Abstract
BACKGROUND
Recurrence rates for atypical and anaplastic meningiomas range between 9% and 50% after gross total resection and between 36% and 83% after subtotal resection. Optimal treatment of recurrent meningiomas exhibiting atypical/anaplastic histology is complicated because they are often refractory to both surgery and radiation.
OBJECTIVE
To evaluate clinical determinants of recurrence and treatment-specific outcomes in patients with recurrent meningiomas exhibiting atypical/anaplastic histology at our institution.
METHODS
A cohort study was conducted using clinical data of all patients treated for meningiomas with atypical/anaplastic histology at first recurrence between January 1985 and July 2014 at a tertiary cancer center. Predictors of second recurrence were analyzed using competing risks regression models.
RESULTS
Nine hundred eighteen patients with meningioma were screened, of whom 60 (55% female) had recurrent disease with atypical/anaplastic histology at a median age of 58.1 yr at diagnosis. The median follow-up from the time of first recurrence was 36.7 mo, with 32 (53%) patients alive at last follow-up. There was no effect of extent of resection at first recurrence on time to a subsequent recurrence. Inclusion of radiation as primary or adjuvant therapy at first recurrence reduced the risk of progression or subsequent recurrence compared to surgery alone (P = .07).
CONCLUSION
Treatment of recurrent meningiomas with atypical/anaplastic histology remains challenging. Our data, from one of the largest cohorts, suggest better tumor control with the addition of radiation and challenges the importance of extent of resection at first recurrence. A multicenter effort is needed to confirm these findings and propose treatment guidelines.
Keywords: Atypical meningioma, Anaplastic meningioma, Recurrent meningioma, Repeat surgery, Radiation therapy
ABBREVIATIONS
- cEBRT
conventional external beam radiation therapy
- CI
confidence interval
- EOR
extent of resection
- GTR
gross total resection
- HR
hazard ratio
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OS
overall survival
- PFS
progression-free survival
- RT
radiation therapy
- SRS
stereotactic radiosurgery
- STR
subtotal resection
Atypical (WHO grade II) and anaplastic (WHO grade III) meningiomas account for 20% to 35% of intracranial meningiomas.1-4 As in other meningioma subtypes, extent of resection (EOR) is a critical factor in establishing local control of treated primary lesions.5-15 Recurrence rates for WHO grade II/III meningiomas remain high, ranging between 9% and 50% after gross total resection (GTR)16-23 and 36% to 83% after subtotal resection (STR).18,24 Our group previously reported a 5-yr actuarial recurrence rate of 35.5% after GTR in 52 patients with atypical meningiomas.23
The roles of radiation therapy (RT) and stereotactic radiosurgery (SRS) have recently been explored for treating both atypical23,25-27 and incompletely resected meningiomas.28-36 Although adjuvant RT is increasingly being considered in the literature following primary resection for atypical histology,26,37-42 there remains significant heterogeneity between centers.43 A recent SEER practice review found 76% of patients with atypical and 34% with anaplastic meningiomas did not receive adjuvant RT following STR, with similar rates after GTR.14 Because many patients with atypical/anaplastic meningiomas receive some form of postoperative RT, repeat surgery for recurrence can be technically challenging due to scarring. Moreover, meningiomas in eloquent areas may be more prone to recurrence, making GTR difficult.44,45
These tumors may therefore be considered refractory to both surgery and radiation and represent an ongoing clinical challenge.43 Published experience with WHO grade II/III meningiomas is limited to small case series and is insufficient to develop meaningful treatment guidelines.46-53 Our objective was to use a retrospective cohort study design to analyze the clinical course of patients treated at a major cancer hospital for recurrent meningiomas displaying atypical/anaplastic histological features and to evaluate treatment-specific outcomes and clinical determinants of subsequent recurrence.
METHODS
Patient Selection
Approval for the study was obtained from the Institutional Review Board. Because existing, deidentified data were used, informed consent was not required. To identify eligible patients for this retrospective cohort study, a prospectively maintained, multidisciplinary clinical database was searched using ICD-9 codes. A total of 918 patients received treatment for intracranial meningioma between January 1985 and July 2014, of whom 81 patients had WHO grade II/III histology at first recurrence. From these 81 patients, 15 patients were excluded due to unavailable pathology (11 patients) or revised tumor grade after applying current WHO (2007) criteria to pathological specimens (4 patients were downgraded from WHO grade II to WHO grade I). Six patients were lost to follow-up after treatment for recurrent disease and were excluded. In total, 60 patients were included in the final analysis.
Data Collection
Clinical, radiographic, and pathological data were collected from electronic medical records. Age, sex, age at first diagnosis, number of recurrences, time to each recurrence, salvage therapy (surgery, radiation, and/or chemotherapy) for each recurrence, and presence of other cancers were recorded. Tumor volumes were quantified using magnetic resonance imaging (MRI) images and the abc/2 relationship for an ellipsoid.54 Anatomic site was categorized as (1) convexity, (2) parasagittal/falcine, (3) sphenoid wing, (4) midline anterior fossa, (5) posterior fossa, or (6) other.37 Recurrence was defined as a new contrast-enhancing nodule within the prior resection cavity or 95% isodose line or progression of tumor remnant in patients having undergone STR. Cause of death was collected from clinical notes or vital statistics records. Pathology reports were reviewed to collect WHO grade and the presence of spontaneous tumor necrosis and bone or brain invasion. Spontaneous necrosis was differentiated from embolization necrosis based on histological grouping pattern of necrotic cells and location within the tumor. Pathological specimens of patients diagnosed before 2000 were re-reviewed by an experienced neuropathologist (MR) and graded using current WHO criteria.1
Treatment Data
Operative reports and postoperative MRI were used to classify EOR as GTR (equivalent to Simpson grade I-II) or STR (equivalent to Simpson grade III-IV). Operative reports were analyzed and surgery was classified as GTR if no residual tumor was noted, or STR if residual tumor was noted in the operative bed. Operative reports were then correlated to postoperative imaging. RT intent (salvage, postoperative, or definitive), type (conventional, SRS, hypofractionated or brachytherapy), radiation dose, fractions, and duration were collected for each recurrence. Chemotherapy type and duration were also recorded. Treatment response was determined according to Response Assessment in Neuro-Oncology criteria.
Statistical Analysis
Descriptive statistics including frequencies, medians, and interquartile ranges (IQRs) were used for patient, tumor, and treatment characteristics. The primary endpoint was progression/recurrence after treatment of first recurrence. Univariate competing risk regression models were generated for second recurrence/progression including the following variables: treatment modality (surgery, surgery plus RT, or RT alone), age, sex, surgical EOR, and pathology characteristics.55 Death from unrelated cause was considered a competing risk. Time-dependent treatment variables were employed to mitigate bias from variation of treatment initiation with respect to time of first recurrence. Time was calculated from first recurrence to second recurrence/progression, death, or last follow-up, whichever occurred first.
Univariate Cox proportional hazards models were used to analyze predictors of overall survival(OS). Treatment variables were time-dependent. P values were 2-sided, with P < .05 considered significant. Statistical analyses and plots were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina), Stata version 13 (StataCorp, College Station, Texas), and R version 3.2.0 (the R Foundation for Statistical Computing).
RESULTS
Participants
Sixty patients were treated for WHO grade II/III meningioma recurrence at our institution between 1985 and 2014. Twenty-seven patients (45%) were male, the median age was 58.1 yr (IQR 50.7-64.0) at first diagnosis, and 13 (22%) had other prior cancers (Table 1). Patients with prior cancers were in remission at the time of meningioma recurrence, and no patients had a clinically documented immunodeficiency or germline neoplastic syndrome.
TABLE 1.
Demographics and Primary Tumor Characteristics
| Number of patients (n) | 60 |
|---|---|
| Male/female ratio | 27/33 |
| Median age at initial diagnosis, years (interquartile range, IQR) | 58.1 (50.7-64.0) |
| Median primary tumor volume, cm3 (IQR) | 27 (14.1-45.0) |
| Lesion site, no. of patients (% of total) | |
| Convexity | 27/60 (45%) |
| Parasagittal | 17 (28) |
| Sphenoid | 7 (12) |
| Other/unknown | 9 (15) |
| Primary tumor histological (WHO) grade, no. (%) | |
| I (benign) | 7/60 (12%) |
| II (atypical) | 45 (75) |
| III (anaplastic/malignant) | 8 (13) |
| Primary tumor treatment modality, no. (%) | |
| Surgery alone | 39/60 (65%) |
| Surgery + radiation therapy (RT) | 20 (33) |
| Surgery + RT + chemotherapy | 1 (2) |
| Extent of resection (EOR) at initial surgery, no. (%) | |
| GTR (gross total resection) | 34/60 (57%) |
| STR (subtotal resection) | 21 (35) |
| Unknown | 5 (8) |
The median number of recurrences was 2 (range 1-5), and patients underwent a median of 2 surgeries (range 1-4) for recurrent disease. Median follow-up from diagnosis was 70.7 mo (IQR 46.2-119.9) and that from first recurrence was 36.7 mo (IQR 17.7-61.5). Thirty-two (54%) patients were alive at last follow-up.
Tumor Anatomy and Pathology
Of 60 tumors, 29 (48%) were located at the convexity, 15 (25%) parasagittal, 7 (12%) sphenoid wing, 1 (1.6%) posterior fossa, 1 (1.6%) was multicompartmental, and 7 had unknown location (Table 1). Median tumor volume was 27 cm3 (IQR 14-45) for primary tumor and 9 cm3 (IQR 5-33.1) for first recurrence (Tables 1 and 2).
TABLE 2.
First Recurrence—Diagnosis and Treatment
| Median time to first recurrence, months (IQR) | 25.6 (14.6-41.1) |
|---|---|
| Median tumor volume at first recurrence, cm3 (IQR) | 9 (5.0-33.1) |
| First recurrence histological (WHO) grade (available for 40 out of 60 patients)a | |
| I (benign), no. of patients (% of 40 pts. with histology) | 0/40 (0%) |
| II (atypical) | 34 (86%) |
| III (anaplastic/malignant) | 6 (14%) |
| Progression of WHO grade from primary tumor → first recurrence | |
| Grade I → grade II, no. (% of 40 pts. with histology) | 7/40 (18%) |
| Grade II → grade III | 1 (3) |
| Treatment modality for first recurrenceb | |
| Surgery alone, no. (% of all patients) | 17/60 (28%) |
| Surgery + RT (18 cEBRT, 3 SRS, 3 Brachy)c | 23 (38) |
| RT alone (10 cEBRT, 7 SRS) | 17 (28) |
| Chemotherapy alone | 2 (3) |
| Observation and serial imaging | 1 (2) |
| Extent of resection (EOR) at surgery for first recurrence (40 patients received surgery) | |
| GTR (gross total resection) | 18/40 (45%) |
| STR (subtotal resection) | 20 (50%) |
| Unknown | 2 (5%) |
Twenty patients received noninvasive treatments for their first recurrence; hence, no tissue was obtained for histological diagnosis.
aHistological grade based on WHO 2007 criteria.
bSeven patients (12%) had adjuvant chemotherapy in addition to surgery and/or RT.
cEBRT = conventional External Beam Radiation Therapy, SRS = Stereotactic Radiosurgery, Brachy = Implanted Brachytherapy (125I).
Of 60 primary lesions, 7 (12%) were WHO grade I, 45 (75%) WHO grade II, and 8 (13%) WHO grade III. Of these, 6 (10%) had bone invasion, 19 (32%) had brain invasion, and 33 (55%) had spontaneous necrosis. Forty (67%) patients underwent reoperation for first recurrence and had histopathological data available; 34/40 (85%) recurred as grade II and 6/40 (15%) recurred as grade III (Tables 2 and 3). This includes 7 patients with grade I primary lesions that recurred as grade II. Another patient with grade II primary progressed to grade III at first recurrence. Of 8 lesions exhibiting histological progression between primary lesion and first recurrence, 4 received surgery alone for their primary tumor and 4 received surgery with adjuvant radiation. At first recurrence, bone invasion was present in 8 (20%), brain invasion in 16 (40%), and spontaneous necrosis in 17 (43%) of 40 surgical specimens.
TABLE 3.
Patient and Histopathological Factors—Competing Risk of Recurrence/Progression and All-Cause Death After First Recurrence
| Variable | Competing risk hazard ratio (HR) for recurrence or Progression | Hazard ratio (HR) for death from any cause |
|---|---|---|
| Extent of resection (EOR) for first recurrence (n = 40 patients) | ||
| GTR (gross total resection) | Ref | Ref |
| STR (subtotal resection), HR (95% CI, P-value) | 1.10 (0.44-3.20, P = .74) | 1.53 (0.68-3.45, P = .31) |
| Age | 0.99 (0.97-1.02, P = .65) | 1.05 (1.01-1.09, P = .02*) |
| Gender | ||
| Female | Ref | Ref |
| Male | 1.32 (0.73-2.41, P = .36) | 2.47 (1.12-5.46, P = .03*) |
| Brain invasion (histology at first recurrencea) | ||
| Absent | Ref | Ref |
| Present | 1.28 (0.62-2.65, P = .50) | 1.76 (0.61-5.13, P = .30) |
| Necrosis (histology at first recurrencea) | ||
| Absent | Ref | Ref |
| Present | 1.28 (0.62-2.65, P = .50) | 1.76 (0.61-5.13, P = .30) |
aAvailable for 38 patients; 20 patients received noninvasive therapy and had no histology, 2 patients had histology reports that did not note presence or absence of invasion, necrosis.
Treatment
For primary tumors, 39 (65%) patients underwent surgery alone, 20 (33%) had surgery plus RT, and 1 (2%) had surgery with concomitant bevacizumab-based chemoradiation. Thirty-four (57%) patients had GTR and 21 (35%) had STR, with no EOR data for 5 (8%) patients. Of 21 patients receiving RT, 19 (90%) received conventional external beam RT (cEBRT, median dose 5940 cGy [IQR 5400-5940] in 30 fractions [IQR 30-33]), while 2 (10%) received SRS (median dose 1600 [IQR 1400-1800] in a single fraction [IQR 1-3]).
Of 60 patients treated for first recurrence, 17 (28%) had surgery alone, 23 (38%) had surgery with adjuvant RT, 17 (28%) had RT alone, and 1 (2%) patient was observed with serial imaging (Table 2). Seven (12%) patients received chemotherapy at first recurrence, either alone (n = 2) or in combination with other modalities (n = 5). Of those undergoing surgery, 18 (45%) had GTR and 20 (50%) had STR, with EOR data unknown in 2 (5%) patients (Table 2). The proportion of patients receiving definitive therapy with surgery and/or radiation decreased with each successive recurrence, from 57/60 (95%) at first recurrence to 9/23 (39%) at third recurrence (Table 3).
Forty (67%) patients received RT alone or in combination with surgery or chemotherapy at first recurrence. Of these, 26 (65%) received cEBRT (median dose 5400 cGy [IQR 2450-5770] in 28.5 fractions [IQR 1-30]), 10 (25%) had SRS (median dose 1650 cGy [IQR 1400-2400]) in a single fraction, 2 (5%) had brachytherapy, and 2 (5%) were treated with hypofractionated RT (median dose 2750 cGy [IQR 2500-3000]) in 5 fractions. Of patients receiving RT at recurrence, 7 (18%) patients had cEBRT after primary resection.
Outcomes
A majority of patients, 47/60 (78%), developed disease recurrence/progression after treatment for first recurrence (Figure 1). Four (7%) patients had stable disease, 8 (13%) died before disease progression or recurrence, and 1 (1.6%) was lost to follow-up. Median time to first recurrence was 25.6 mo (IQR 14.6-41.1) after treatment, 10.7 mo (IQR 4.5-36.6) for second, 11.1 mo (IQR 4.9-17.8) for third, 9.5 mo (IQR 6.4-18.0) for fourth, and 2.9 mo for the fifth recurrence. The probability of a second recurrence was 49.9% (95% confidence interval [CI]: 36.8-62.9) at 2 yr after treatment for first recurrence (Figure 2A). The probability of a third recurrence increased to 70% (95% CI: 54.3-86.4) at 2 yr after treatment of second recurrence.
FIGURE 1.
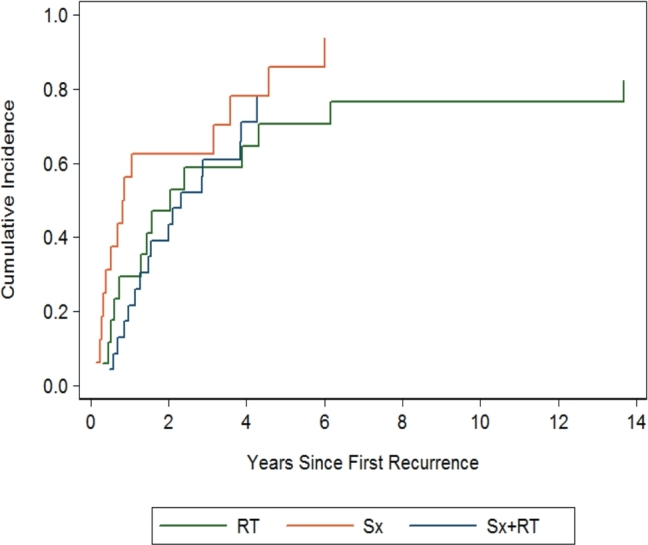
Treatment-dependent cumulative incidence of recurrence/progression after first recurrence. Patients who were treated with surgery alone were twice as likely to experience a second progression or recurrence compared to those who either had RT alone or surgery with RT (HR 2.13, 95% CI 0.94-4.83, P = 0.07).
FIGURE 2.
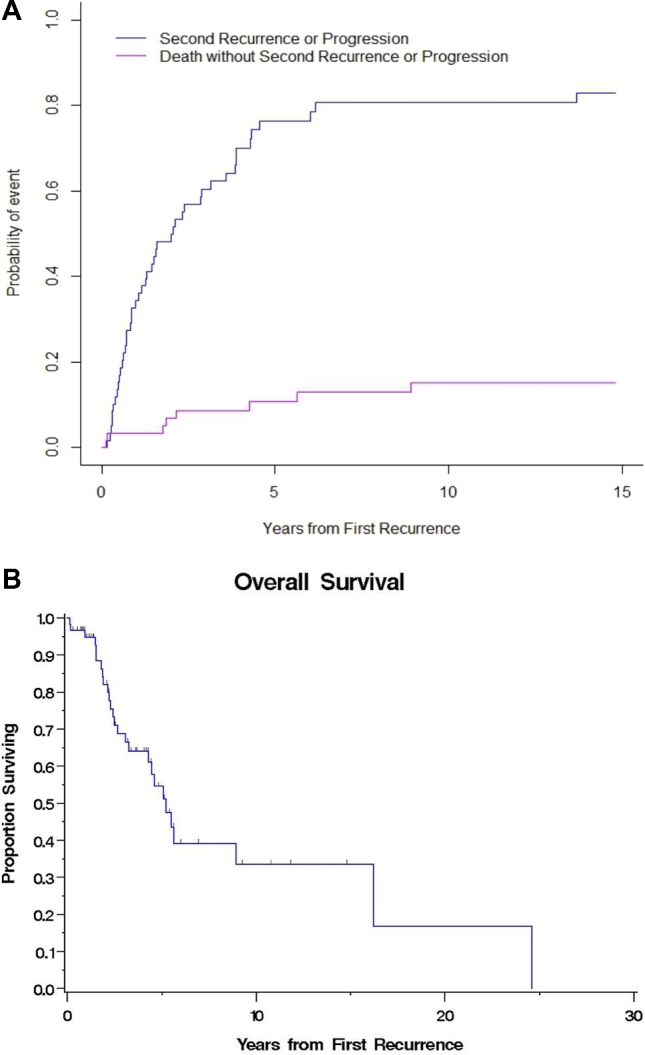
Subsequent recurrence and OS after first recurrence. A, Competing risks regression model for recurrence/progression after first recurrence. Death was considered a competing risk. Half the patients were likely to experience a second recurrence within 2 yr of being treated for a first recurrence. B, Kaplan–Meier estimates of OS after first recurrence. OS decreased from 5.2 yr after first recurrence to 3.7 yr after a second recurrence.
The median OS after first recurrence was 5.2 yr (95% CI: 3.3-16.2). The Kaplan–Meier 2- and 5-yr OS after first recurrences were 82.1% (95% CI: 71.4-92.8) and 54.6% (95% CI: 39.2-70; Figure 2B). After a second recurrence, 2- and 5-yr OS dropped to 60% and 30%, respectively.
Follow-up
At the conclusion of the study period, 28 (47%) patients were deceased; 11 (39%) succumbed to disease progression, while 17 (61%) died of unrelated or unknown causes. Of those alive at last follow-up, 20 (63%) patients had disease progression while 12 (37%) had stable disease.
Clinical Predictors of Second Recurrence and Progression
Tables 4 and 5 summarize the findings for predictors of progression or second recurrence. Though not reaching significance, there was a trend toward a higher likelihood of a second recurrence or progression when comparing surgery alone to surgery with RT (hazard ratio [HR] 2.13, 95% CI: 0.94-4.83, P = .07, Figure 1) and surgery alone to RT alone (HR 2.01, 95% CI: 0.87-4.62, P = .10). Surgery with RT carried a similar risk of second recurrence or progression as RT alone (HR 0.94, 95% CI: 0.48-1.84, P = .86).
TABLE 4.
Treatment Factors—Competing Risk of Recurrence/Progression and All-Cause Death After First Recurrence
| Competing risk hazard ratio (HR) for recurrence or progression | |||
|---|---|---|---|
| Treatment modality | vs Surgery alone | vs RT alone | vs Surgery + RT |
| Surgery alone, HR (95% CI, P-value) | Ref | 2.01 (0.87-4.62, P = .10a) | 2.13 (0.94-4.83, P = .07a) |
| Radiation therapy (RT) alone | 0.50 (0.22-1.15, P = .10a) | Ref | 1.06 (0.54-2.07, P = .86) |
| Surgery + RT | 0.47 (0.22-1.07, P = .07a) | 0.94 (0.48-1.84, P = .86) | Ref |
| Hazard Ratio (HR) for Death from any cause | |||
|---|---|---|---|
| Treatment modality | vs Surgery alone | vs RT alone | vs Surgery + RT |
| Surgery alone, HR (95% CI, P-value) | Ref | 0.89 (0.23-3.42, P = .87) | 0.49 (0.13-1.82, P = .29) |
| Radiation therapy (RT) alone | 1.12 (0.29-4.31, P = .87) | Ref | 0.55 (0.23-1.35, P = .19) |
| Surgery + RT | 2.03 (0.55-7.55, P = .29) | 1.81 (0.74-4.44, P = .19) | Ref |
aTrend towards significance.
TABLE 5.
Treatment of Successive Recurrences
| Recurrence | No. of Pts. | None | Surgerya | Surgery + RTa | RTa | Chemo | Adjuvant Chemo |
|---|---|---|---|---|---|---|---|
| First | n = 60 | 1 (2%) | 17 (28%) | 23 (38%) | 17 (28%) | 2 (3%) | 7 (12%) |
| Second | n = 43 | 8 (19%) | 11 (26%) | 11 (26%) | 11 (26%) | 2 (5%) | 10 (23%) |
| Third | n = 23 | 8 (36%) | 4 (17%) | 1 (5%) | 4 (17%) | 6 (27%) | 9 (39%) |
| Fourth | n = 7 | 3 (43%) | 1 (14%) | 0 | 2 (29%) | 1 (14%) | 1 (14%) |
| Fifth | n = 1 | 1 (100%) | 0 | 0 | 0 | 0 | 0 |
RT: radiation therapy.
aIncludes patients that received chemotherapy in combination.
“Adjuvant Chemo” category denotes patients who had chemotherapy in combination with any other treatment modalities (ie, Surgery, Surgery + RT, or RT)
None: Patients were observed with serial imaging and did not receive any treatment for the recurrence.
Increased EOR (GTR vs STR, HR 1.19, 95% CI: 0.44-3.20, P = .74) did not reduce risk of second recurrence or progression after surgery for first recurrence. Age (HR 0.99, 95% CI: 0.97-1.02, P = .65), gender (HR 1.32, 95% CI: 0.73-2.41, P = .36), brain invasion (HR 1.28, 95% CI: 0.62-2.65, P = .50), and spontaneous necrosis at first recurrence (HR 1.06, 95% CI: 0.52-2.14, P = .88) did not confer a higher risk of subsequent recurrence/progression.
Clinical Predictors of OS and Death
Treatment modality did not impact OS (HR 2.03, 95% CI: 0.55-7.55, P = .29; Tables 4 and 5). Age (HR 1.05, 95% CI: 1.01-1.09, P = .02) and male gender (HR 2.47, 95% CI: 1.12-5.46, P = .03) were significant predictors of overall mortality after first recurrence. EOR (STR vs GTR, HR 1.53, 95% CI: 0.68-3.45, P = .31), presence of brain invasion (HR 1.76, 95% CI: 0.61-5.13, P = .30), and necrosis on pathology (HR 0.52, 95% CI: 0.17-1.53, P = .23) did not predict OS.
DISCUSSION
Key Findings
No clinical benefit was derived from increased EOR at treatment for first recurrence, suggesting local control of these tumors may depend on factors other than aggressive surgical removal alone. This contradicts a recent single-center report retrospectively examining the prognosis and treatment response of 41 meningiomas with atypical histology at recurrence that concluded GTR (defined as Simpson grade I or II) conveyed a significant benefit in both progression-free survival (PFS) and OS compared to STR (Simpson grade III or IV).56 We found no significant difference between GTR and STR groups in time to second recurrence/progression (P = .74) or OS (P = .31).
Our study highlights the inherent difficulties of a single-center approach in answering questions regarding the benefits, or lack thereof, of RT and aggressive EOR in recurrent meningiomas with atypical/anaplastic characteristics. There was an observed trend towards reduced risk of progression or subsequent recurrence with RT at first recurrence compared to surgery alone. However, this finding failed to reach statistical significance despite a larger sample size than any prior studies of this patient population in the literature.
Interpretation
Benefit from RT in the treatment of recurrent meningioma has been suggested by recently published reports. Buglione et al.57 described 37 patients with recurrent meningiomas (including 10 atypical and 5 anaplastic) treated with cEBRT (mean dose 60 Gy) and found a trend toward better OS and local recurrence (LC) rates in patients treated with RT at first recurrence compared to RT at the second (or later) recurrence. Dzuik et al.58 concluded adjuvant RT improved 2-yr PFS from 50% to 89% in 23 anaplastic meningioma patients with recurrent disease, without affecting 5-yr PFS. Similarly, Taylor et al.59 described a 10-yr actuarial LC rate of 30% in re-resected recurrent meningiomas compared to 89% with adjuvant RT after salvage surgery. Kokubo et al.60 described 20 patients with recurrent meningiomas (4 atypical and 6 anaplastic) receiving RT after salvage surgery and reported 5- and 8-yr LC rates of 30% and 0% for atypical and anaplastic tumors with 5- and 8-yr OS of 50% and 0%. Li et al.48 recommended aggressive surgery including dura and bone flap removal followed by RT in their series of 15 patients with recurrent atypical meningiomas. Based on these data, salvage surgery plus RT is better than surgery alone and RT should be given at the time of first recurrence, rather than at second or later recurrences.36,57,59 In the context of this evidence, current data suggest that patients receiving surgery alone at first recurrence may be twice as likely to have a second recurrence or progression compared with RT alone or surgery plus RT. Because the difference did not reach significance in our study, however, the possibility remains that RT does not provide clinical benefit. Although surgery plus RT and RT alone subgroups carried a similar risk for a second recurrence, surgery is often necessary to relieve swelling and mass effect.
Chemotherapy is generally ineffective for recurrent high-grade meningiomas.49,61 A recent prospective phase II study of imatinib in radiation- and surgery-refractory recurrent atypical meningiomas was prematurely terminated due to lack of accrual and efficacy, and Kaley et al.61 recently published a comprehensive meta-analysis showing disappointing survival outcomes.62 Forty percent of our patients received chemotherapy at some point, but patient numbers prevented efficacy analysis.
Limitations
The major limitations of this study arise from the relative disease rarity, which necessitates inclusion criteria laxity for sufficient accrual and resultant heterogeneity in treatments and tumor biology. While this is the largest study to date regarding recurrences in atypical/anaplastic meningiomas, a retrospective study design carries risk of detection and selection biases. Additionally, our series spans several iterations of treatment and classification standards, complicating direct comparisons between groups.
Several changes in histological meningioma grading occurred during the period captured by this series. The major updates in the WHO 2000 classification system were (1) reduced relative importance of brain invasion in designating malignancy and (2) numerical rather than subjective categorization (eg, >4 mitoses/hpf vs “extensive mitoses”).4 Therefore, atypical and malignant lesions diagnosed before the year 2000 had pathological specimens re-reviewed in this study. As a tertiary and quaternary referral center, patients are frequently not diagnosed at our institution and several patients without specimens available for reinterpretation by our pathologist (MR) were excluded. The WHO 2007 changes were not deemed significant enough to warrant re-review of pathological specimens for patients diagnosed between 2000 and 2007. These changes increased the overall proportion of meningiomas qualifying for WHO grade II (“atypical”), primarily through the upgrade of tumors previously categorized as WHO grade I (“benign”).1,3,4 Such lesions “under-graded” prior to 2000 would not be captured in the current series, as their identification would require direct review of several decades of pathological specimens with marginal benefit. Similarly, patients may not have completed their entire follow-up at our institution and had more recurrences than those captured by our database. For this reason, we focused on risk factors for second recurrence rather than total number of recurrences.
Generalizability
Recurrent atypical/anaplastic meningiomas are challenging to treat, and management options include chemotherapeutic agents,47,49,50,52,53,62-66 surgical resection,46,48,67 conventional radiation,57,60,68 and SRS.51,69 National Comprehensive Cancer Network guidelines recommend surgical treatment when feasible followed by radiation to the tumor bed, but the evidence level for this practice is limited.46-53,59 While methodological limitations necessitate appropriate caution when clinically applying the results of the current study, they are typical for studies investigating WHO grade II/III meningioma recurrence, and the heterogeneity of the study population mirrors clinical practice at major cancer hospitals.48,51,56,68,70,71
The larger benefit of this study is hypothesis generation for future prospective, multicenter trials. Firmly establishing the benefit, if any, derived from addition of RT and increased EOR in managing these recurrent tumors is important because of the morbidity of these treatments and likelihood of numerous successive interventions in this population.
CONCLUSION
Recurrence rates in atypical/anaplastic meningiomas remain high. Multiple single-center studies have failed to definitively establish the relative efficacy of different treatment strategies in this patient population. In the current study, increased EOR at first recurrence did not affect time to recurrence or overall mortality in patients with meningioma recurrence with atypical/anaplastic histology. RT may improve local control and should be considered in the treatment plan, though the effect was no more than a trend (P = .07). Further prospective, multicenter studies are needed to investigate the role of RT and EOR at first recurrence in this population, as it is unlikely any single center will accrue case numbers sufficient to generate class I/II evidence.
Disclosures
Statistical support for this study was provided by MSK Institutional Core Grant (P30 CA008748). The authors have no personal, financial, orinstitutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgment
The authors would like to thank Shahiba Q. Ogilvie, MPh (Translational Research and Operations Manager, Brain Tumor Center and Department of Neurosurgery, Memorial Sloan Kettering Cancer Center) for her assistance in IRB submission and data acquisition.
Notes
This study was presented, in part, at the Annual Meeting of the Congress of Neurological Surgeons in New Orleans, Louisiana, September 26 to 30, 2015.
COMMENTS
This retrospective review of patients with recurrent atypical and anaplastic meningiomas evaluates the important outcomes with surgery, combining surgery with adjuvant therapy and primary radiotherapy in patients with recurrent Type II and Type III meningiomas.
Although there have been several smaller studies, this particular series is one of the larger ones reported to date, and provides a thorough examination of clinical outcomes and determinants. The obvious limitation is that of changes to the WHO grading of meningiomas, which may limit interpretation of the results.
The findings, although not significantly correlated, are provocative with respect to the current literature. Larger, multi-institutional collaborative efforts may be necessary to fully elucidate outcomes of the aforementioned strategies.
The authors here suggest “the importance of adjuvant RT in the treatment of recurrent meningiomas” despite non-significant statistical results. It appears that this may reflect a changing paradigm that may alter clinical practice of neurosurgery in the future with respect to atypical and anaplastic meningiomas.
Isaac Yang
Los Angeles, California
This is a retrospective analysis of a single institution series of 60 recurrent meningiomas with WHO grades II or III. The authors looked for prognostic variables as to outcome after first recurrence using univariate statistics and analyzing for factors like extent of resection, adjuvant radiotherapy, sex, location, etc. The authors could not show any significant effect of extent of resection or of adjuvant radiotherapy though they claim an effect, but their P value is .07. Extent of resection is estimated from operative reports and MRIs without employing standard volumetry.
Radiation was applied at different time points using different radiotherapy fractionations as well as stereotactic radiosurgery and brachytherapy, further complicating the analysis and rendering a useful assessment of these different techniques virtually impossible.
It took 29 years to collect these 60 patients illustrating the rarity of WHO II and III meningiomas at this institution. In a retrospective single institution series selection bias is obvious and without an appropriately powered multivariate analysis - which is difficult in 60 patients - the data are very hard to interpret. This represents at best class III evidence.
In summary, the paper demonstrates the limited value of retrospective reports from single institutions where patients are treated inconsistently – which the authors acknowledge. A prospective multicenter study and not a registry is the only way forward.
Peter C. Warnke
Chicago, Illinois
The authors have extracted data on WHO grade II and III meningiomas from nearly 3 decades of experience at their institution. They recognized the retrospective nature of the data and all the biases and limits this introduces and then analyzed the data available in an honest and conservative fashion. They were able to come up with a couple of conclusions including a suggestion that extent of resection at first recurrence does not affect time to next recurrence. They also found a trend toward benefit from the addition of radiation therapy in diminishing future tumor growth. The small number of grade III neoplasms did not allow for separate and meaningful analysis of this difficult histology. Overall the data here adds to the stack of case series on these 2 tumor types suggesting surgery and radiation are the treatment options in their management.
Jeffrey J. Olson
Atlanta, Georgia
REFERENCES
- 1. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK et al. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008;132(6):993-1007. [DOI] [PubMed] [Google Scholar]
- 2. Pearson BE, Markert JM, Fisher WS et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3. [DOI] [PubMed] [Google Scholar]
- 3. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99(3):393-405. [DOI] [PubMed] [Google Scholar]
- 4. Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31(2):141-149. [DOI] [PubMed] [Google Scholar]
- 5. Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58(1):51-56. [DOI] [PubMed] [Google Scholar]
- 6. Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26(5):461-469. [DOI] [PubMed] [Google Scholar]
- 7. Kallio M, Sankila R, Hakulinen T, Jaaskelainen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31(1):2-12. [DOI] [PubMed] [Google Scholar]
- 8. Mahmood A, Caccamo DV, Tomecek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33(6):955-963. [DOI] [PubMed] [Google Scholar]
- 9. Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir. 1994;126(2-4):53-58. [DOI] [PubMed] [Google Scholar]
- 10. Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18-24. [DOI] [PubMed] [Google Scholar]
- 11. Sankila R, Kallio M, Jaaskelainen J, Hakulinen T. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70(6):1568-1576. [DOI] [PubMed] [Google Scholar]
- 12. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046-2056. [DOI] [PubMed] [Google Scholar]
- 14. Aizer AA, Bi WL, Kandola MS et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015;121(24):4376-4381. [DOI] [PubMed] [Google Scholar]
- 15. Endo T, Narisawa A, Ali HS et al. A study of prognostic factors in 45 cases of atypical meningioma. Acta Neurochir. 2016;158(9):1661-1667. [DOI] [PubMed] [Google Scholar]
- 16. Hardesty DA, Wolf AB, Brachman DG et al. The impact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. J Neurosurg. 2013;119(2):475-481. [DOI] [PubMed] [Google Scholar]
- 17. Lee KD, DePowell JJ, Air EL, Dwivedi AK, Kendler A, McPherson CM. Atypical meningiomas: is postoperative radiotherapy indicated? Neurosurg Focus. 2013;35(6):E15. [DOI] [PubMed] [Google Scholar]
- 18. Mair R, Morris K, Scott I, Carroll TA. Radiotherapy for atypical meningiomas. J Neurosurg. 2011;115(4):811-819. [DOI] [PubMed] [Google Scholar]
- 19. Park HJ, Kang HC, Kim IH et al. The role of adjuvant radiotherapy in atypical meningioma. J Neuro Oncol. 2013;115(2):241-247. [DOI] [PubMed] [Google Scholar]
- 20. Sun SQ, Kim AH, Cai C et al. Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery. 2014;75(4):347-354. [DOI] [PubMed] [Google Scholar]
- 21. Vranic A, Popovic M, Cor A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery. 2010;67(4):1124-1132. [DOI] [PubMed] [Google Scholar]
- 22. Durand A, Labrousse F, Jouvet A et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neuro Oncol. 2009;95(3):367-375. [DOI] [PubMed] [Google Scholar]
- 23. Komotar RJ, Iorgulescu JB, Raper DM et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012;117(4):679-686. [DOI] [PubMed] [Google Scholar]
- 24. Choi CY, Soltys SG, Gibbs IC et al. Cyberknife stereotactic radiosurgery for treatment of atypical (WHO grade II) cranial meningiomas. Neurosurgery. 2010;67(5):1180-1188. [DOI] [PubMed] [Google Scholar]
- 25. Akeyson EW, McCutcheon IE. Management of benign and aggressive intracranial meningiomas. Oncology. 1996;10(5):747-756; discussion 756-749. [PubMed] [Google Scholar]
- 26. Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34(4):817-822. [DOI] [PubMed] [Google Scholar]
- 27. Wilson CB. Meningiomas: genetics, malignancy, and the role of radiation in induction and treatment. The richard C. schneider lecture. J Neurosurg. 1994;81(5):666-675. [DOI] [PubMed] [Google Scholar]
- 28. Abdelaziz OS, Kandil A, El-Assaal S, Abdelaziz A, Rostom Y, Rashed Y. Linear accelerator-based stereotactic radiosurgery of intracranial meningiomas: results of the first 5 years of clinical practice. Neurosurg Rev. 2011;34(1):87-99. [DOI] [PubMed] [Google Scholar]
- 29. Hakim R, Alexander E 3rd, Loeffler JS et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery. 1998;42(3):446-453; discussion 453-444. [DOI] [PubMed] [Google Scholar]
- 30. Kano H, Park KJ, Kondziolka D et al. Does prior microsurgery improve or worsen the outcomes of stereotactic radiosurgery for cavernous sinus meningiomas? Neurosurgery. 2013;73(3):401-410. [DOI] [PubMed] [Google Scholar]
- 31. dos Santos MA, de Salcedo JB, Gutierrez Diaz JA et al. Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2011;81(5):1436-1441. [DOI] [PubMed] [Google Scholar]
- 32. Shin M, Kurita H, Sasaki T et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95(3):435-439. [DOI] [PubMed] [Google Scholar]
- 33. Duma CM, Lunsford LD, Kondziolka D, Harsh GRt, Flickinger JC. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32(5):699-704; discussion 704-695. [DOI] [PubMed] [Google Scholar]
- 34. Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80(2):195-201. [DOI] [PubMed] [Google Scholar]
- 35. Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18(4):755-761. [DOI] [PubMed] [Google Scholar]
- 36. Miralbell R, Linggood RM, de la Monte S, Convery K, Munzenrider JE, Mirimanoff RO. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neuro Oncol. 1992;13(2):157-164. [DOI] [PubMed] [Google Scholar]
- 37. Aghi MK, Carter BS, Cosgrove GR et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56-60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 38. Hug EB, Devries A, Thornton AF et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neuro Oncol. 2000;48(2):151-160. [DOI] [PubMed] [Google Scholar]
- 39. Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809-816. [DOI] [PubMed] [Google Scholar]
- 40. Hoffmann W, Muhleisen H, Hess CF et al. Atypical and anaplastic meningiomas–does the new WHO-classification of brain tumours affect the indication for postoperative irradiation? Acta Neurochir. 1995;135(3-4):171-178. [DOI] [PubMed] [Google Scholar]
- 41. Pasquier D, Bijmolt S, Veninga T et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71(5):1388-1393. [DOI] [PubMed] [Google Scholar]
- 42. Rogers L, Barani I, Chamberlain M et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chamberlain MC. Is there effective systemic therapy for recurrent surgery- and radiation-refractory meningioma? CNS Oncol. 2013;2(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boker DK, Meurer H, Gullotta F. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci. 1985;29(1):11-17. [PubMed] [Google Scholar]
- 45. Yamashita J, Handa H, Iwaki K, Abe M. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14(1):33-40. [PubMed] [Google Scholar]
- 46. Feng R, Che X, Hu J, Pan L, Cui D, Yang L. Surgical treatment of recurrent torcular meningiomas: case report and review of the literature. J Neurolog Surg A Cent Eur Neurosurg. 2013;74(suppl 1):e266-e270. [DOI] [PubMed] [Google Scholar]
- 47. Horak P, Wohrer A, Hassler M, Hainfellner J, Preusser M, Marosi C. Imatinib mesylate treatment of recurrent meningiomas in preselected patients: a retrospective analysis. J Neuro Oncol. 2012;109(2):323-330. [DOI] [PubMed] [Google Scholar]
- 48. Li F, Lai ZP, Lin JK, Zhu G, Feng H. Radical treatment strategies improve the long-term outcome of recurrent atypical meningiomas. Chin Med J. 2011;124(15):2387-2391. [PubMed] [Google Scholar]
- 49. Chamberlain MC, Barnholtz-Sloan JS. Medical treatment of recurrent meningiomas. Expert Rev Neurother. 2011;11(10):1425-1432. [DOI] [PubMed] [Google Scholar]
- 50. Johnson MD, Sade B, Milano MT, Lee JH, Toms SA. New prospects for management and treatment of inoperable and recurrent skull base meningiomas. J Neuro Oncol. 2008;86(1):109-122. [DOI] [PubMed] [Google Scholar]
- 51. Mattozo CA, De Salles AA, Klement IA et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J Neurosurg. 2007;106(5):846-854. [DOI] [PubMed] [Google Scholar]
- 52. Rosenthal MA, Ashley DL, Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9(2):156-158. [DOI] [PubMed] [Google Scholar]
- 53. Kaba SE, DeMonte F, Bruner JM et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40(2):271-275. [DOI] [PubMed] [Google Scholar]
- 54. Kothari RU, Brott T, Broderick JP et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304-1305. [DOI] [PubMed] [Google Scholar]
- 55. Sun SQ, Cai C, Murphy RK et al. Management of atypical cranial meningiomas, part 2: predictors of progression and the role of adjuvant radiation after subtotal resection. Neurosurgery. 2014;75(4):356-363; discussion 363. [DOI] [PubMed] [Google Scholar]
- 56. Cao X, Hao S, Wu Z et al. Treatment response and prognosis after recurrence of atypical meningiomas. World Neurosurg. 2015;84(4):1014-1019. [DOI] [PubMed] [Google Scholar]
- 57. Buglione M, De Bari B, Trevisan F et al. Role of external beam radiotherapy in the treatment of relapsing meningioma. Med Oncol. 2014;31(3):866. [DOI] [PubMed] [Google Scholar]
- 58. Dziuk TW, Woo S, Butler EB et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neuro Oncol. 1998;37(2):177-188. [DOI] [PubMed] [Google Scholar]
- 59. Taylor BW Jr, Marcus RB Jr, Friedman WA, Ballinger WE Jr, Million RR. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15(2):299-304. [DOI] [PubMed] [Google Scholar]
- 60. Kokubo M, Shibamoto Y, Takahashi JA et al. Efficacy of conventional radiotherapy for recurrent meningioma. J Neuro Oncol. 2000;48(1):51-55. [DOI] [PubMed] [Google Scholar]
- 61. Kaley T, Barani I, Chamberlain M et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wen PY, Yung WK, Lamborn KR et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01–08). Neuro Oncol. 2009;11(6):853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson DR, Kimmel DW, Burch PA et al. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011;13(5):530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Norden AD, Raizer JJ, Abrey LE et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neuro Oncol. 2010;96(2):211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sioka C, Kyritsis AP. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J Neuro Oncol. 2009;92(1):1-6. [DOI] [PubMed] [Google Scholar]
- 66. Simo M, Argyriou AA, Macia M et al. Recurrent high-grade meningioma: a phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol. 2014;73(5):919-923. [DOI] [PubMed] [Google Scholar]
- 67. Ware ML, Larson DA, Sneed PK, Wara WW, McDermott MW. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54(1):55-63; discussion 63-54. [DOI] [PubMed] [Google Scholar]
- 68. Wojcieszynski AP, Ohri N, Andrews DW, Evans JJ, Dicker AP, Werner-Wasik M. Reirradiation of recurrent meningioma. J Clin Neurosci. 2012;19(9):1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pollock BE. Defining the best management for patients with intracranial world health organization grade II meningiomas. World Neurosurg. 2014;81(5-6):712-713. [DOI] [PubMed] [Google Scholar]
- 70. Simo M, Izquierdo C, Bruna J. Systemic treatment of recurrent meningioma. Eur Assoc Neurooncol Magazine. 2013;3(3):132-138. [Google Scholar]
- 71. El-Khatib M, El Majdoub F, Hoevels M et al. Stereotactic LINAC radiosurgery for incompletely resected or recurrent atypical and anaplastic meningiomas. Acta Neurochir. 2011;153(9):1761-1767. [DOI] [PubMed] [Google Scholar]


