Abstract
BACKGROUND: Glioblastoma patients can develop hydrocephalus, either obstructive, typically at diagnosis as a result of mass effect, or communicating, usually later in the disease.
OBJECTIVE: To characterize the indications and efficacy of ventriculoperitoneal (VP) shunting for patients with glioblastoma-associated hydrocephalus.
METHODS: Retrospective review was conducted of 841 glioblastoma patients diagnosed from 2004 to 2014, 64 (8%) of whom underwent VP shunting for symptomatic hydrocephalus, to analyze symptoms and outcomes after shunting. Overall survival and postshunt survival were analyzed with Kaplan-Meier methods, with predictors evaluated by use of Cox proportional hazards.
RESULTS: Of the 64 patients who underwent shunting, 42 (66%) had communicating hydrocephalus (CH) and 22 (34%) had obstructive hydrocephalus (OH). CH patients underwent more preshunt craniotomies than those with noncommunicating hydrocephalus, with a mean of 2.3 and 0.7 surgeries, respectively (P < .001). Ventricular entry during craniotomy occurred in 52% of CH patients vs 59% of those with OH (P = .8). After shunting, 61% of all patients achieved symptomatic improvement, which was not associated with hydrocephalus variant (P > .99). Hydrocephalus symptom improvement rates were as follows: headache, 77%; lethargy, 61%; and altered cognition or memory, 54%. Symptomatic improvement was more likely in patients who were younger at shunt placement (hazard ratio, 0.96; P = .045). Symptomatic improvement, shorter time between glioblastoma diagnosis and shunt placement, and CH rather than OH led to improved postshunt survival (hazard ratio = 0.24-0.99; P = .01-.04).
CONCLUSION: VP shunting improves symptoms in most glioblastoma patients with suspected CH or OH, specifically younger patients. Symptomatic improvement, shorter duration between glioblastoma diagnosis and shunt placement, and CH rather than OH improve postshunt survival.
Keywords: Glioblastoma, Hydrocephalus, Ventriculoperitoneal shunt
ABBREVIATIONS
- CH
communicating hydrocephalus
- CI
confidence interval
- EVD
external ventricular drain
- HR
hazard ratio
- OH
obstructive hydrocephalus
- OS
overall survival
- VP
ventriculoperitoneal
Glioblastoma, the most common adult primary brain tumor, is marked by uniformly poor prognosis despite current standard of care, including surgical resection, radiation therapy, and temozolomide chemotherapy. Overall survival (OS) from diagnosis has gradually increased over the last decade to 22 months1 from approximately 15 months.2 As glioblastoma patients continue to survive longer and undergo more craniotomies, a larger proportion will likely exhibit long-term sequelae of their therapies or progressive disease such as hydrocephalus.
Hydrocephalus is the abnormal accumulation of cerebrospinal fluid (CSF) within the ventricular system and can be categorized into obstructive hydrocephalus (OH; secondary to a physical blockage of CSF drainage) or communicating hydrocephalus (CH; secondary to increased production or decreased absorption of CSF) variants. Glioblastoma-associated OH can be due to mass effect or surrounding edema from the tumor itself (typically in the posterior fossa, brainstem, or thalamic region) and can present at any point in the disease course. Glioblastoma-associated CH may reflect accumulation of scarring in the ventricular CSF absorptive system after radiation and multiple craniotomies, which would lead to this phenomenon occurring in recurrent or posttreated patients.3,4 In either variant, patients present with some combination of symptoms, typically associated with increased intracranial pressure, including headache, nausea/vomiting, motor disturbances, cognitive decline, or gait instability.5 On magnetic resonance imaging, patients will typically have ventriculomegaly, either throughout the ventricular system (communicating) or proximal to the CSF outflow blockage (obstructive).
Rates of hydrocephalus in glioblastoma patients are presumed to be low but are challenging to report accurately, often because symptoms present late in the disease course when there is coincident tumor progression. One study from 2003 reported that 5 of 50 patients with supratentorial glioblastoma developed CH after treatment.6 Montano et al3 evaluated 124 glioblastoma patients between 2005 and 2009 and identified 7 cases of posttreatment CH. A more recent study that evaluated 151 glioblastoma patients treated between 2007 and 2011 reported 11 cases of CH.4 In 1 series of 41 patients with thalamic gliomas, OH was the presenting symptom in 33% to 56% of cases, depending on tumor grade.7
Reported risk factors for the development of CH include surgical entrance into the ventricular system3 and leptomeningeal tumor spread.4 Hydrocephalus is typically managed surgically, with short-term options including extraventricular or lumbar drainage, usually as a bridge to more permanent solutions such as endoscopic third ventriculostomy or ventriculoperitoneal (VP) shunt placement. Some case series have explored the benefit of VP shunting in patients with glioblastoma. Roth et al5 reviewed outcomes in 16 patients who presented with nonobstructive ventriculomegaly not explained by progressive glioblastoma and found that shunting was effective for restoring both motor and cognitive function. They did, however, report a fairly high rate of shunt-related morbidity, including early and late infections.
We sought to contribute to the understanding of hydrocephalus in glioblastoma patients by reviewing our institutional experience over the past decade. The aim of this study was to determine which symptoms are most likely to improve after shunting of glioblastoma patients with OH or CH and what features, if any, predict symptomatic improvement and OS outcomes in glioblastoma patients who undergo VP shunt placement.
METHODS
Study Design, Setting, and Participants
Using a departmental neurosurgical database of patients treated at our center between 2004 and the first 6 months of 2014, we retrospectively reviewed 841 glioblastoma patients who underwent surgical resection of their tumor. All consecutive patients who fit the eligibility criteria of histologically confirmed World Health Organization grade IV glioblastoma and underwent surgical resection of their tumor were included. No patients were excluded. Of these 841 glioblastoma patients, 64 (8%) underwent VP shunt placement for either communicating or non-CH at some point during their glioblastoma course. Hydrocephalus was determined from radiographic assessment. The size of the ventricular system was compared with earlier scans for patients with established brain tumor diagnosis or compared with the radiologist's impression of standard ventricular sizes in that patient's age group for patients diagnosed at the time of presentation. All patients treated with VP shunt had radiologic diagnosis of either CH or non-CH before the intervention. This study was approved by the University of California at San Francisco Institutional Review Board (No. 11-06160). All patients had pathologically confirmed World Health Organization grade IV glioblastoma or gliosarcoma diagnosed after either surgical resection or biopsy.8
Variables, Data Sources, and Measurement
Identified patients were analyzed for various demographic and clinical parameters, including age, sex, tumor location, tumor mutational status, presenting clinical symptoms of hydrocephalus, number of previous craniotomies before shunting, evidence of tumoral leptomeningeal dissemination on contrast-enhanced magnetic resonance imaging, ventricular entrance during craniotomy, placement of an extraventricular or a lumbar drain before shunting, number of shunt revisions, and infectious complications secondary to shunting. Tumor mutation analysis obtained from pathology reports was not available for all cases because of the timing of when these tests became routine relative to the 2004 start of our cohort. Epidermal growth factor receptor (EGFR) gene amplification was assessed after 2008 by immunostaining because most cases predate use of fluorescence in situ hybridization at our center (n = 31 shunted glioblastomas, n = 376 nonshunted glioblastomas). Phosphatase and tensin homolog (PTEN) alteration was assessed after 2008 by immunostaining (n = 27 shunted glioblastomas, n = 203 nonshunted glioblastomas). Tumor protein p53 (TP53) mutations were assessed by immunostaining intermittently starting in 2008 (n = 8 shunted glioblastomas, n = 95 nonshunted glioblastomas). Isocitrate dehydrogenase 1 (IDH1) mutations were assessed by DNA sequencing starting in 2011 (n = 14 shunted glioblastomas, n = 170 nonshunted glioblastomas).
Our 2 outcome measures were improvement of clinical symptoms after shunt placement and survival after shunt placement. Clinical improvement was evaluated qualitatively with the use of follow-up notes that indicated subjective improvement or resolution of clinical symptoms. Postshunt radiographic imaging identifying improvement or resolution of ventriculomegaly was also used as a more objective method of identifying clinical improvement to reduce bias. Dates of death were obtained by documented clinical notes or by searching through publically available databases. Kaplan-Meier analysis for patients whose dates of death could be verified was performed on control glioblastoma patients without hydrocephalus (n = 160) and our cohort of patients with both OH and CH (n = 64).
Statistical Methods
Clinical or outcome differences between OH and CH patients were evaluated with the Fisher exact test for proportions derived from categorical variables, and the unpaired, 2-tailed Student t test was used to compare continuous variables between groups. Binary logistic regression analysis was performed to determine whether there was any correlation between various clinical predictors and symptomatic outcome after placement of a VP shunt. Survival after shunt placement was estimated with the Kaplan-Meier method, with hazard differences between patients with each variant of hydrocephalus assessed with the log-rank test. Univariate and multivariate Cox proportional hazard analyses were used to assess whether these clinical predictors were associated with increased overall postshunt survival. Patients missing tumor mutational analysis were excluded from the respective portion of the analysis. For all statistical analyses, a value of P < .05 was deemed to be significant. All statistical tests were performed with SPSS Statistics 22 (IBM, Armonk, New York) or GraphPad Prism 6 (GraphPad Software, La Jolla, California). One potential source of bias involves the qualitative assessment of symptomatic improvement post–VP shunt placement. All measures were taken to reduce this potential bias, including relying on documented symptomatic improvement via clinic notes.
RESULTS
Participants and Descriptive Data
We identified 841 patients who were treated surgically for glioblastoma at our center between 2004 and mid-2014, of whom 64 (8%) underwent at least 1 VP shunt placement. Glioblastoma patients requiring VP shunt placement were younger than patients not requiring VP shunt (49 vs 57 years; P < .001; Table 1). In terms of tumor locations, patients requiring shunts were less likely to have lobar tumors (P = .02) and more likely to have thalamic tumors (P = .002) than patients not requiring shunts. In terms of procedures, shunted patients were less likely to undergo gross total resection (P < .001).
TABLE 1.
Cohort Characteristics by Hydrocephalus Varianta
| P Value, | ||||||
|---|---|---|---|---|---|---|
| Nonshunted | Shunted | Nonshunted | P Value, | |||
| Patients | Patients | vs Shunted | CH | OH | CH vs OH | |
| Patients, n | 777 | 64 | N/A | 42 (66) | 22 (34) | N/A |
| Male sex, n (%) | 458 (59) | 37 (58) | .9 | 27 (64) | 10 (45) | .2 |
| Mean (range) age at glioblastoma diagnosis, y | 56.6 (16-88) | 49.3 (6-85) | <.001b | 50.1 (7.0-74.5) | 46.6 (6.3-85.2) | .2 |
| Original tumor hemisphere, n (%) | ||||||
| Left | 311 (40) | 28 (44) | .6 | 21 (50) | 7 (32) | .2 |
| Right | 396 (51) | 31 (48) | .7 | 19 (45) | 12 (55) | .6 |
| Bilateral | 70 (9) | 5 (8) | .9 | 2 (4) | 3 (14) | .4 |
| Original tumor lobar location, n (%) | ||||||
| Lobar | 651 (84) | 46 (72) | .02b | 39 (93) | 7 (32) | <.001b |
| Thalamus | 39 (5) | 10 (16) | .002b | 1 (2) | 9 (41) | <.001b |
| Cerebellum | 16 (2) | 3 (5) | .2 | 0 | 3 (14) | .04b |
| Intraventricular | 16 (2) | 2 (3) | .6 | 0 | 2 (9) | .1 |
| Multifocal | 45 (6) | 2 (3) | .6 | 1 (2) | 1 (5) | .9 |
| Brainstem | 10 (1) | 1 (2) | .6 | 1 (2) | 0 | .9 |
| Extent of first resection, n (%) | ||||||
| Gross total | 513 (66) | 8 (13) | <.001b | 8 (19) | 0 | .04b |
| Subtotal | 187 (24) | 37 (58) | <.001b | 27 (64) | 10 (45) | .2 |
| Biopsy | 77 (10) | 19 (30) | <.001b | 7 (17) | 12 (55) | .003b |
| Received chemotherapy before developing hydrocephalus, n (%) | N/A | 54 (84) | N/A | 40 (95) | 14 (64) | .002 |
| Received radiation before developing hydrocephalus, n (%) | N/A | 57 (89) | N/A | 42 (100) | 15 (68) | <.001b |
| Mean (range) craniotomies before shunt, n | N/A | 1.7 (0-5) | N/A | 2.3 (0-5) | 0.7 (0-4) | <.001b |
| Ventricular system was entered during any surgery, n (%) | N/A | 35 (55) | N/A | 22 (52) | 13 (59) | .8 |
| Trial of extraventricular drain before VP shunt, n (%) | Not applicable | 29 (45) | N/A | 21 (50) | 8 (36) | .4 |
| Trial of lumbar drain before VP shunt, n (%) | Not applicable | 6 (9) | N/A | 5 (12) | 1 (5) | .7 |
| Median (range) duration of time between glioblastoma diagnosis and shunt placement, mo | Not applicable | 7.3 (0-461) | N/A | 10.6 (0.3-461) | 0.4 (0-25.6) | .07 |
aCH, communicating hydrocephalus; OH, obstructive hydrocephalus; VP, ventriculoperitoneal. Table represents patient and tumor characteristics for those who did not end up needing VP shunts vs those who developed OH vs CH.
bSignificant.
Shunted vs nonshunted glioblastoma patients had equal male preponderance and did not differ in terms of frequency of bilateral tumors or tumors of the cerebellum, entry into the ventricular system, multifocal tumors, or brainstem tumors (P = .2 to P = .9; Table 1). In terms of mutational profile, of the glioblastomas in patients requiring VP shunt placement vs those not requiring VP shunt placement, 45% vs 41% exhibited EGFR amplification (P = .7), 25% vs 9% exhibited TP53 mutations (P = .2), 22% vs 33% (P = .3) exhibited PTEN alteration, and 7% vs 6% (P = .6) exhibited IDH1 mutation. In terms of follow-up, no patients were lost to follow-up, and all patients were followed up until death (n = 54) or censored at the time of their last magnetic resonance imaging before this report (n = 10; median, 12 months of follow-up after VP shunt placement).
Outcome Data: Timing of Shunt Placement
Of those patients who underwent VP shunt placement, 42 patients (66%) had CH with a shunt placed a median of 10.6 months (range = 0.3-461 months) after glioblastoma diagnosis, and 22 patients (34%) had OH with shunt placement a median of 0.4 months (range = 0-25.6 months) after glioblastoma diagnosis (Table 1 and Figure 1). Patients with CH were more likely to have lobar tumors (P < .001) and thalamic tumors (P < .001) than patients with OH (Table 1).
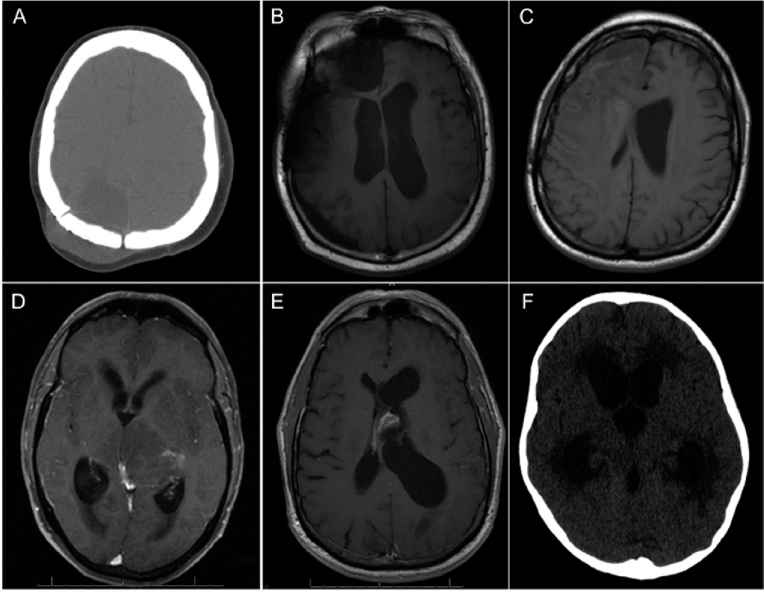
FIGURE 1.
Representative imaging for communicating and noncommunicating hydrocephalus. Representative brain imaging for various presentations of 3 cases of glioblastoma-associated communicating hydrocephalus (A-C) and 3 cases of glioblastoma-associated obstructive hydrocephalus (D-F). A, noncontrast computed tomography (CT) of a 38-year-old woman with parietal glioblastoma found to have significant subgaleal and extra-axial fluid collection compatible with pseudomeningocele. B, T1-weighted magnetic resonance imaging (MRI) for a 60-year-old man with right frontal glioblastoma who developed a significant extra-axial fluid collection with associated ventriculomegaly. C, T1-weighted MRI for a 52-year-old woman with right frontal glioblastoma found to have ventriculomegaly and bilateral subependymal lateral ventricular enhancement after developing gait instability and urinary incontinence. D, axial T1-weighted postcontrast MRI of a 60-year-old man diagnosed with left thalamic glioblastoma extending laterally through the basal ganglia with obstruction at the cerebral aqueduct, resulting in enlarged lateral and third ventricles but normal fourth ventricular size. E, axial T1-weighted postcontrast MRI of a 60-year-old man with history of intraventricular glioblastoma who presented with several days of severe headache and development of shuffling gate who was found to have tumor recurrence obstructing the left foramen of Monro. F, noncontrast head CT scan of a 6-year-old girl with history of right cerebellar glioblastoma who became increasingly nonverbal and somnolent; she was found to have severe obstructive hydrocephalus proximal to the third ventricular outflow.
Five patients (8%), all with OH, received a VP shunt at initial surgery (these surgeries were all biopsies), and 10 (16%) additional patients (2 with CH, 8 with OH) were shunted within 1 month of pathological diagnosis. Overall, patients underwent an average of 1.7 craniotomies (range = 0-5) before the development of hydrocephalus, with an average of 2.3 (range = 0-5) in the communicating group and 0.7 (range = 0-4) in the obstructive group (P < .001). Nineteen patients (30%) underwent a biopsy alone for their initial diagnostic procedure, 6 of whom (32%) never underwent an additional craniotomy (2 with OH, 4 with CH). Of the 19 biopsied patients, 5 (all with OH) were shunted during their initial surgery, and an additional 6 (5 with OH, 1 with CH) were shunted within 1 month of diagnostic biopsy.
Only 7 patients (11%) in our cohort had evidence of leptomeningeal gadolinium enhancement on magnetic resonance imaging before shunt placement, with 4 of these patients having CH (10% of CH patients) and 3 having OH (14% of OH patients). Entry into the ventricular system during craniotomy occurred in 35 patients (55%; 52% of CH patients vs 59% of OH patients; P = .8).
Outcome Data: Hydrocephalus Presentation
In our cohort, 24 patients (38%) had radiographic evidence of hydrocephalus at the time of initial clinical presentation before obtaining tissue diagnosis (9 CH and 15 OH). Of these 24 patients who presented with radiographic hydrocephalus, 12 patients (50%; 11 with OH, 1 with CH) were shunted within 20 days of their diagnosis. Specifically, 5 patients underwent VP shunt placement at diagnosis during their initial procedure, which was a biopsy. For the remaining 7, 1 had postbiopsy intraventricular hemorrhage requiring therapeutic shunting, 1 had tumor growth after biopsy causing compression, 1 had a failed endoscopic third ventriculostomy, and the remaining 4 became symptomatic in short course after their biopsies. Of the 16 patients who received a biopsy initially, 8 patients (50%; 8 with OH, 0 with CH) had radiographic evidence of hydrocephalus at the time of the biopsy. Twelve patients (8 with CH, 4 with OH) had early radiographic evidence of hydrocephalus but were not shunted within a month of diagnosis. In general, these patients did not develop significant clinical evidence of hydrocephalus until after their first surgery, or they developed a pseudomeningocele or subgaleal hygroma requiring VP shunt drainage.
Three additional patients underwent VP shunt placement about 2 weeks postoperatively who did not have radiographic suspicion of hydrocephalus on their prediagnosis scan. Two patients had ventricular entry during their first resection and subsequently failed external ventricular drain (EVD) weaning trials, necessitating VP shunt placement for new CH. The third patient had an intraventricular lesion and also failed EVD weaning after resection. Therefore, in total, 15 patients (23% of cohort) were shunted perioperatively within 20 days, leaving 49 patients (77%) who developed symptomatic hydrocephalus later in their disease course.
Of these 49 patients (40 with CH, 9 with OH) who were shunted in a delayed manner relative to diagnosis or developed hydrocephalus later in the course of their disease, 44 patients (90%; 38 CH patients, 6 OH patients) had received adjuvant radiotherapy. Forty-two patients (86%; 36 CH patients, 6 OH patients) had received prior temozolomide chemotherapy. These 49 patients were shunted a median of 8.6 months (range = 1.2-461 months) after diagnosis. These 49 patients underwent an average of 2.1 open craniotomies before the development of delayed hydrocephalus, with an average of 2.3 (range = 0-5) for patients with CH and 1.2 (range = 0-4) for patients with OH (P = .04).
The most common presenting symptoms in our cohort that were potentially attributable to hydrocephalus were altered cognition or memory (26 patients, 41%), headache (22 patients, 34%), and lethargy (18 patients, 28%; Table 2). Altered mental status (31% of those with CH, 59% of those with OH; P = .04), lethargy (19% of those with CH, 45% of those with OH; P = .04), nausea and vomiting (12% of those with CH, 50% of those with OH; P = .002), and visual disturbances (0% of those with CH, 14% of those with OH; P = .04) were more prominent in patients with OH, whereas pseudomeningocele or CSF leak (40% of those with CH, 0% of those with OH, P < .001) was more common in patients with CH (Table 2).
TABLE 2.
Presenting Symptoms of Hydrocephalus and Associated Outcomesa
| Entire | Type of Hydrocephalus, n (%) | P Value, Incidence | Symptoms | Type of Hydrocephalus, n (%) | P Value, Symptomatic | |||
|---|---|---|---|---|---|---|---|---|
| Presenting | Cohort, | CH | OH | of Symptoms in CH | Improved After | Improvement in CH | ||
| Symptoms | n (%) | (n = 42) | (n = 22) | vs OH | Shunt, n (%)b | CH | OH | vs OH |
| Altered mental status | 26 (41) | 13 (31) | 13 (59) | .04c | 14 (59) | 8 (62) | 6 (46) | .7 |
| Headache | 22 (34) | 12 (29) | 10 (45) | .3 | 17 (77) | 9 (75) | 8 (80) | >.99 |
| Lethargy | 18 (28) | 8 (19) | 10 (45) | .04c | 11 (61) | 4 (50) | 7 (70) | .6 |
| Pseudomeningocele/CSF leak | 17 (27) | 17 (40) | 0 (0) | <.001c | 10 (59) | 10 (59) | 0 (0) | >.99 |
| Nausea/vomiting | 16 (25) | 5 (12) | 11 (50) | .002c | 12 (75) | 4 (80) | 8 (73) | >.99 |
| Gait disturbances | 15 (23) | 8 (19) | 7 (32) | .4 | 8 (63) | 4 (50) | 4 (57) | >.99 |
| Speech disturbances | 8 (13) | 4 (10) | 4 (18) | .7 | 5 (63) | 3 (75) | 2 (50) | >.99 |
| Urinary incontinence | 5 (8) | 5 (12) | 0 (0) | .16 | 2 (40) | 2 (40) | 0 (0) | >.99 |
| Visual disturbances | 3 (5) | 0 (0) | 3 (14) | .04c | 3 (100) | 0 (0) | 3 (100) | >.99 |
| Hemiparesis | 2 (3) | 0 (0) | 2 (9) | .1 | 2 (100) | 0 (0) | 2 (100) | >.99 |
| Seizure | 1 (2) | 1 (2) | 0 (0) | >.99 | 1 (100) | 1 (100) | 0 (0) | >.99 |
| Tremor | 1 (2) | 1 (2) | 0 (0) | >.99 | 1 (100) | 1 (100) | 0 (0) | >.99 |
aCH, communicating hydrocephalus; CSF, cerebrospinal fluid; OH, obstructive hydrocephalus. Clinical symptoms on initial presentation of hydrocephalus in our cohort and the number in each group who improved after shunting are listed here. Of note, many patients had multiple presenting symptoms, all of which are classified here.
bColumn heading indicates number and percentage of patients within each symptom grouping who improved after shunting.
cSignificant.
Trials of non–VP shunt management were attempted in 34 patients (53%) before shunt placement to better understand whether symptoms were attributable to increased intracranial pressure. Specifically, 4 patients (6%) underwent solely lumbar drainage, 28 patients (44%) had solely EVD placement, and 2 patients (3%) received both lumbar drain and EVD. Of the 6 patients who had preshunt lumbar drainage, 5 patients had CH (83%) and 1 patient had OH (17%). Of the 30 patients who underwent preshunt EVD trial, 21 patients had CH (70%) and 9 patients had OH (30%). The 2 patients who underwent both interventions had the communicating variant. Of the 34 patients who underwent CSF drainage trials, symptomatic improvement occurred in 20 patients (59%) compared with 19 patients (63%) who had symptomatic improvement without a preceding CSF drainage trial (P = .8), suggesting that these trials did not increase the chances of symptomatic improvement beyond that achieved by radiographic or clinical suspicion alone.
Main Results: Symptomatic Improvement
After shunting, 39 patients (61%) had symptomatic improvement, and 25 patients (39%) experienced no benefit or worsened symptoms. We report 26 patients with CH (62%) and 13 patients with OH (59%) who improved (P > .99). Symptom resolution after shunt placement occurred in 14 of 26 patients (54%) with altered cognition or memory, 17 of 22 patients (77%) with headache, 11 of 18 patients (61%) with lethargy, 10 of 17 patients (59%) with pseudomeningocele or CSF leak, 12 of 16 patients (75%) with nausea and vomiting, 8 of 15 patients (53%) with gait disturbances, 5 of 8 patients (63%) with speech disturbances, 2 of 5 patients (40%) with urinary incontinence, 3 of 3 patients (100%) with visual disturbances, and 2 of 2 patients (100%) with hemiparesis (Table 2).
Univariate and multivariate binary logistic regression for symptomatic improvement was performed using OS, duration between glioblastoma diagnosis and shunt placement, age at shunt placement, sex, number of craniotomies before shunt placement, ventricular opening during surgery, and hydrocephalus variant (Table 3). This revealed that younger age predicted symptomatic improvement on both univariate (hazard ratio [HR], 0.98; 95% confidence interval [CI], 0.9-1.0; P = .047) and multivariate (HR, 0.96; 95% CI, 0.9-1.0; P = .045) analyses.
TABLE 3.
Binary Logistic Regression Modeling for Symptomatic Improvement After Ventriculoperitoneal Shunt Placementa
| Univariate Binary Logistic Regression | Multivariate Binary Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio of Symptomatic Improvement | 95% CI | P | Odds Ratio of Symptomatic Improvement | 95% CI | P |
| Sex | ||||||
| Female | 0.885 | 0.321-2.444 | .81 | 0.635 | 0.207-1.948 | .43 |
| Male | Referent | Referent | ||||
| Ventricle surgically entered | ||||||
| Yes | 0.917 | 0.334-2.517 | .87 | 0.948 | 0.327-2.752 | .92 |
| No | Referent | Referent | ||||
| Type of hydrocephalus | ||||||
| OH | 0.889 | 0.310-2.549 | .83 | 1.038 | 0.254-3.690 | .97 |
| CH | Referent | Referent | ||||
| No. of craniotomies | 1.078 | 0.758-1.534 | .68 | 1.109 | 0.723-1.703 | .64 |
| Age at shunt placement | 0.967 | 0.933-1.002 | .047b | 0.963 | 0.928-0.999 | .045b |
| Time between glioblastoma diagnosis and shunt placement | 1.004 | 0.990-1.019 | .56 | 1.003 | 0.990-1.017 | .61 |
aCH, communicating hydrocephalus; CI, confidence interval; OH, obstructive hydrocephalus.
b P values of significance in univariate or multivariate analysis.
Other Analyses: Survival
OS was estimated for glioblastoma patients who did and did not experience hydrocephalus. For the entire cohort, the median OS was 14.7 months: 14.4 months for patients who did not experience hydrocephalus and 15.5 months for patients with hydrocephalus. There was no significant difference in median OS between the control cohort and the combined cohort of all patients who experienced hydrocephalus. Similarly, there was no difference between the control cohort and patients with OH whose median OS was 8.25 months; however, patients with CH had an improved OS of 17.9 months compared with the control cohort (P = .01). When median OS is compared between hydrocephalus variants, OS for patients with CH was better than that for patients with OH (P = .007).
We then calculated patient survival from the time of first VP shunt placement. For the entire cohort, median postshunt survival was 5.1 months: 7.6 months for patients with CH and 4.4 months for patients with OH (univariate HR, 1.73; 95% CI, 1.05-3.12; P > .99; Table 4). Figure 2 shows a Kaplan-Meier plot of OS and postshunt survival by control cohort and hydrocephalus variant. We investigated the impact of additional variables on postshunt survival through univariate proportional hazard analysis (Table 4), of which sex, surgical entry into the ventricle, number of craniotomies, and mutations were not associated with postshunt survival. The 4 variables with univariate P ≤ .15 (type of hydrocephalus, age at shunt placement, time between glioblastoma diagnosis and shunt placement, and symptomatic improvement) were then subjected to multivariate analyses, revealing 3 variables to affect postshunt survival: Symptomatic improvement increased postshunt survival (HR, 0.24; 95% CI, 0.11-0.46; P = .02), whereas longer duration between glioblastoma diagnosis and shunt placement (HR, 1.007; 95% CI, 1.002-1.0013; P = .01) and OH (HR, 1.87; 95% CI, 1.03-3.44; P = .04) worsened postshunt survival.
TABLE 4.
Cox Proportional Hazard Modeling for Overall Survival After Ventriculoperitoneal Shunt Placementb
| Univariate Cox Proportional Hazards | Multivariate Cox Proportional Hazards | |||||
|---|---|---|---|---|---|---|
| Variable | HR of Postshunt OS | 95% CI | P | HR of Postshunt OS | 95% CI | P |
| Sex | ||||||
| Female | 0.89 | 0.54-1.49 | .7 | |||
| Male | Referent | |||||
| Ventricle surgically entered | ||||||
| Yes | 0.83 | 0.5-1.37 | .5 | |||
| No | Referent | |||||
| Type of hydrocephalus | ||||||
| OH | 1.73 | 1.05-3.12 | .14b | 1.87 | 1.03-3.44 | .04b |
| CH | Referent | Referent | ||||
| No. of craniotomies | 0.96 | 0.81-1.14 | .6 | |||
| Age at shunt placement | 1.013 | 1.002-1.03 | .15b | 1.017 | 0.998-1.037 | .08 |
| Time between glioblastoma diagnosis and shunt placement | 1.016 | 1.000-1.032 | .047b | 1.007 | 1.002-1.013 | .01b |
| Symptomatic improvement | ||||||
| Yes | 0.29 | 0.2-0.5 | .03b | 0.24 | 0.11-0.46 | .02b |
| No | Referent | Referent | ||||
| Tumor mutation | ||||||
| IDH1 mutation | 0.78 | 0.11-4.15 | .8 | |||
| EGFR amplification | 1.16 | 0.63-2.15 | .6 | |||
| PTEN deletion | 1.21 | 0.56-2.62 | .6 | |||
| TP53 mutation | 0.70 | 0.13-3.93 | .7 | |||
aCH, communicating hydrocephalus; CI, confidence interval; HR, hazard ratio; OH, obstructive hydrocephalus; OS, overall survival.
bVariables with univariate P values ≤ .15 (shown on the left) underwent multivariate analysis. Significant P values in multivariate analyses are shown on the right.
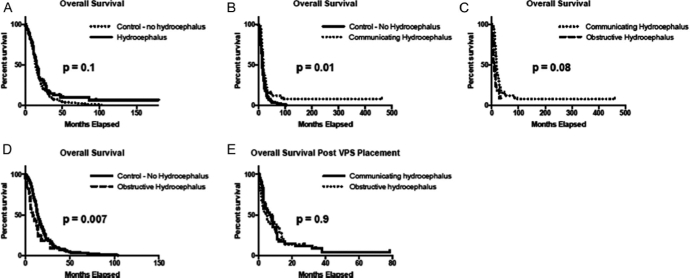
FIGURE 2.
Overall survival (OS) and post–ventriculoperitoneal (VP) shunt survival. Kaplan-Meier analysis of overall survival in glioblastoma patients with and without hydrocephalus, which is further subdivided into communicating hydrocephalus (CH) and obstructive hydrocephalus (OH). A, median OS for patients without hydrocephalus (n = 160) was 14.4 vs 15.5 months for combined hydrocephalus patients (n = 64; P = 0.1). B, there was a significant difference between control patients and those with CH, who had an improved median OS of 17.9 months (n = 42; P = .01). C, no difference was seen between control patients and the median OS of 8.3 months seen in OH (n = 22; P = .08). D, directly comparing median OS between hydrocephalus variants indicated an improved median OS with CH compared with OH (P = .007). E, Kaplan-Meier analysis of post–VP shunt survival indicated no significant difference between 7.6 months in CH and 4.4 months in OH (P = .9).
Nineteen patients (29%) had at least 1 shunt failure necessitating surgical revision, of whom 9 (14%) developed an infectious complication, 3 (5%) underwent shunt removal, and 3 (5%) required multiple revisions. Infection complications occurred in 9 of 64 shunted patients (14%), of whom 7 patients (11%) presented with ventriculitis or meningitis, 1 (2%) presented with an abdominal wound infection, and 1 (2%) presented with an infected and broken-down shunt cranial wound. Seven of 9 infected shunts were externalized and replaced after antibiotic treatment, and 2 of 9 were removed and not replaced. The third and final patient whose shunt was removed suffered from uninfected subdural collections secondary to shunt overdraining.
In the 3 patients receiving multiple revisions, 1 patient was switched to a programmable valve for persistently enlarged ventricles and symptomatic hydrocephalus without evidence of tumor progression. Later, this shunt required a second revision for a blockage at the peritoneal catheter. The second patient underwent a series of revisions, initially valvular changes for overshunting. Later, the shunt was removed to treat ventriculitis, which had developed. The replacement shunt then became blocked from an intraventricular hemorrhage secondary to enoxaparin treatment for coincident pulmonary embolism, leading to another revision, which was later replaced by an EVD because septated membranes developed within the ventricles, preventing them from communicating and necessitating a fenestration procedure. The final patient's catheter eroded through the skin, so she was taken to the operating room for revision through the contralateral ventricle; however, she developed shunt failure 1 day after the new device was implanted, necessitating another surgical revision.
DISCUSSION
Key Results
Hydrocephalus is a relatively rare phenomenon in glioblastoma patients that can occur at diagnosis or later in the course of the disease. Hydrocephalus as a sequela of glioblastoma is poorly understood because of a limited amount of studies and small sample sizes. Previous reports have documented that CH contributes to neurological worsening in approximately 10% (range = 3%-26%) of patients who have pathologically confirmed glioblastoma by either surgical resection or biopsy.3,5,6,9,10 We identified 64 patients (7.6% of our total sampling of 841 patients) who received a VP shunt for hydrocephalus associated with glioblastoma, aligning with previous epidemiological estimates.
Forty-two patients, 66% of shunted patients and 5% of total sampling, developed symptomatic CH, whereas 22 patients, 34% of shunted patients and 2.6% of total sampling, suffered from OH. To the best of our knowledge, this is the largest case series of glioblastoma patients who also exhibited clinical and radiographic signs of CH treated with shunt. Until now, the largest sampling in the literature was 16 patients.5 Although OH in glioblastoma patients invariably occurs secondary to mass effect from the tumor itself, the pathogenesis of CH in glioblastoma patients remains unclear. Although it has been hypothesized to be due to leptomeningeal or intraventricular dissemination of tumor cells causing physical blockage of CSF outflow,6,11,12 in our series, leptomeningeal dissemination did not seem to be an important contributing factor to the development of CH relative to OH and overall occurred in a small number of shunted glioblastoma patients. This finding is consistent with previous studies from Montano et al3 and Roth et al,5 which showed no evidence of periventricular or subarachnoid gadolinium enhancement on magnetic resonance imaging, an indicator of tumor cells that have disseminated into the CSF. CH must be differentiated from hydrocephalus ex vacuo or ventriculomegaly caused by loss of brain tissue, which is a known phenomenon after whole-brain radiotherapy.13 Radiation has also been theorized to cause mildly increased intracranial pressure by causing fibrosis of the arachnoid granulations, thus decreasing CSF absorption, and could be a risk factor for the development of CH in glioblastoma patients, whose timing after glioblastoma diagnosis and subsequent radiation was comparable to that of other radiation-induced changes seen in glioblastoma patients.14,15
Identifying and correcting factors contributing to poor survival and quality of life in glioblastoma patients remains crucial to improving patient outcomes.16-18 Glioblastoma patients who developed CH had significantly improved OS compared with the control cohort, which may simply indicate that the patients with a propensity to survive longer have a greater risk of developing any complication, one of which is hydrocephalus. In our study, younger patients were better candidates for VP shunt placement because they were more likely to receive symptomatic benefit from the procedure. Patients had improved postshunt survival when the time between glioblastoma diagnosis and shunt placement was shorter, indicating the need to follow up patients closely to monitor for any complications of hydrocephalus and to decide on the need for a shunt earlier rather than later. It is possible, however, that poorer survival in patients with delayed VP shunt placement is due to more advanced disease progression. Our data suggest that developing late-stage, posttreatment hydrocephalus is a poor prognostic sign. Although hydrocephalus variant did not predict symptomatic improvement, more than half of our cohort experienced symptomatic improvement after the procedure, and this symptomatic improvement was associated with improved postshunt survival in these patients.
Limitations
There are, of course, several limitations to our study. The retrospective nature of this analysis makes it subject to weakness in the accuracy of the archived data, whereas the single-institution nature of the study makes it influenced by management strategies of glioblastoma patients that might be unique to our center. Another limitation of our work is that we were unable to say definitively whether surgical intraventricular entry was associated with increased risk of hydrocephalus because we did not have documentation of the rate of ventricular entry in our nonshunted cohort.
Interpretation
The most significant findings from this study are that the rates of symptomatic improvement we noted were higher than the morbidity rate, which consisted primarily of shunt infections in this immunocompromised patient population, and that symptomatic improvement was associated with improved postshunt survival in these patients. In the absence of being able to identify factors predictive of symptomatic improvement, this would argue for offering VP shunt placement for glioblastoma patients with symptomatic hydrocephalus.
Generalizability
Given the importance of maximizing quality of life during the short life expectancy faced by all glioblastoma patients, our findings would be strengthened if we identified ways to distinguish patients who benefited from VP shunt placement from those who did not. One study aimed at determining which patients are at a higher risk of developing postoperative hydrocephalus suggests that 5-aminolevulinic acid fluorescence of the ventricular wall is predictive, and those authors recommended that these patients receive postoperative radiotherapy encompassing the entire ventricular system.19 Ventricular opening during surgical resection has been shown to be a risk factor for the development of CH in these patients.3 We can speculate that it is an important risk factor because 35 shunted glioblastoma patients (55%) had prior surgical entry into the ventricular system in at least 1 of their operations, a rate that would seem to exceed the rate of surgical entry into the ventricular system in glioblastoma patients in general. For glioblastoma with intraventricular resection, the benefit of resecting tumor adjacent to the ventricular system should be weighed against the risks of developing hydrocephalus and eventually necessitating shunt placement.
CONCLUSION
The potential causes of neurologic decline in a glioblastoma patient are numerous and include mass effect from tumor progression, intratumoral or extratumoral hemorrhage, stroke, infection, treatment effect, leptomeningeal spread of tumor, and hydrocephalus.20 Although the majority of neurological changes will prompt clinicians to suspect tumor progression or treatment effect, it is important to consider hydrocephalus as a cause of worsening symptoms, especially when taking into account that younger patients and shorter duration between diagnosis and shunt placement predict better outcomes. Hydrocephalus in glioblastoma patients can be radiographically subtle if it is communicating in nature. Thus, the scrutiny of imaging in glioblastoma patients must include not only assessment for tumor-associated imaging changes with the use of modern criteria21 but also awareness of potential hydrocephalus and the recognition that symptoms otherwise attributed to tumor progression might actually reflect aberrant CSF flow or obstruction. The majority of patients will experience clinical improvement after VP shunting, and those who receive clinical benefit will experience improved postshunt survival; however, the clinical team must balance the tangible risk of infectious complications.
Disclosures
B.A. Castro is a Howard Hughes Medical Institute Medical Research Fellow. B. S. Imber was supported through the National Center for Advancing Translational Sciences, National Institutes of Health via UCSF-CTSI grant TL1 TR000144. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Clark AJ, Lamborn KR, Butowski NA et al. Neurosurgical management and prognosis of patients with glioblastoma that progresses during bevacizumab treatment. Neurosurgery. 2012;70(2):361-370. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 3. Montano N, D'Alessandris QG, Bianchi F et al. Communicating hydrocephalus following surgery and adjuvant radiochemotherapy for glioblastoma. J Neurosurg. 2011;115(6):1126-1130. [DOI] [PubMed] [Google Scholar]
- 4. Fischer CM, Neidert MC, Peus D et al. Hydrocephalus after resection and adjuvant radiochemotherapy in patients with glioblastoma. Clin Neurol Neurosurg. 2014;120:27-31. [DOI] [PubMed] [Google Scholar]
- 5. Roth J, Constantini S, Blumenthal DT, Ram Z. The value of ventriculo-peritoneal shunting in patients with glioblastoma multiforme and ventriculomegaly. Acta Neurochir (Wien). 2008;150(1):41-46; discussion 46-47. [DOI] [PubMed] [Google Scholar]
- 6. Inamasu J, Nakamura Y, Saito R et al. Postoperative communicating hydrocephalus in patients with supratentorial malignant glioma. Clin Neurol Neurosurg. 2003;106(1):9-15. [DOI] [PubMed] [Google Scholar]
- 7. Sandhya G, Reddy PB, Kumar KA, Sridhar Reddy B, Prasad N, Kiran G. Surgical management of oro-antral communications using resorbable GTR membrane and FDMB sandwich technique: a clinical study. J Maxillofac Oral Surg. 2013;12(3): 254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marquardt G, Setzer M, Lang J, Seifert V. Delayed hydrocephalus after resection of supratentorial malignant gliomas. Acta Neurochir (Wien). 2002;144(3):227-231; discussion 231. [DOI] [PubMed] [Google Scholar]
- 10. Smith KA, Ashby LS, Gonzalez LF et al. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;(suppl 109):106-117. [DOI] [PubMed] [Google Scholar]
- 11. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Symptomatic cerebrospinal fluid dissemination of cerebral glioblastoma: computed tomographic findings in 11 cases. Neuroradiology. 1990;32(2):146-150. [DOI] [PubMed] [Google Scholar]
- 12. Ando S, Moritake K. Communicating hydrocephalus occurring in the postoperative course of glioblastoma multiforme. Nihon Geka Hokan. 1989;58(6):508-515. [PubMed] [Google Scholar]
- 13. Thiessen B, DeAngelis LM. Hydrocephalus in radiation leukoencephalopathy: results of ventriculoperitoneal shunting. Arch Neurol. 1998;55(5):705-710. [DOI] [PubMed] [Google Scholar]
- 14. Perrini P, Scollato A, Cioffi F, Mouchaty H, Conti R, Di Lorenzo N. Radiation leukoencephalopathy associated with moderate hydrocephalus: intracranial pressure monitoring and results of ventriculoperitoneal shunting. Neurol Sci. 2002;23 (5):237-241. [DOI] [PubMed] [Google Scholar]
- 15. Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648-657. [DOI] [PubMed] [Google Scholar]
- 16. Lacroix M, Abi-Said D, Fourney DR et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. [DOI] [PubMed] [Google Scholar]
- 17. Chaichana KL, Halthore AN, Parker SL et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection: clinical article. J Neurosurg. 2011;114(3):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1): 113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi Y, Nakada M, Tanaka S et al. Implication of 5-aminolevulinic acid fluorescence of the ventricular wall for postoperative communicating hydrocephalus associated with cerebrospinal fluid dissemination in patients with glioblastoma multiforme: a report of 7 cases. J Neurosurg. 2010;112(5):1015-1019. [DOI] [PubMed] [Google Scholar]
- 20. Silbergeld DL, Rostomily RC, Alvord EC Jr. The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10(2):179-185. [DOI] [PubMed] [Google Scholar]
- 21. van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol. 2009;27(18):2905-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]