Abstract
Cannabidiol (CBD) has gained much attention in the past several years for its therapeutic potential in the treatment of drug-resistant epilepsy, such as Dravet syndrome. Although CBD has shown anecdotal efficacy in reducing seizure frequency, little is known regarding the potential adverse side effects of CBD on physiology, development, organogenesis, or behavior. The goal of this project was to compare the relative morphological, behavioral, and gene expression phenotypes resulting after a developmental exposure to Δ9-tetrahydrocannabinol (THC) or CBD. Zebrafish were exposed from blastula through larval stage (96 h postfertilization [hpf]) to 0.3, 0.6, 1.25, 2.5, 5 mg/l (1, 2, 4, 8, 16 µM) THC or 0.07, 0.1, 0.3, 0.6, 1.25 mg/l CBD (0.25, 0.5, 1, 2, 4 µM). Despite the similarity in THC and CBD dysmorphologies, ie, edemas, curved axis, eye/snout/jaw/trunk/fin deformities, swim bladder distention, and behavioral abnormalities, the LC50 for CBD (0.53 mg/l) was nearly 7 times lower than THC (3.65 mg/l). At 96 hpf, c-fos, dazl, and vasa were differentially expressed following THC exposure, but only c-fos expression was significantly increased by CBD. Cannabidiol was more bioconcentrated compared with THC despite higher THC water concentrations. This work supports the potential for persistent developmental impacts of cannabinoid exposure, but more studies are needed to assess latent effects and their molecular mechanisms of toxicity.
Keywords: cannabis, cannabidiol, Δ9-tetrahydrocannabinol, zebrafish, development, toxicity
Cannabidiol (CBD) has gained much attention in the past several years for its therapeutic potential in the treatment of many ailments, including drug-resistant epilepsy, such as Dravet syndrome (Devinsky et al., 2017). Additionally, CBD legalization is increasing in the United States due to potential therapeutic indications ranging from depression, schizophrenia, chronic pain, and even cancer (reviewed by McKenna, 2014). States with legalized medical cannabis, such as Washington, currently offer over 800 CBD products available for purchase, and CBD sales in the United States are projected to reach $2.1 billion by 2020 (Murphy and Ooyen, 2016). Numerous studies suggest CBD contains therapeutic potential (reviewed by Cridge and Rosengren, 2013; Devinsky et al., 2014, 2017); however, the toxicology community has yet to scrutinize its possible adverse effects. Most importantly, CBD is currently being administered to toddler through adult-aged patients of Dravet syndrome, with little empirical evidence of its potential developmental or reproductive consequences. Moreover, CBD is perceived to possess a safer toxicological profile than Δ9-tetrahydrocannabinol (THC) due to its weak affinity for cannabinoid receptor 1 (CB1); therefore, lacking a psychotropic effect. This study aims to define the relative adverse developmental effects of CBD compared with the more well-known cannabis constituent, THC, which does cause reported developmental, teratogenic, and behavioral abnormalities (Akhtar et al., 2013; Becker et al., 2010; Brunet et al., 2006; Crane et al., 2013; Deiana et al., 2012; Fontes et al., 2011; Geber and Schramm, 1969; Hisaoka, 1958; Hurd et al., 2005; Maccarrone and Finazzi-Agro, 2004; Paria et al., 1995; Ruhl et al., 2014; Stewart and Kalueff, 2014; Thomas, 1975; Wright et al., 1976).
Distribution of cannabinoid receptors differ; in vertebrates CB1 is currently thought to primarily concentrate in the central and peripheral nervous systems, whereas CB2 is primarily expressed in the immune system (Lam et al., 2006; Pertwee, 2006). Cannabinoid receptor 2 functions are less known, but include immune regulation and potential adversities related to neurodegeneration (Szaflarski and Martina Bebin, 2014). The endocannabinoid system among mammals and zebrafish is highly conserved (Krug and Clark, 2015). Whole-larval homogenate has shown CB1 mRNA expression present during the 3 somite stage through the 25 somite stage (Migliarini and Carnevali, 2009), whereas whole-mount in situ hybridization localized CB1 mRNA throughout the zebrafish brain with highest expression in the telencephalon at 96 h postfertilization (hpf) (Lam et al., 2006). In addition, CB1 protein has been observed in both larval zebrafish brain homogenates beginning around 48 hpf through 15 days postfertilization (Migliarini and Carnevali, 2009). Cannabinoid receptor 2 has also been detected throughout the adult zebrafish gills, heart, retina, intestine, spleen, brain, and pituitary, but less is known regarding CB2’s expression patterns throughout development and adolescence (Rodriguez-Martin et al., 2007).
Dating back to the 1960s, animal models have been used to elucidate cannabinoid toxicity (Persaud and Ellington, 1968). Primary literature has focused on prenatal and postnatal exposure in rodents via oral or subcutaneous injection measuring various endpoints from behavior and endocrine function to neuropathology (Scallet, 1991). Adverse outcomes in rodents of developmental exposure include craniofacial (Bloch et al., 1986) and behavior abnormalities (Onaivi et al., 1995; Wright et al., 1976) similar to those reported in zebrafish (Akhtar et al., 2013; Thomas, 1975), but very few publications offer insight into CBD toxicity. Recent literature has primarily focused on the protective and therapeutic potential of CBD; however, some results suggest that CBD exposure during neuronal development might lead to sensitization to neurotoxicants (Schönhofen et al., 2015). Rats and adult zebrafish were used to demonstrate CBD’s behavior abnormalities (Nazario et al., 2015; Resstel et al., 2009), but little information has been offered with regard to developmental adversities, potential onset of adult disorders, or early neurogenesis and maturation, which may result from a teratogenic or pediatric exposure.
The goal of this project was to use the highly relevant zebrafish model to study the potential morphological and behavioral toxicities of CBD compared with a known developmental toxicant, THC (Akhtar et al., 2013; Thomas, 1975). Developmental biomarkers were also screened to elucidate potential mechanistic roles. Additionally, we provide evidence that via water-based exposures, lipophilic cannabinoids (THC log P: 6.97; CBD log P: 5.79) bioaccumulated in fish embryos (Thomas et al., 1990) and caused developmental toxicity.
MATERIALS AND METHODS
Zebrafish care and exposure.
Tg(fli1:EGFP) zebrafish were purchased from Zebrafish International Resource Center (ZFIN, Eugene, Oregon). Healthy adult zebrafish were placed in aerated breeding units containing water from an Aquatic Habitats Zebrafish Flow-through System (Aquatic Habitats, Apopka, Florida), pH 7.5–8.0, dissolved oxygen 7.2–7.8 mg/l, conductivity 730–770 µS, and temperature 27°C–29 °C. The next morning, eggs were collected, debris removed, and randomly sorted into scintillation vials (n = 3 vials; 10 embryos per vial) containing embryo water (sterilized deionized water; pH of 7.4–7.7; 60 ppm Instant Ocean, Cincinnati, Ohio). Scintillation vials were then placed into a stand-up incubator at 27°C–29 °C. Exposed eggs were screened every 24 h to assess overall health and to remove any dead embryos. All culture and exposure protocols were in accordance with approved Institutional Animal Care and Use Committee (IACUC) guidelines and recommendations. At approximately 2 hpf, original transfer water was removed and replaced with 0.3125, 0.625, 1.25, 2.5, 5 mg/l (1, 2, 4, 8, 16 µM) THC (0.05% DMSO), 0.075, 0.15, 0.3, 0.6, 1.2 mg/l (0.25, 0.5, 1, 2, 4 µM) CBD (0.05% dimethylsulfoxide; DMSO), or 0.05% DMSO control water. Embryos were exposed in scintillation vials at a 0.6:1 (mL water:fish) ratio in static conditions without a water change during the exposure period. Every 24 h, mortalities, debris, and sloughed chorions were removed from vials. Δ9-Tetrahydrocannabinol and CBD were provided by the NIDA Drug Supply Program (Research Triangle Park, NC).
Morphological and behavior screening.
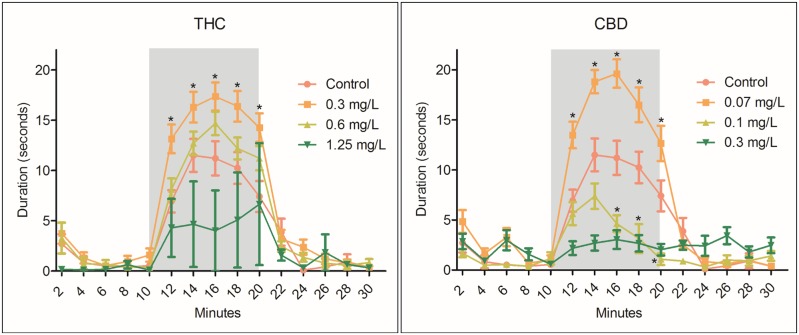
At 96 hpf, larvae were transferred from scintillation vials into 96-well plates (one larva per well). Touch response, yolk sac and pericardial edema, axis curvature, eye/snout/jaw/trunk deformities, swim bladder inflation, and pectoral fin dysmorphologies were all qualitatively analyzed single-blinded as either deformed (yes) or not deformed (no). LC50 values were calculated using the Environmental Protection Agency’s (EPA) LC50 calculation program at the conclusion of the 96 hpf assessment (Hamilton et al., 1977). Lowest observed adverse effect levels (LOAELs) were calculated as a 20% dysmorphologic occurrence greater than controls (eg, if curved axis occurrence in control fish was 5%, the LOAEL concentration = ≥ 25% curved axis occurrence). Following morphological screening, zebrafish with a touch response were directly transferred to an isolated behavioral screening room kept in full light at 27°C–29 °C and allowed to acclimate for 5 min. Touch response was assessed by touching the larvae’s tail with the end of a 10 µl pipette tip. Larvae either reacted to the mechanical impact by avoidance swimming or did not respond. Zebrafish were then monitored using a ViewPoint ZebraBox (ViewPoint, Montreal, Canada) for 30 min (0–10 min, 100% light [8000 lux]; 10–20 min, dark, 0% light [0 lux]; and 20–30 min, 100% light) (Kirla et al., 2016). Travel duration at a velocity ≥5 mm/s was collected per well for each dosing parameter. Duration mean for each pooled vial was calculated and statistical significance was calculated per vial (n = 3) using two-way analysis of variance with repeated measure followed by Bonferroni post hoc test (p ≤ .05) (n = 3 replicates; 7–10 larvae per replicate for morphology and behavioral screens). Δ9-Tetrahydrocannabinol (1.25 mg/l) and CBD (0.3 mg/l) were excluded from statistical analysis due to a lack of fish (n < 3) healthy enough to perform in the behavioral analysis; however, they are still depicted in Figure 2.
Figure 2.
Zebrafish larvae at 96 hpf were monitored using a ViewPoint ZebraBox for 30 min (0–10 min, light; 10–20 min, dark [grey box]; 20–30 min, light). Duration of movement (seconds) at a velocity ≥ 5 mm/s was collected at 2 min intervals and compared with control. Behavioral assessment was specific only to larvae displaying a touch response and uninhibited phenotypes; therefore, the full range of concentrations-response is not available. Larvae exposed to 0.3 mg/l THC exhibited significant (*) hyperlocomoter activity compared with control in dark periods (12–20 min); however, 1.25 mg/l THC reduced overall duration of movement at 16 min. Similarly, CBD significantly stimulated locomotor activity at 0.07 mg/l, but larvae exposed to 0.1 and 0.3 mg/l CBD exhibited hypolocomoter characteristics. Data were analyzed on the average distance per vial (n = 3) using two-way analysis of variance with repeated measure followed by Bonferroni post hoc test ± SEM (p ≥ .05) (n = 3 vials; 7–10 larvae per vial).
RT-qPCR.
Following the behavioral screen, larval zebrafish were pooled (n = 3 vials; 10 fish per vial) in RNAlater and stored at −80 °C until RNA isolation and processing. Whole-larval RNA was isolated utilizing TRIzol (Invitrogen #A33251; Waltham, Massachusetts), RNase-Free DNase set (Qiagen #79254; Valencia, California), and RNeasy mini kit (Qiagen #74004) according to manufacturer’s protocol. Extracted RNA was then quantified and assessed for acceptable 260:280 ratio on a NanoDrop 2000 (ThermoFisher Scientific, Waltham, Massachusetts) followed by cDNA (10 µg/µl) substock preparation (Invitrogen #4304134). RT-qPCR was performed on an Applied Biosystems 7200 using SYBR Green chemistry (Applied Biosystems #4309155; Waltham, Massachusetts) with the following parameters: 95 °C for 10 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s dissociation curve. Primer optimization and confirmation was performed as previously described by Fang et al. (2013). Final concentrations in the reaction mix were forward and reverse primer 0.2 µM, template cDNA 0.4 ng/µl, and SYBR Green PCR Master Mix according to manufacturer’s protocol (Applied Biosystems). All samples (n = 3) were screened in duplicate and evaluated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). RT-qPCR results were analyzed using 3 pooled (10 fish) homogenate biological replicates (n = 3). For statistical significance, we used one-way analysis of variance followed by Tukey’s post hoc test with statistical significance being met at p ≤ .05. Additional RT-qPCR information available in Supplementary Table 1.
Gas chromatography-mass spectrometry analysis.
To confirm exposure concentrations and better understand the exposure kinetics of THC and CBD, water and tissue concentrations were verified at 0 hpf (Ti) and 96 hpf (Tf), as previously described (Kudo et al., 1995). Briefly, deuterated THC-d3 (Sigma Aldrich, Saint Louis, Missouri) was added to 2 ml (10 mg/l) of exposure water along with 1 ml of 2 M NaOH followed by a solvent: water extraction using hexane:ethyl acetate (9:1, vol/vol) repeated 2 times. Once in solvent, samples were evaporated to dryness using nitrogen in a water bath at 55 °C. Samples were then reconstituted and derivatized in N, O-bis(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane (ThermoScientific) at 90 °C for 1 h. Following derivatization, samples were evaporated to dryness and reconstituted in 50 µl isooctane and transferred directly to the gas chromatography-mass spectrometry (Agilent Technologies 6890N; Mass Spectrometer 5973) with DB-5MS column (Agilent Technologies, Santa Clare, California) for analysis as previously described (Kemp et al., 1995). Retention times and ions [quantitative; qualitative] for quantifying THC-d3, THC, and CBD were as follows: 8.142 min [374; 389 m/z], 8.167 min [386; 371 m/z], and 6.936 min [390; 458 m/z].
For tissue extractions, dry larvae were weighed and then homogenized in hexane:ethyl acetate (9:1, vol/vol) (containing THC-d3) with 100 pulses of a Teflon pestle. Following homogenization, extractions were evaluated as described above for water. Measured water and tissue concentrations of THC or CBD were calculated using a relative response factor derived from the surrogate standard recovery (e.g. initially measured THC or CBD concentration/surrogate standard recovery). Bioconcentration factors were calculated by dividing the measured concentration of compound in tissue samples at 96 hpf by the measured concentration of compound in exposure water at 0 hpf, bioconcentration factor (BCF = Ctissue(Tf)/Cwater(Ti)). To measure extraction recoveries pooled whole zebrafish larvae (n = 10 per replicate; 2–10 replicates) at 96 hpf and hexane:ethyl acetate (9:1, vol/vol) were spiked with 10 mg/l THC, CBD, or THC-d3 and then extracted as described above. Recoveries were calculated by comparing the area under the curve of ion peaks to a nonextracted standard curve 0.016–50 mg/l for each compound. Recoveries were as follows: THC 83.7 ± 11.6%; CBD 54.2 ± 0.14%; and THC-d3 65.5 ± 8.6%.
RESULTS
Water and Tissue Analysis
Actual THC concentrations in water at Ti (0 hpf) were between 64% and 88% of expected and declined to between 16% and 32% of Ti at 96 hpf (Tf) (Table 1). Actual CBD concentrations were only 33%–40% of nominal at Ti and decreased to either not detected or 3% of Ti at Tf (Table 1). Δ9-Tetrahydrocannabinol and CBD tissue concentrations were assessed at 96 hpf following homogenization and solvent extraction. Following 96 hpf exposure to 0.313 or 1.24 µg/ml THC, the calculated BCFs were approximately 1.4 and 0.65, respectively (Table 1). Following 96 hpf exposure to 0.075 or 0.3 CBD, the calculated BCFs were 39 and 790, respectively (Table 1). After 96 h of exposure to nominally 0.3 µg/ml THC or CBD, tissue concentrations in larvae were 0.28 and 79 µg/g, respectively.
Table 1.
Cannabinoid Water and Tissue Concentrations
| Compound | hpf | Nominal Water Concentration, µg/ml | Measured Water Concentration, µg/ml ± SD | % Nominal | Fish Massa, g ± SD | Measured Tissue Concentration, µg/g ± SD | BCFb |
|---|---|---|---|---|---|---|---|
| Δ9-Tetrahydrocannabinol | |||||||
| 0 | 0c | nd | — | ||||
| 0.313 | 0.2 ± 0.01 | 64 | |||||
| 1.25 | 1.1 ± 0.2 | 88 | |||||
| 96 | 0c | nd | — | 0.01 ± 0.0 | nd | — | |
| 0.313 | 0.05 ± 0.008 | 16 | 0.007 ± 0.001 | 0.28 ± 0.14 | 1.4 | ||
| 1.25 | 0.4 ± 0.03 | 32 | 0.001 ± 0.008 | 0.71 ± 0.72 | 0.65 | ||
| Cannabidiol | |||||||
| 0 | 0c | nd | — | ||||
| 0.075 | 0.03 ± 0.01 | 40 | |||||
| 0.3 | 0.1 ± 0.03 | 33 | |||||
| 96 | 0c | nd | — | 0.003 ± 0.003 | nd | — | |
| 0.075 | nd | — | 0.002 ± 0.0 | 1.2 ± 0.16 | 39 | ||
| 0.3 | 0.01 ± 0.004 | 3 | 0.003 ± 0.001 | 79 ± 51 | 790 | ||
nd = not detected.
3–7 larvae.
BCF = Ctissue(time 96)/Cwater(time 0).
Control (0.05% DMSO).
Lethality and Morphology
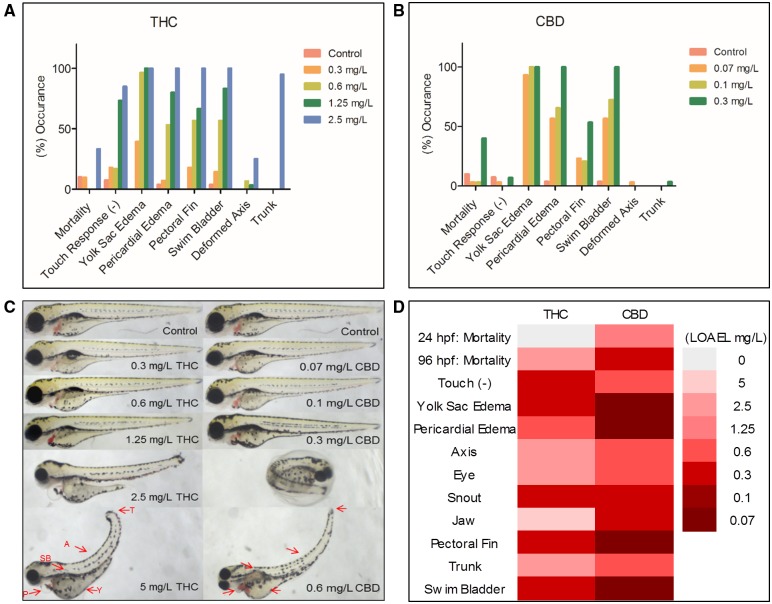
Following a waterborne exposure to CBD and THC, 96 hpf zebrafish displayed concentration-dependent morphological (Figure 1) and behavioral toxicities (Figure 2). The CBD LC50 of 0.53 mg/l was nearly 7 times lower than that of THC (3.65 mg/l). Cannabidiol and THC shared similar adverse morphologic outcomes, ie, yolk sac and pericardial edema, pectoral fins missing, and swim bladder distention; however, the LOAEL for these observed dysmorphologies varied (Figure 1D). For example, the LOAEL for pericardial edema following THC exposure was approximately 0.6 mg/l, whereas CBD was more than 10 times lower at 0.07 mg/l (Figure 1D). Similarly, LOAELs for jaw malformations (5 mg/l THC, 0.3 mg/l CBD), axis curvature (2.5 mg/l THC, 0.6 mg/l CBD), and trunk degradation (2.5 mg/l THC, 0.6 mg/l CBD) were all lower in CBD exposed larvae compared with THC.
Figure 1.
Following 96 h postfertilization (hpf) exposure to Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD) larvae were plated in 96-well plates (one per well) and qualitatively assessed for common developmental dysmorphologies. All morphology endpoints were designated either a yes (abnormal) or no (normal) and quantitatively calculated from the total larvae measured for both THC (A) and CBD (B). Higher concentrations with 100% occurrence in all endpoints are depicted. Subsequent phenotypical pictures were taken following the morphology screening (C), red arrows (see online version) indicate a few key morphologic endpoints (T = trunk, A = axis, SB = swim bladder, P = pericardial edema, Y = yolk sac edema). In addition to phenotypic malformations following 0.6 mg/l CBD exposure, we also observed numerous 96 hpf larvae that were unhatched. Heat-map portrayal (D) of the lowest observed adverse effect level (LOAEL) after 96 hpf exposure to THC and CBD (n = 3 replicates consisting of 10 fish per replicate).
Behavior
Behavior is assessed via movement during dark conditions compared with bright light conditions. Typically, zebrafish larvae exhibit hyperlocomoter behavior during dark periods and hypolocomoter behavior during bright conditions, especially during initial cycling periods (Kirla et al., 2016). Larvae exposed to 0.3 mg/l THC or 0.07 mg/l CBD exhibited a significantly increased duration of movement (seconds of duration ≥ 5 mm/s) during dark periods compared with control. In contrast, 1.25 mg/l THC and 0.1–0.3 mg/l CBD significantly reduced duration compared with control, which is indicative of hypolocomoter activity (Figure 2). All duration times were compared using two-way ANOVA with the Bonferroni post hoc test ± SEM. All larvae lacking a touch response or phenotypically not able to swim were excluded from behavioral assessment; therefore, high concentrations of compound tended to result in smaller sample sizes (n =3), which lead to increased standard errors. However, even with increased error, larvae exposed to 1.25 mg/l THC still exhibited significantly decreased duration during dark periods compared with control larvae (16 min; Figure 2).
RT-qPCR
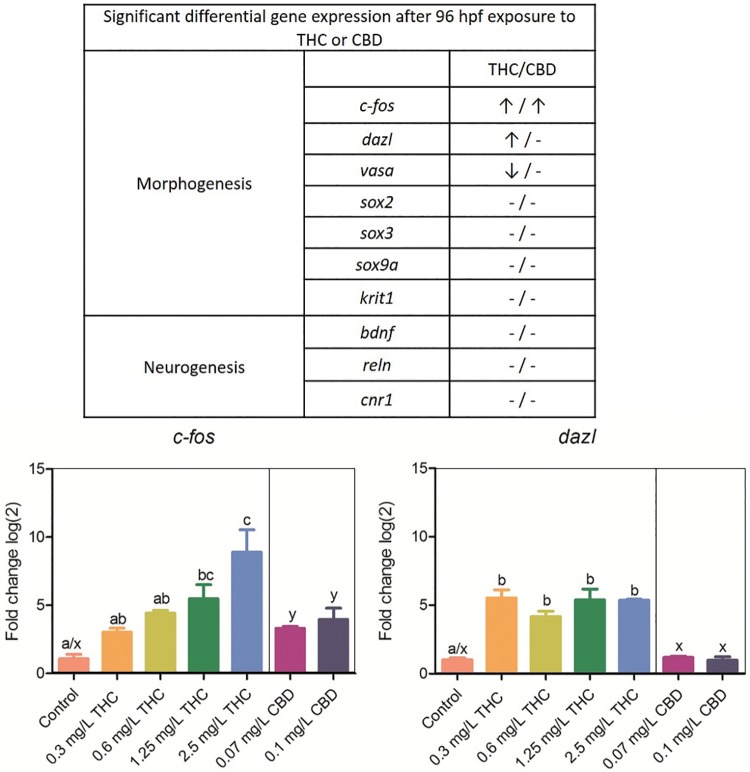
Following a 96 hpf exposure to THC or CBD, larval zebrafish were screened for potential differential expression of 10 key morphogenic or neurogenic genes. c-fos was differentially upregulated in a concentration-dependent manner following a developmental exposure to both THC (1.25 and 2.5 mg/l) and CBD (0.07 and 0.1 mg/l) (Figure 3). Δ9-Tetrahydrocannabinol (0.3, 0.6, 1.25, and 2.5 mg/l), but not CBD exposure, resulted in dazl upregulation (Figure 3). In addition, expression of vasa, sox2, sox3, sox9a, bdnf, reln, krit1, and cannabinoid-expressing gene cnr1 was measured; however, no statistically significant differential expression of these genes compared with control was detected.
Figure 3.
Differential expression via RT-qPCR of 10 genes, as compared with reference gene 18s, following a 96 hpf developmental exposure to THC, CBD, or DMSO. (↑/↓) represents significance (p < .05) using analysis of variance and Tukey’s post hoc test (n = 3 pooled replicates). c-fos was differentially expressed in a concentration-dependent manner in both THC and CBD exposed larvae, whereas dazl and vasa were only differentially expressed by THC. CBD is depicted in only 2 concentrations due to LC50 limitations. Bars with the same letter (THC a–c; CBD x, y) are not significantly different.
DISCUSSION
An important result of this study was that similar concentration-dependent morphological and locomotor behavioral outcomes following a 96 hpf developmental exposure to both CBD and THC were observed. Zebrafish larvae exposed to THC displayed very similar adverse outcomes to those previously published (Akhtar et al., 2013; Thomas, 1975). Toxicities included pericardial/yolk sac edemas and curved axis as well as increased locomotor activity at low concentrations of THC; however, implications for larval morphology and behavior following CBD exposure were previously unknown.
Cannabidiol exposure at blastula through larval stage increased developmental dysmorphologies, especially jaw malformation, at relatively lower LOAELs (0.3 mg/l) than THC (5 mg/l). Previously embryo abnormalities such as craniofacial deformities have been reported in rodents following gestational exposure to THC (Bloch et al., 1986; Harbison et al., 1977; reviewed by Rosenkrantz, 1999). A/J strain mice exposed to as little as 60 mg/kg THC between gestational days 11–14 experienced increased frequency of cleft palate (Bloch et al., 1986). These results were also found previously using Swiss-Webster mice at much higher doses of THC (300 mg/kg) (Harbison et al., 1977). Although numerous studies have been conducted on THC developmental abnormalities in rodents, doses, routes of administration, and species differences vary. For example, newborn albino rat fetuses experienced few abnormalities when parents were exposed prior to mating to low concentrations of THC (0.5–5 mg/kg) (Wright et al., 1976). Comparative studies have been conducted relating development genes during early morphologic stages in the mouse (embryonic day 12) and zebrafish (48 hpf) models (reviewed by Wullimann and Mueller, 2004). Additionally, genes such as sox9 have been implicated in craniofacial deformities in both zebrafish (Yan et al., 2005) and mice (Bi et al., 2001), but in our study differential gene expression of sox9 was not detected in 96 hpf THC or CBD-treated larvae.
An additional endpoint that significantly differed between CBD and THC was the relative LOAELs for pericardial edema. The THC LOAEL for pericardial edema (0.6 mg/l) was roughly 10 times higher than CBD (0.07 mg/l). Enlarged pericardium in larval zebrafish can be caused by a number of attributes, including deregulated cardiovascular development–oriented genes (krit1), heart failure, or morphologies related to improperly placed heart chambers (Chen et al., 1996). In mice, research has shown krit1 mutations were present in cerebral cavernous malformations, which encompassed vascular lesion irregularities, although no connection to cardiovascular morphology was present in current literature (Wüstehube et al., 2010). However, we did not observe differentially expressed krit1 in 96 hpf whole-larval homogenates. This result could be attributed to time specific–differential expression that should precede cardiovascular adverse outcomes; future work should assess gene expression at multiple developmental time-points.
Molecular reporters for morphogenesis or neurogenesis aberration were a point of focus due to the observed organogenesis and behavioral changes in exposed fish. bdnf and rln are both essential for normal brain development and function, for example, bdnf differential expression has been observed in patients with Parkinson’s disease, Alzheimer’s disease, and dementia whereas rln assists in neuronal cell migration during brain development (Fatemi et al., 2005; Vairo et al., 2015). We did not find any significance in bdnf or rln expression following THC or CBD exposure, but will continue to explore the possible ramification of cannabinoids on brain development and adult onset of neurological disorders in zebrafish. vasa, sox2, and sox3 have been previously studied in our laboratory as potential markers of impaired organogenesis or embryogenesis (Corrales et al., 2014; Fang et al., 2013) and are important molecular biomarkers for assessing the adverse reproductive effects of cannabis exposure. Although we observed no differential expression in sox2 and sox3, vasa expression was decreased between the 0.3 and 1.25 mg/l THC treatments (Supplementary Figure 1), but not statistically different than control. vasa is highly expressed in spermatocytes as well as mature oocytes and is highly conserved in both invertebrates (eg, Drosophila) and vertebrates (eg, Xenopus, chick, mouse, and human) during germ cell development (reviewed by Raz, 2000).
Behavioral abnormalities following acute and chronic exposure to THC are well established in both rodents and zebrafish (Akhtar et al., 2013; Champagne et al., 2010; Long et al., 2010; Rodríguez et al., 2017). Neonatal THC exposure to C57/BL6 mice caused neurotoxicity during early developmental periods leading to schizophrenia-suggestive behavior in early adulthood (Rodríguez et al., 2017). CB1-knockout mice have increased anxiety-like behavior and more aggressive tendencies than wild type, which indicates possible CB1-mediated anxiogenic properties when disrupted (Haller et al., 2004). Zebrafish have conserved behavior to rodents following THC exposure (Champagne et al., 2010). Following developmental low-concentration THC exposure, zebrafish exhibit an increased locomotor activity, but increased concentrations have a normalizing effect (Akhtar et al., 2013). Our results indicate similar neurotoxic effects following THC and CBD exposure. Whether or not these results represent anxiogenic-like behavior requires further analysis. Stress or anxiogenic-like behavior in larval zebrafish is usually represented by a reversal in characteristic light:dark behavior (Ellis et al., 2012). We observed hypolocomoter activity in dark periods at high concentrations in both THC and CBD, but also observed hypolocomoter activity during dark periods, which suggests a neurotoxic event in neuronal connectivity possibly void of stressful/anxiogenic properties (Kim et al., 2013). We did find concentration-dependent increases in c-fos expression, which is correlated to increased neural activity and hyperlocomoter behavior in zebrafish (Baraban et al., 2005; Morgan et al., 2016). Our initial hypothesis predicted CBD to significantly reduce c-fos expression in correlation with hypolocomoter behavior due to its anti-convulsant indications (Devinsky et al., 2017); however, both THC and CBD upregulated c-fos in a manner that was inconsistent with behavioral outcomes. This outcome may be due to a neurotoxic event affecting molecular signaling and behavioral phenotypes.
Another major goal of this study was to study the basic pharmacokinetic/toxicokinetic characteristics of a cannabis waterborne exposure scenario. Although zebrafish is an established model for assessing developmental toxicities (Henry et al., 1997) and behavioral phenotypes (Nazario et al., 2015), water-based exposures require water concentration and tissue bioconcentration validation. With advancements of CBD cultivation and pharmaceutical application, it is important to formulate a model that meets high standards for both organismal translatability and high-throughput potentiality. Prior studies have subjected adult zebrafish to IP injections of CBD, which is time-consuming, stressful, and not applicable for high-throughput applications.
Previous studies have documented developmental toxicities without providing confirmation of cannabinoid concentrations in water or proof of whether or not cannabinoids were concentrated in larvae tissue during the exposure period (Akhtar et al., 2013; Thomas, 1975). We found that CBD, even when used at much lower concentrations than THC, tended to bioconcentrate in tissue. Although this is counterintuitive due to the higher polarity of CBD and lower log P compared with THC, the significantly lower CBD LC50 may be attributed to bioconcentration rather than potency.
CONCLUSIONS
Our investigation of CBD toxicity is highly relevant to a wide range of medical fields of research including toxicology, epilepsy, and cancer (Blair et al., 2015; Cridge and Rosengren, 2013). Initially, we hypothesized CBD would be the less toxic cannabis constituent compared with THC primarily due to its non-psychotropic properties and weak CB1 affinity; however, CBD mirrored THC developmental and behavioral toxicities at strikingly lower concentrations. Additionally, CBD bioconcentrated more readily than THC regardless of its lower log P. Because of the developmental toxicities that we detected and the overwhelming outcry for novel cannabis-based therapeutics, more research is necessary to elucidate the molecular mechanisms and potential downstream endocannabinoid system targets or unrelated endocannabinoid system off-target effects that may contribute to neurological or developmental basis for both developmental and adult-onset disease.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Department of BioMolecular Sciences at The University of Mississippi—School of Pharmacy for their support and The University of Mississippi—Center of Research Excellence in Natural Products Neuroscience and Stephen Cutler for providing initial support for this project.
FUNDING
Research reported in this publication was supported by the National Institute on Drug Abuse and the National Institute of General Medical Sciences under Award Numbers R21DA044473-01 and P20GM104932, respectively. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Akhtar M. T., Ali S., Rashidi H., van der Kooy F., Verpoorte R., Richardson M. K. (2013). Developmental effects of cannabinoids on zebrafish larvae. Zebrafish 10, 283–293. [DOI] [PubMed] [Google Scholar]
- Baraban S. C., Taylor M. R., Castro P. A., Baier H. (2005). Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131, 759–768.http://dx.doi.org/10.1016/j.neuroscience.2004.11.031 [DOI] [PubMed] [Google Scholar]
- Becker B., Wagner D., Gouzoulis-Mayfrank E., Spuentrup E., Daumann J. (2010). Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology (Berl) 209, 361–374. [DOI] [PubMed] [Google Scholar]
- Bi W., Huang W., Whitworth D. J., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl. Acad. Sci. U.S.A. 98, 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R. E., Deshpande L. S., DeLorenzo R. J. (2015). Cannabinoids: Is there a potential treatment role in epilepsy? Expert Opin. Pharmacother. 16, 1911–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E., Fishman R. H., Morrill G. A., Fujimoto G. I. (1986). The effect of intragastric administration of delta 9-tetrahydrocannabinol on the growth and development of fetal mice of the A/J strain. Toxicol. Appl. Pharmacol. 82, 378–382. [DOI] [PubMed] [Google Scholar]
- Brunet B., Doucet C., Venisse N., Hauet T., Hébrard W., Papet Y., Mauco G., Mura P. (2006). Validation of Large White Pig as an animal model for the study of cannabinoids metabolism: Application to the study of THC distribution in tissues. Forensic Sci. Int. 161, 169–174. [DOI] [PubMed] [Google Scholar]
- Champagne D. L., Hoefnagels C. C. M., de Kloet R. E., Richardson M. K. (2010). Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 214, 332–342. [DOI] [PubMed] [Google Scholar]
- Chen J. N., Haffter P., Odenthal J., Vogelsang E., Brand M., van Eeden F. J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C. P., et al. (1996). Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123, 293–302. [DOI] [PubMed] [Google Scholar]
- Corrales J., Fang X., Thornton C., Mei W., Barbazuk W. B., Duke M., Scheffler B. E., Willett K. L. (2014). Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 163, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane N. A., Schuster R. M., Gonzalez R. (2013). Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. J. Int. Neuropsychol. Soc. 19, 1009–1015.http://dx.doi.org/10.1017/S135561771300088X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cridge B. J., Rosengren R. J. (2013). Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag. Res. 5, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S., Watanabe A., Yamasaki Y., Amada N., Arthur M., Fleming S., Woodcock H., Dorward P., Pigliacampo B., Close S., et al. (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl) 219, 859–873. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Cilio M. R., Cross H., Fernandez-Ruiz J., French J., Hill C., Katz R., Di Marzo V., Jutras-Aswad D., Notcutt W. G., et al. (2014). Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 55, 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Cross J. H., Laux L., Marsh E., Miller I., Nabbout R., Scheffer I. E., Thiele E. A., Wright S. (2017). Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 376, 2011–2020. [DOI] [PubMed] [Google Scholar]
- Ellis L. D., Seibert J., Soanes K. H. (2012). Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 1449, 46–59.http://dx.doi.org/10.1016/j.brainres.2012.02.022 [DOI] [PubMed] [Google Scholar]
- Fang X., Thornton C., Scheffler B. E., Willett K. L. (2013). Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environ. Toxicol. Pharmacol. 36, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S. H., Snow A. V., Stary J. M., Araghi-Niknam M., Reutiman T. J., Lee S., Brooks A. I., Pearce D. A. (2005). Reelin signaling is impaired in autism. Biol. Psychiatry 57, 777–787. [DOI] [PubMed] [Google Scholar]
- Fontes M. A., Bolla K. I., Cunha P. J., Almeida P. P., Jungerman F., Laranjeira R. R., Bressan R. A., Lacerda A. L. T. (2011). Cannabis use before age 15 and subsequent executive functioning. Br. J. Psychiatry 198, 442–447. [DOI] [PubMed] [Google Scholar]
- Geber W. F., Schramm L. C. (1969). Effect of marihuana extract on fetal hamsters and rabbits. Toxicol. Appl. Pharmacol. 14, 276–282.http://dx.doi.org/10.1016/0041-008X(69)90108-2 [DOI] [PubMed] [Google Scholar]
- Haller J., Varga B., Ledent C., Barna I., Freund T. F. (2004). Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 19, 1906–1912. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A., Russo R. C., Thurston R.V. (1977). Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 12, 417. [Google Scholar]
- Harbison R. D., Mantilla-Plata B., Lubin D. J. (1977). Alteration of delta 9-tetrahydrocannabinol-induced teratogenicity by stimulation and inhibition of its metabolism. J. Pharmacol. Exp. Ther. 202, 455–465. [PubMed] [Google Scholar]
- Henry T. R., Spitsbergen J. M., Hornung M. W., Abnet C. C., Peterson R. E. (1997). Early life stage toxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 142, 56–68. [DOI] [PubMed] [Google Scholar]
- Hisaoka K. K. (1958). The effects of 2-acetylaminofluorene on the embryonic development of the zebrafish: I. Morphological studies. Cancer Res. 18, 527–535. [PubMed] [Google Scholar]
- Hurd Y. L., Wang X., Anderson V., Beck O., Minkoff H., Dow-Edwards D. (2005). Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 27, 221–229. [DOI] [PubMed] [Google Scholar]
- Kemp P. M., Abukhalaf I. K., Manno J. E., Manno B. R., Alford D. D., Abusada G. A. (1995). Cannabinoids in humans. I. Analysis of delta 9-tetrahydrocannabinol and six metabolites in plasma and urine using GC-MS. J. Anal. Toxicol. 19, 285–291. [DOI] [PubMed] [Google Scholar]
- Kim K. T., Zaikova T., Hutchison J. E., Tanguay R. L. (2013). Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol. Sci. 133, 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirla K. T., Groh K. J., Steuer A. E., Poetzsch M., Banote R. K., Stadnicka-Michalak J., Eggen R. I. L., Schirmer K., Kraemer T. (2016). Zebrafish larvae are insensitive to stimulation by cocaine: Importance of exposure route and toxicokinetics. Toxicol. Sci. 154, 183–193. [DOI] [PubMed] [Google Scholar]
- Krug R. G., Clark K. J. (2015). Elucidating cannabinoid biology in zebrafish (Danio rerio). Gene 570, 168–179.http://dx.doi.org/10.1016/j.gene.2015.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K., Nagata T., Kimura K., Imamura T., Jitsufuchi N. (1995). Sensitive determination of Ag-tetrahydrocannabinol in human tissues by GC-MS. J. Anal. Toxicol. 19, 87–90. [DOI] [PubMed] [Google Scholar]
- Lam C. S., Rastegar S., Strähle U. (2006). Distribution of cannabinoid receptor 1 in the CNS of zebrafish. Neuroscience 138, 83–95. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408.http://dx.doi.org/10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Long L. E., Chesworth R., Huang X.-F., McGregor I. S., Arnold J. C., Karl T. (2010). A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 13, 861–876. [DOI] [PubMed] [Google Scholar]
- Maccarrone M., Finazzi-Agro A. (2004). Anandamide hydrolase: A guardian angel of human reproduction? Trends Pharmacol. Sci. 25, 353–357. [DOI] [PubMed] [Google Scholar]
- McKenna G. J. (2014). The current status of medical marijuana in the United States. Hawaii J. Med. Public Health 73, 105–108. [PMC free article] [PubMed] [Google Scholar]
- Migliarini B., Carnevali O. (2009). A novel role for the endocannabinoid system during zebrafish development. Mol. Cell. Endocrinol. 299, 172–177.http://dx.doi.org/10.1016/j.mce.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. O. M. (2016). Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237, 192–197. [DOI] [PubMed] [Google Scholar]
- Murphy S., Ooyen C. (2016). The CBD report. Hemp Bus. J. [Google Scholar]
- Nazario L. R., Antonioli R., Capiotti K. M., Hallak J. E. C., Zuardi A. W., Crippa J. A. S., Bonan C. D., Da Silva R. S. (2015). Caffeine protects against memory loss induced by high and non-anxiolytic dose of cannabidiol in adult zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 139, 134–140. [DOI] [PubMed] [Google Scholar]
- Onaivi E. S., Chakrabarti A., Gwebu E. T., Chaudhuri G. (1995). Neurobehavioral effects of delta 9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav. Brain Res. 72, 115–125. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Das S. K., Dey S. K. (1995). The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 92, 9460–9464.http://dx.doi.org/10.1073/pnas.92.21.9460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud T. V., Ellington A. C. (1968). Teratogenic activity of cannabis resin. Lancet 2, 406–407. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G. (2006). The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. (Lond.) 30(Suppl. 1), S13–S18. [DOI] [PubMed] [Google Scholar]
- Raz E. (2000). The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 1, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel L. B. M., Tavares R. F., Lisboa S. F. S., Joca S. R. L., Corrêa F. M. A., Guimarães F. S. (2009). 5-HT 1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 156, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin I., Herrero-Turrion M. J., Marron Fdez de Velasco E., Gonzalez-Sarmiento R., Rodriguez R. E. (2007). Characterization of two duplicate zebrafish Cb2-like cannabinoid receptors. Gene 389, 36–44. [DOI] [PubMed] [Google Scholar]
- Rodríguez G., Neugebauer N. M., Yao K. L., Meltzer H. Y., Csernansky J. G., Dong H. (2017). Δ9-tetrahydrocannabinol (Δ9-THC) administration after neonatal exposure to phencyclidine potentiates schizophrenia-related behavioral phenotypes in mice. Pharmacol. Biochem. Behav. 159, 6–11. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H. (1999). Chapter 33 Effects of cannabis on fetal development of rodents, Marihuana and Medicine. Humana Press Inc., Totowa, NJ. [Google Scholar]
- Ruhl T., Prinz N., Oellers N., Seidel N. I., Jonas A., Albayram O., Bilkei-Gorzo A., von der Emde G. (2014). Acute administration of THC impairs spatial but not associative memory function in zebrafish. Psychopharmacology (Berl) 231, 3829–3842. [DOI] [PubMed] [Google Scholar]
- Scallet A. C. (1991). Neurotoxicology of cannabis and THC: A review of chronic exposure studies in animals. Pharmacol. Biochem. Behav. 40, 671–676.http://dx.doi.org/10.1016/0091-3057(91)90380-K [DOI] [PubMed] [Google Scholar]
- Schönhofen P., de Medeiros L. M., Bristot I. J., Lopes F. M., De Bastiani M. A., Kapczinski F., Crippa J. A. S., Castro M. A. A., Parsons R. B., Klamt F. (2015). Cannabidiol exposure during neuronal differentiation sensitizes cells against redox-active neurotoxins. Mol. Neurobiol. 52, 26–37. [DOI] [PubMed] [Google Scholar]
- Stewart A. M., Kalueff A. V. (2014). The behavioral effects of acute Δ9-tetrahydrocannabinol and heroin (diacetylmorphine) exposure in adult zebrafish. Brain Res. 1543, 109–119. [DOI] [PubMed] [Google Scholar]
- Szaflarski J. P., Martina Bebin E. (2014). Cannabis, cannabidiol, and epilepsy—From receptors to clinical response. Epilepsy Behav. 41, 277–282. [DOI] [PubMed] [Google Scholar]
- Thomas B. F., Compton D. R., Martin B. R. (1990). Characterization of the lipophilicity of natural and synthetic analogs of delta 9-tetrahydrocannabinol and its relationship to pharmacological potency. J. Pharmacol. Exp. Ther. 255, 624–630. [PubMed] [Google Scholar]
- Thomas R. J. (1975). The toxicologic and teratologic effects of delta9-tetrahydrocannabinol in the Zebrafish embryo. Toxicol. Appl. Pharmacol. 32, 184–190.http://dx.doi.org/10.1016/0041-008X(75)90209-4 [DOI] [PubMed] [Google Scholar]
- Vairo F., Sperb-Ludwig F., Wilke M., Michellin-Tirelli K., Netto C., Neto E. C., Doederlein Schwartz I. V. (2015). Brain-derived neurotrophic factor expression increases after enzyme replacement therapy in Gaucher disease. J. Neuroimmunol. 278, 190–193. [DOI] [PubMed] [Google Scholar]
- Wright P. L., Smith S. H., Keplinger M. L., Calandra J. C., Braude M. C. (1976). Reproductive and teratologic studies with delta9-tetrahydrocannabinol and crude marijuana extract. Toxicol. Appl. Pharmacol. 38, 223–235. [DOI] [PubMed] [Google Scholar]
- Wullimann M. F., Mueller T. (2004). Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 475, 143–162.http://dx.doi.org/10.1002/cne.20183 [DOI] [PubMed] [Google Scholar]
- Wüstehube J., Bartol A., Liebler S. S., Brütsch R., Zhu Y., Felbor U., Sure U., Augustin H. G., Fischer A. (2010). Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 12640–12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.-L., Willoughby J., Liu D., Crump J. G., Wilson C., Miller C. T., Singer A., Kimmel C., Westerfield M., Postlethwait J. H. (2005). A pair of Sox: Distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 132, 1069–1083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.