pink. The results are shown in Table 3.
The MIC for mycobacteria was defined as a 90% or greater (IC90) reduction of growth in comparison with the control. The MIC/IC90 value is routinely and widely used in bacterial assays and is a standard detection limit according to the Clinical and Laboratory Standards Institute (CLSI, www.clsi.org/).
Acknowledgements
This study was supported by the Polish Ministry of Science N405 178735, by the Ministry of Education of the Czech Republic MSM 6215712403 and by the Irish Department of Education and Science TSR Strand1-06/CR08 and by Sanofi-Aventis Pharma Slovakia.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Roth H.J., Fenner H. Arzneistoffe. 3rd. Deutscher Apotheker Verlag; Stuttgart, Germany: 2000. pp. 51–114. [Google Scholar]
- 2.Harris C.R., Thorarensen A. Advances in the discovery of novel antibacterial agents during the year 2002. Curr. Med. Chem. 2004;11:2213–2243. doi: 10.2174/0929867043364658. [DOI] [PubMed] [Google Scholar]
- 3.Andries K., Verhasselt P., Guillemont J., Gohlmann H.W., Neefs J.M., Winkler H., van Gestel J., Timmerman P., Zhu M., Lee E., Williams P., de Chaffoy D., Huitric E., Hoffner S., Cambau E., Truffot-Pernot C., Lounis N., Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 4.Vangapandu S., Jain M., Jain R., Kaur S., Singh P.P. Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorg. Med. Chem. 2004;12:2501–2508. doi: 10.1016/j.bmc.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Carta A., Piras S., Palomba M., Jabes D., Molicotti P., Zanetti S. Anti-mycobacterial activity of quinolones. Triazoloquinolones a new class of potent anti-mycobacterial agents. Anti-Infective Agents Med. Chem. 2008;7:134–147. doi: 10.2174/187152108783954641. [DOI] [Google Scholar]
- 6.Sissi C., Palumbo M. The quinolone family: From antibacterial to anticancer agents. Curr. Med. Chem. Anti-Canc. Agents. 2003;3:439–450. doi: 10.2174/1568011033482279. [DOI] [PubMed] [Google Scholar]
- 7.Bossu E., Agliano A.M., Desideri N., Sestili I., Porra R., Grandilone M., Quaglia M.G. LTB4 as marker of 5-LO inhibitory activity of two new N-ethoxycarbonyl-4-quinolones. J. Pharm. Biomed. Anal. 1999;19:539–549. doi: 10.1016/S0731-7085(98)00250-7. [DOI] [PubMed] [Google Scholar]
- 8.Ko T.C., Hour M.J., Lien J.C., Teng C.M., Lee K.H., Kuo S.C., Huang L.J. Synthesis of 4-alkoxy-2-phenylquinoline derivatives as potent antiplatelet agents. Bioorg. Med. Chem. Lett. 2001;11:279–282. doi: 10.1016/S0960-894X(00)00652-1. [DOI] [PubMed] [Google Scholar]
- 9.Jampilek J., Dolezal M., Kunes J., Vichova P., Jun D., Raich I., O´Connor R., Clynes M. Synthesis of (2E)-2-methyl-3-(4-{[4-(quinolin-2-ylmethoxy)phenyl]sulfanyl}phenyl)prop-2-enoic acid (VUFB 20609) and 2-methyl-3-(4-{[4-(quinolin-2-ylmethoxy)phenyl]sulfanyl}phenyl)propionic acid (VUFB 20584) as potential antileukotrienic agents. J. Pharm. Pharmacol. 2004;56:783–794. doi: 10.1211/0022357023484. [DOI] [PubMed] [Google Scholar]
- 10.Jampilek J., Dolezal M., Kunes J., Vichova P., Jun D., Raich I., O´Connor R., Clynes M. Preparation of 2-(4-{[4-(quinolin-2-ylmethoxy)phenyl]sulfanyl}phenyl)propionic acid (VUFB 20615) and 2-methyl-2-(4-{[4-(quinolin-2-ylmethoxy)phenyl]sulfanyl}phenyl)propionic acid (VUFB 20623) as potential antileukotrienic agents. Curr. Org. Chem. 2004;8:1235–1243. doi: 10.2174/1385272043370041. [DOI] [PubMed] [Google Scholar]
- 11.Jampilek J., Dolezal M., Opletalova V., Hart J. 5-Lipoxygenase, leukotrienes biosynthesis and potential antileukotrienic agents. Curr. Med. Chem. 2006;13:117–126. doi: 10.2174/092986706775197935. [DOI] [PubMed] [Google Scholar]
- 12.Polanski J., Zouhiri F., Jeanson L., Desmaele D., d’Angelo J., Mouscadet J.F., Gieleciak R., Gasteiger J., Le Bret M. Use of the Kohonen neural network for rapid screening of ex vivo anti-HIV activity of styrylquinolines. J. Med. Chem. 2002;45:4647–4654. doi: 10.1021/jm020845g. [DOI] [PubMed] [Google Scholar]
- 13.Polanski J., Niedbala H., Musiol R., Tabak D., Podeszwa B., Gieleciak R., Bak A., Palka A., Magdziarz T. Analogues of styrylquinoline and styrylquinazoline HIV-1 integrase inhibitors: Design and synthetic problems. ActaPoloniae Pharm. Drug Res. 2004;61:3–4. [PubMed] [Google Scholar]
- 14.Polanski J., Niedbala H., Musiol R., Podeszwa B., Tabak D., Palka A., Mencel A., Finster J., Mouscadet J.F., Le Bret M. 5-Hydroxy-8-nitro-6-quinaldic acid as a novel molecular scaffold for HIV-1 integrase inhibitors. Lett. Drugs Des. Disc. 2006;3:175–178. doi: 10.2174/157018006776286934. [DOI] [Google Scholar]
- 15.Polanski J., Niedbala H., Musiol R., Podeszwa B., Tabak D., Palka A., Mencel A., Mouscadet J.F., Le Bret M. Fragment based approach for the investigation of HIV-1 integrase inhibition. Lett. Drugs Des. Disc. 2007;4:99–105. [Google Scholar]
- 16.Vargas L.Y., Castelli M.V., Kouznetsov V.V., Urbina J.M., Lopez S.N., Sortino M., Enriz R.D., Ribas J.C., Zacchino S. In vitro antifungal activity of new series of homoallylamines and related compounds with inhibitory properties of the synthesis of fungal cell wall polymers. Bioorg. Med. Chem. 2003;11:1531–1550. doi: 10.1016/s0968-0896(02)00605-3. [DOI] [PubMed] [Google Scholar]
- 17.Jampilek J., Dolezal M., Kunes J., Buchta V., Kralova K. Quinaldine Derivatives: Preparation and Biological Activity. Med. Chem. 2005;1:591–599. doi: 10.2174/157340605774598108. [DOI] [PubMed] [Google Scholar]
- 18.Musiol R., Jampilek J., Buchta V., Niedbala H., Podeszwa B., Palka A., Majerz-Maniecka K., Oleksyn B., Polanski J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006;14:3592–3598. doi: 10.1016/j.bmc.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Musiol R., Jampilek J., Kralova K., Richardson D.R., Kalinowski D., Podeszwa B., Finster J., Niedbala H., Palka A., Polanski J. Investigating biological activity spectrum fornovel quinoline analogues. Bioorg. Med. Chem. 2007;15:1280–1288. doi: 10.1016/j.bmc.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Musiol R., Tabak D., Niedbala H., Podeszwa B., Jampilek J., Kralova K., Dohnal J., Finster J., Mencel A., Polanski J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis inhibiting activity. Bioorg. Med. Chem. 2008;16:4490–4499. doi: 10.1016/j.bmc.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 21.Jampilek J., Musiol R., Pesko M., Kralova K., Vejsova M., Carroll J., Coffey A., Finster J., Tabak D., Niedbala H., Kozik V., Polanski J., Csollei J., Dohnal J. Ring-substituted 4-hydroxy-1H-quinolin-2-ones: Preparation and biological activity. Molecules. 2009;14:1145–1159. doi: 10.3390/molecules14031145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jampilek J., Musiol R., Finster J., Pesko M., Carroll J., Kralova K., Vejsova M., O'Mahony J., Coffey A., Dohnal J., Polanski J. Investigating biological activity spectrum for novel styrylquinazoline analogues. Molecules. 2009;14:4246–4265. doi: 10.3390/molecules14104246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podeszwa B., Niedbala H., Polanski J., Musiol R., Tabak D., Finster J., Serafin K., Wietrzyk J., Boryczka S., Mol W., Jampilek J., Dohnal J., Kalinowski D., Richardson D.R. Investigating the antiproliferative activity of quinoline-5,8-dione analogues on tumour cell lines. Bioorg.Med. Chem. Lett. 2007;17:6138–6141. doi: 10.1016/j.bmcl.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Global Tuberculosis Control – Surveillance, Planning, Financing. WHO Report 2008. [accessed on 28 October 2009]. Available online: http://www.who.int/tb/publications/global_report/2008/summary/en/index.html/
- 25.Field S.K., Cowie R.L. Lung disease due to the more common nontuberculous mycobacteria. Chest. 2006;129:1653–1672. doi: 10.1378/chest.129.6.1653. [DOI] [PubMed] [Google Scholar]
- 26.Wagner D., Young L.S. Nontuberculous mycobacterial infections: A clinical review. Infection. 2004;32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 27.Morrone N., Cruvinel M.C., Morrone N., Jr., Freire J.A., Oliveira L.M., Goncalves C. Pneumopatia causada por Mycobacterium kansasii. J. Pneumol. 2003;29:341–349. doi: 10.1590/S0102-35862003000600005. [DOI] [Google Scholar]
- 28.Doctor Fungus. [accessed on 28 October 2009]. Available online: http://www.doctorfungus.org/
- 29.Gershon H., Gershon M., Clarke D.D. Synergistic mixtures of fungitoxicmonochloro- and dichloro-8-quinolinols against five fungi. Mycopathologia. 2004;158:131–135. doi: 10.1023/b:myco.0000038427.42852.6a. [DOI] [PubMed] [Google Scholar]
- 30.Dardari Z., Lemrani M., Bahloul A., Sebban A., Hassar M., Kitane S., Berrada M., Boudouma M. Antileishmanial activity of a new 8-hydroxyquinoline derivative designed 7-[5′-(3′-phenylisoxazolino)methyl]-8-hydroxyquinoline: Preliminary study. Farmaco. 2004;59:195–199. doi: 10.1016/j.farmac.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Draber W., Tietjen K., Kluth J.F., Trebst A. Herbicides in photosynthesis research. Angew. Chem. Int. Ed. Engl. 1991;3:1621–1633. [Google Scholar]
- 32.Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim. Biophys. Acta. 1977;460:113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- 33.Trebst A., Draber W. Structure activity correlations of recent hrbicides in photosynthetic reactions. In: Greissbuehler H., editor. Advances in Pesticide Science. Pergamon Press; Oxford, UK: 1979. pp. 223–234. [Google Scholar]
- 34.Bowyer J.R., Camilleri P., Vermaas W.F.J. In: Herbicides, Topics in Photosynthesis. Baker N.R., Percival M.P., editors. Vol. 10. Elsevier; Amsterdam, The Netherlands: 1991. pp. 27–85. [Google Scholar]
- 35.Kralova K., Sersen F., Kubicova L., Waisser K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3-and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Techn. 2000;18:251–256. [Google Scholar]
- 36.Kralova K., Sersen F., Miletín M., Dolezal M. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 2002;56:214–217. [Google Scholar]
- 37.Dolezal M., Palek L., Vinsova J., Buchta V., Jampilek J., Kralova K. Substituted pyrazinecarboxamides: Synthesis and biological evaluation. Molecules. 2006;11:242–256. doi: 10.3390/11040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerns E.H., Li D. Drug-Like Properties: Concept, Structure Design and Methods. Elsevier; San Diego, CA, USA: 2008. [Google Scholar]
- 39.Honda I., Yoneyama K., Iwamura H., Konnai M., Takahashi N., Yoshida S. Structure-activity relationship of 3-nitro-2,4,6-tri-hydroxybenzamide derivatives in photosynthetic electron transport inhibition. Agric. Biol. Chem. 1990;54:1227–1233. [Google Scholar]
- 40.Messinger P., Meyer H. The reaction of iodohydroxyquinolinesulfonic acid with nitrite. Arch. Pharm. 1976;309:1009–1010. doi: 10.1002/ardp.19763091212. [DOI] [PubMed] [Google Scholar]
- 41.Ponikiewski L., Nycz J.E. 3-[(E)-2-(5,7-Dichloro-8-hydroxyquinolin-2-yl)vinyl]-4-hydroxyphenyl acetate. ActaCryst. E. 2009;65:515. [Google Scholar]
- 42.Nycz J.E., Ponikiewski L. Unpublished data, 2009. Detailed analysis of the structure of these compounds will be published elsewhere.
- 43.Sheehan D.J., Espinel-Ingroff A., Steele M., Webb C.D. Antifungal susceptibility testing of yeasts: A brief overview. Clin. Infect. Dis. 1993;17:494–500. doi: 10.1093/clinids/17.Supplement_2.S494. [DOI] [PubMed] [Google Scholar]
- 44.Reference Method for Broth Dilution Testing of Yeasts: Approved StandardM27-A2. 2nd. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2002. National Committee for Clinical Laboratory Standards. [Google Scholar]
- 45.Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2004. National Committee for Clinical Laboratory Standards. [Google Scholar]
- 46.Masarovicova E., Kralova K. Approaches to measuring plant photosynthesis activity. In: Pessarakli M., editor. Handbook of Photosynthesis. 2nd. Taylor & Francis Group; Boca Raton, London/New York/Singapore: 2005. pp. 617–656. [Google Scholar]
- 47.Kralova K., Sersen F., Sidoova E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992;46:348–350. [Google Scholar]
- 48.Fedke C. Biochemistry and Physiology of Herbicide Action. Springer Verlag; Berlin, Germany: 1982. [Google Scholar]