Glioblastoma multiforme (GBM) is a highly malignant cancer due to its invasive nature and resistance to current therapies. The long-term prognosis for GBM patients remains poor, with median survival of less than 2 years despite the addition of recently approved treatments such as Avastin and tumor-treating electrical fields. Promising new therapeutic strategies are being explored, such as the exciting research area of using viruses in anticancer therapies.
For over 20 years, viruses have been studied in potential therapies for glioblastoma. Martuza et al1 first engineered herpes simplex virus (HSV) to selectively target GBM cells. Even though the engineered HSV variant lacked thymidylate kinase, some toxicity and pathogenicity to adjacent neural tissue were still observed. Much effort has continued in refining the selectivity of viral therapies for GBM cells while sparing normal brain tissue by advances in understanding tumor biology and its microenvironment, and by creating new viral vectors.
A recent exciting advance in GBM virotherapy is reported by Zhu et al2 that the virus implicated in the 2015 microcephaly outbreak, Zika virus (ZIKV), has oncolytic activity against glioblastoma stem cells (GSCs). The aggressive nature of glioblastoma is now thought to be due to a fraction of self-renewing tumor precursor cells called GSCs. Targeting these cells is key to developing new treatments that prevent local recurrence while reducing adjacent neural damage. Since ZIKV tends to infect and destroy CNS precursor cells, researchers hypothesized that the virus would preferentially target GSCs rather than differentiated tumor cells and normal neuronal cells. This selective ZIKV targeting of glioblastoma might spare normal adult brain tissue.
In this study, patient-derived GSCs were infected with ZIKV and caused significant loss of GSC self-renewal, decreased proliferation, and increased apoptosis when compared with differentiated glioma cells after 7 days (measured by immunofluorescence microscopy). To confirm that ZIKV is specific for GSCs, both in vitro human glioblastoma organoid model and human GBM surgical specimens were infected with ZIKV, with observation via immunofluorescence analysis showing ZIKV preferentially infected cells that expressed the GSC marker SOX2. As shown before, decreases in proliferation and increased apoptosis were noted in GSCs vs differentiated glioma cells.
To follow-up the promising in vitro results, mouse models of high-grade glioma were treated via direct intratumoral injection of a mouse-adapted ZIKV strain. Histological examination demonstrated that ZIKV-treated tumors were significantly smaller in size compared with control saline-treated tumors. Notably, ZIKV therapy successfully extended the survival of tumor-bearing mice in a dose-dependent fashion with increasing viral inoculum (Figure). The ZIKV specificity of this response was also observed by SOX2+ expression in the majority of ZIKV-infected cells. Further studies are needed to specifically examine ZIKV oncolytic effects on in vivo tumor xenografts generated from patient-derived GSC lines.
Figure.
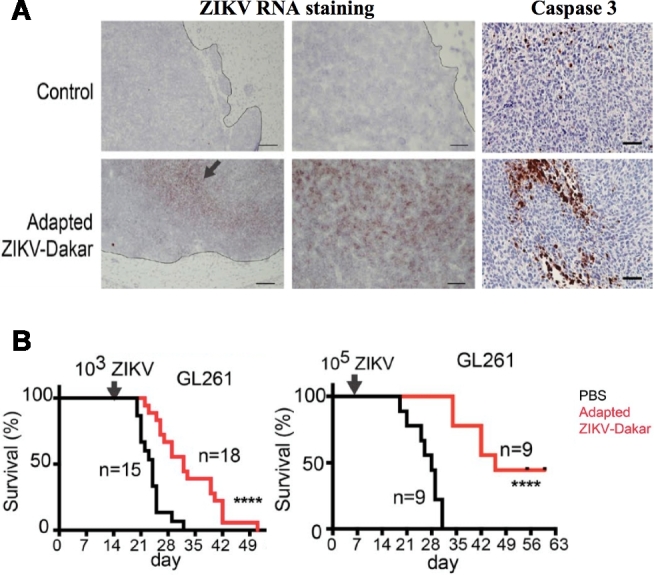
Mouse-adapted ZIKV-Dakar attenuates growth of mouse glioma cells and prolongs survival of mice with glioma in vivo. A, Representative low- and high-power images of in situ hybridization staining for viral RNA in mice with CT2A glioma 2 wk after treatment with ZIKV-Dakar or PBS (representative of 2 experiments). Arrow indicates positive staining. Representative high-power images of cleaved caspase-3 staining on the same tumors indicate increased apoptosis in oncolytic treatment group. B, Mice bearing GL261 glioma were treated with phosphate buffer solution (PBS) control, 103 FFU, or 105 FFU of the ZIKV-Dakar mouse-adapted strain. There is a dose-dependent increase in survival with increasing viral inoculum. Reprinted with permission from Rockefeller University Press from Zhu et al; permission conveyed through Copyright Clearance Center, Inc.2
Although further studies are needed to evaluate the safety of ZIKV before it can be translated for GBM therapy, this promising addition to the oncolytic GBM virotherapy armamentarium could increase treatment options for this devastating disease. Shown to be practical and feasible in ongoing clinical trials of analogous GBM therapies with other viral vectors,3,4 modified ZIKV viral vectors could be injected into surgical margins to target invasive and residual GSCs to prevent local recurrence. This is a promising direction that takes advantage of the natural predilection of ZIKV to target precursor cells, and builds on the work of many others who have bioengineered and adapted viruses for anti-GBM therapies.
REFERENCES
- 1. Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854-856. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Z, Gorman MJ, McKenzie LD et al. . Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med. 2017;214(10):2843-2857. doi:10.1084/jem.20171093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cloughesy TF, Landolfi J, Hogan DJ et al. . Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowenstein PR, Castro MG. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of malignant brain tumors: from mice to clinical trials for glioma patients. Clin Immunol. 2017;7 doi:10.1016/j.clim.2017.07.006 [Corrected proof, Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


