Abstract
BACKGROUND
Minimally invasive thrombolytic evacuation of intracerebral hematoma is being investigated in the ongoing phase III clinical trial of Minimally Invasive Surgery plus recombinant Tissue plasminogen activator for Intracerebral hemorrhage Evacuation (MISTIE III).
OBJECTIVE
To assess the accuracy of catheter placement and efficacy of hematoma evacuation in relation to surgical approach and surgeon experience.
METHODS
We performed a trial midpoint interim assessment of 123 cases that underwent the surgical procedure. Accuracy of catheter placement was prospectively assessed by the trial Surgical Center based on prearticulated criteria. Hematoma evacuation efficacy was evaluated based on absolute volume reduction, percentage hematoma evacuation, and reaching the target end-of-treatment volume of <15 mL. One of 3 surgical trajectories was used: anterior (A), posterior (B), and lobar (C). Surgeons were classified based on experience with the MISTIE procedure as prequalified, qualified with probation, and fully qualified.
RESULTS
The average hematoma volume was 49.7 mL (range 20.0-124), and the mean evacuation rate was 71% (range 18.4%-99.8%). First placed catheters were 58% in good position, 28% suboptimal (but suitable to dose), and 14% poor (requiring repositioning). Posterior trajectory (B) was associated with significantly higher rates of poor placement (35%, P = .01). There was no significant difference in catheter placement accuracy among surgeons of varying experience. Hematoma evacuation efficacy was not significantly different among the 3 surgical approaches or different surgeons’ experience.
CONCLUSION
Ongoing surgical education and quality monitoring in MISTIE III have resulted in consistent rates of hematoma evacuation despite technical challenges with the surgical approaches and among surgeons of varying experience.
Keywords: Intracerebral hemorrhage, Minimally invasive, MISTIE, Tissue plasminogen activator, Alteplase
ABBREVIATIONS
- CT
computed tomography
- DICOM
Digital Imaging and Communications in Medicine
- ICH
intracerebral hemorrhage
- MISTIE
Minimally Invasive Surgery plus recombinant Tissue plasminogen activator for Intracerebral hemorrhage Evacuation
- rtPA
recombinant tissue plasminogen activator
Contemporary management of intracerebral hemorrhage (ICH) has evolved over time, with a resurging interest in surgical hematoma evacuation aimed at abating secondary insults after ICH. Nevertheless, most studies on surgery for ICH evacuation have been, at best, neutral, suggesting that the benefit of hematoma evacuation may be potentially neutralized by the requisite surgical trauma.1-4 This has driven investigators to design and test novel surgical techniques that can achieve hematoma evacuation while minimizing brain injury by avoidance of wide exposure, retraction, and manipulation.5,6 The minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation (MISTIE) technique addresses these goals using combined mechanical and pharmacological approaches. The technique involves a single pass image-guided cannulation of the hematoma, through which an initial 1-time aspiration is performed and a catheter is placed, followed by irrigation with serial doses of thrombolytic agent [recombinant tissue plasminogen activator (rtPA)] with ongoing passive drainage.7
Several studies have advocated the feasibility, safety, and efficacy of the novel technique culminating with the MISTIE phase II trial.7-9 The results confirmed safety and apparent outcome benefit among 96 subjects from 26 sites with stable, sizable ICH, randomized to minimally invasive surgery and rtPA vs best medical treatment.10 The phase II experience also demonstrated the importance of accurate catheter location in mediating clot evacuation efficacy and potential benefits of the technique. In contrast to thrombolysis in intraventricular hemorrhage, in which the thrombolytic agent is administered via a standard ventriculostomy catheter, the surgical technique in MISTIE could be rather challenging for neurosurgeons of varying experience levels, including the execution of different trajectories of targeting the ICH and direction of catheter to an entire “volume target” rather than a point target. The ongoing MISTIE III trial has incorporated protocols of surgeon education and mentoring guidance about the choice of different surgical trajectories for ICH in various locations, and quarterly ongoing assessment of catheter placement accuracy and the efficacy of the surgical task.5 With the ongoing phase III trial surpassing the midpoint of enrollments, we have assessed the impact of the different surgical trajectories and surgeon experience on actual surgical performance.
METHODS
Protocol, Patients, and Surgeon Experience
The inclusion/exclusion criteria and protocol of the MISTIE III trial (Clinicaltrials.gov NCT01827046), approved by each local ethics committee prior to site activation, have been published and reviewed elsewhere.6,11,12 MISTIE is a first-of-kind phase III, explanatory trial with a PROBE design.13 The trial was launched in January 2014 and is designed to enroll 500 subjects with 30 mL or greater supratentorial ICH after verified stability of clot volume, reversal of coagulopathy, and exclusion of underlying vascular etiology other than presumed hypertension or amyloid angiopathy. Enrolled subjects are randomized equally to receive the MISTIE surgical intervention or otherwise be managed per guidelines-driven best medical and critical care management. We performed a midpoint interim assessment of 125 subjects randomized to the surgical arm among the first 250 cases enrolled in the trial. We excluded from analysis 2 subjects who were randomized to the surgical arm but did not actually receive the intervention, one because of reconsideration of consent by the patient and investigator, and another because of rapid clinical deterioration and emergent open surgical evacuation. Our analysis therefore involved 123 subjects who underwent the MISTIE surgical task.
Surgeons were categorized based on their experience with the technique at the time of performance of the surgical task as (1) prequalified, (2) qualified with probation, and (3) fully qualified. Prequalified surgeons are fully trained neurosurgeons, privileged by their respective hospitals or local authority, who have performed at least 3 image-guided or stereotactic catheter aspiration or placement procedures outside MISTIE and completed the MISTIE surgical training modules. Surgeons are promoted to “qualified with probation” or “fully qualified” after successfully performing the MISTIE procedures on up to 3 or more than 3 cases, respectively. Prequalified surgeons are mentored by a fully qualified trial surgeon on site, or by telephone or internet, with the mentor reviewing the steps of the procedure and answering any technical questions that may arise.
Surgical Approaches and Catheter Placement Accuracy
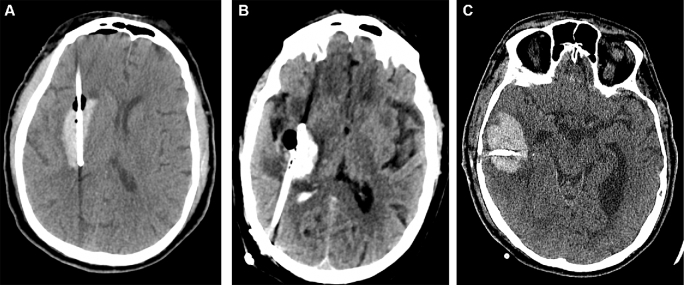
The MISTIE protocol specified 3 main surgical approaches for evacuating intracerebral hematomas of different anatomic location and morphological features.14 These are trajectory A (anterior), B (posterior), and C (lobar). Surgical approach A is used for a deep-seated ICH occupying the anterior basal ganglia and has an entry point at the forehead; the trajectory of the catheter must be along the longitudinal axis of the hematoma. Approach B is used for a deep-seated ICH occupying the posterior basal ganglia or thalamus and has an entry point in the parietal-occipital area, frequently several centimeters lateral from the midline to avoid the occipital ventricular horn; the trajectory of the catheter has to be along the longitudinal axis of the hematoma. Approach C is used for lobar ICH and has an entry point at the superficial area closest to the hematoma with a trajectory along the widest, or “equatorial,” axis of the clot (Figure 1).
FIGURE 1.
Surgical approaches in MISTIE III. A, Trajectory A is used when ICH epicenter is at the caudate, putamen, or anterior capsule, with an entry point at the forehead. B, Trajectory B is used when ICH epicenter is posterior capsular or thalamic, with an entry point in the posterior parietal-occipital area. C, Trajectory C is used for lobar ICH and has an entry point at the superficial area of the ICH closest to the cortical surface, with a trajectory along the widest, or “equatorial,” axis of the clot.
Catheter placement was assessed pragmatically in real time by the trial's Surgical Center, overseen personally by IAA or MZ (rotating on call). Catheter was deemed in a good position when placed along the entire longitudinal axis (defined as at least two-thirds of longitudinal length) of the hematoma with the fenestrated segment in the epicenter of the clot, circumferentially surrounded by the hematoma.10 Suboptimal placement was defined as a catheter over or undershooting the target position or in eccentric location, yet fully engaging the ICH and suitable for dosing. Poorly placed catheters were defined as not engaging the ICH (ie, catheter perforations not in contact with the clot), not allowing safe/effective dosing, hence requiring replacement. In cases in which catheter replacement was done, the initial catheter placement was used for accuracy assessment in our analyses.
Efficacy of Hematoma Evacuation
All determinations of ICH volume were centrally assessed by the trial Reading Center based on computed tomography (CT) scans uploaded electronically as Digital Imaging and Communications in Medicine (DICOM) images by the enrolling sites, and results were stored on the trial's database. Stability ICH volume was that assessed at least 6 h after a prior scan demonstrating no further expansion of ICH by 5 mL or greater. End-of-treatment ICH volume was assessed on the CT scan performed 1 d after removal of the hematoma catheter. Three parameters were used to assess efficacy of hematoma evacuation: (1) absolute volume reduction, representing the stability minus end-of-treatment ICH volume; (2) fraction of ICH evacuated, representing the ratio of absolute volume reduction to the stability volume; and (3) fraction of subjects reaching a target end-of-treatment volume of less than 15 mL.
Statistical Methods
Data was retrieved from the MISTIE III trial database (VISION EDC system, software provided by Prelude Dynamics Inc., Austin, Texas; study-specific implementation design developed by Emissary International LLC, Austin, Texas). The prevalence of cases with “poor” catheter placement was assessed in the whole cohort and in cases with different surgical approaches and surgeons’ experience. The 3 measures of ICH evacuation efficacy were evaluated in the whole cohort and in subgroups with different surgical trajectory and surgeon experience (see below). Individual and cumulative hematoma evacuation rates were plotted for the whole cohort to elucidate any improvement over time.
To assess placement accuracy in each surgical trajectory, a Pearson chi-square test was used to compare the proportion of cases with poor placement vs those with good and suboptimal placement, and these were used to calculate the corresponding relative risk of poor catheter placement per trajectory. Similar analysis was used to compare poor placement by prequalified surgeons, those qualified with probation, and fully qualified surgeons. We used Fisher's exact test to compare the rates of poor catheter placements between surgeon qualification groups in each of the 3 trajectories.
For the efficacy of ICH evacuation analysis, a general linear model was conducted, considering the unbalanced sample sizes to compare absolute reduction, fraction ICH evacuation, and fraction of subjects reaching a target end-of-treatment volume of less than 15 mL. Comparison was performed between trajectories A, B, and C and again between prequalified, qualified with probation, and fully qualified surgeons. SAS 9.4 (by SAS Institute Inc., Cary, North Carolina) was used to conduct the statistical analyses. A P value of .05 or smaller determined statistical significance.
RESULTS
Our trial midpoint interim analysis was based on 123 subjects enrolled in the MISTIE III trial, randomized to the surgical arm and receiving the surgical intervention. Average stability volume prior to randomization was 49.7 mL (20.0-124.1), and the average absolute hematoma volume reduction was 71% (range 18.4%-99.8%). Seventy-two percent of subjects achieved the target end-of-treatment volume of less than 15 mL by the end of the dosing protocol. Surgeons performing the procedure were prequalified in 56% of cases (n = 69), qualified with probation in 23% (n = 28), and fully qualified in 21% (n = 26). Surgical approaches A, B, and C were used in 58 (47%), 26 (21%), and 39 (32%) cases, respectively.
Placement Accuracy
Based on Surgical Center assessment of first placed catheters, 71 (58%) were in good position, 35 (28%) suboptimal, and 17 (14%) in poor position. Except for 2 cases in which dosing was omitted (sufficient hematoma was aspirated mechanically), all catheters deemed in poor position were replaced before dosing. Table 1 summarizes catheter placement accuracy per trajectory and surgeon experience levels. Trajectory C was the most accurate among surgical approaches, with the highest ratio of catheters in good position (67%) and a low incidence of poor placement (10%). Trajectory A, the most commonly utilized surgical approach, showed a comparable rate of good catheter placements (62%) and the lowest incidence of poor placements among the 3 approaches (7%; difference of respective rates of placement accuracy between approaches A and C not statistically significant). Trajectory B was associated with a significantly higher proportion of poor catheter placement compared to the other approaches (35% relative risk 2.5 confidence interval 1.26-4.99 P = .01).
TABLE 1.
Catheter Placement Accuracy in Different Surgical Approaches and Among Different Surgeon Experience Levels
| n | Good | Suboptimal | Poor | RR* | CI | P-value* | |
|---|---|---|---|---|---|---|---|
| Trajectory A | 58 | 36 (62) | 18 (31) | 4 (7) | 0.50 | 0.18-1.42 | .17 |
| Trajectory B | 26 | 9 (35) | 8 (31) | 9 (35) | 2.50 | 1.26-4.99 | .01 |
| Trajectory C | 39 | 26 (67) | 9 (23) | 4 (10) | 0.74 | 0.27-2.07 | .56 |
| Surgeon prequalified | 69 | 41 (59) | 16 (23) | 12 (17) | – | – | – |
| Surgeon qualified with probation | 28 | 14 (50) | 10 (36) | 4 (14) | 0.82 | 0.29-2.33 | .71 |
| Surgeon fully qualified | 26 | 16 (62) | 9 (35) | 1 (4) | 0.22 | 0.03-1.62 | .09 |
*Relative risk of poor catheter placement is calculated by comparing each trajectory to the whole group. For surgeons’ experience, fully qualified and qualified-with-probation surgeons were compared to prequalified surgeons as a reference.
CI, confidence interval; RR, relative risk.
When stratified based on surgeon experience, good catheter position on initial placement was achieved by 59% of prequalified surgeons, 50% of qualified-with-probation, and 62% of fully qualified surgeons (differences not statistically significant). The incidence of poor catheter placement requiring replacement trended inversely with surgeon experience, showing a 17%, 14%, and 4% rate for prequalified, qualified with probation, and fully-qualified surgeons, respectively, but this trend did not reach statistical significance (P = .09).
We then queried the impact of surgeon experience level on placement accuracy in each surgical approach individually. Fully qualified surgeons were found to demonstrate lower rates of poor catheter placement compared to prequalified surgeons; however, this did not reach statistical significance (0% vs 11.1% in trajectory A, 20% vs 54% in trajectory B, and 0% vs 10% in trajectory C).
Hematoma Evacuation
Table 2 summarizes the 3 measures of hematoma evacuation efficacy per trajectory and surgeon experience levels. Trajectory B had the highest hematoma evacuation efficacy based on mean absolute and percent volume reductions of 38.2 mL and 74%, respectively. The highest rate of achieving the target end-of-treatment volume <15 mL was seen in trajectory A (79.3%). These differences in hematoma evacuation efficacy among the different approaches were not statistically significant.
TABLE 2.
Efficacy of Hematoma Evacuation in Different Surgical Approaches and Among Different Surgeon Experience Levels
| Trajectory | Trajectory | Trajectory | Qualified with | Fully | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | P-value | Prequalified | probation | qualified | P-value | |
| n | 58 | 26 | 39 | – | 69 | 28 | 26 | – |
| Absolute reduction (mL) | 34.7 | 36.8 | 32.7 | .63 | 34.9 | 31.2 | 37.3 | .42 |
| Fraction evacuated (%) | 72.9 | 71.7 | 68.4 | .58 | 71.8 | 66.4 | 74.9 | .32 |
| % of subjects reaching <15 mL | 79.3 | 61.5 | 66.7 | .18 | 69.6 | 67.9 | 80.8 | .50 |
Fully qualified surgeons were the most efficient in hematoma evacuation, achieving largest mean absolute volume reduction (37.3 mL), largest mean relative reduction (74.9%), and target end-of-treatment volume of <15 mL (80.8%). Differences in hematoma evacuation efficacy among surgeons of different levels of experience were not statistically significant.
Overall measures of hematoma evacuation efficacy have been consistent during the course of the trial to date (Figure 2).
FIGURE 2.
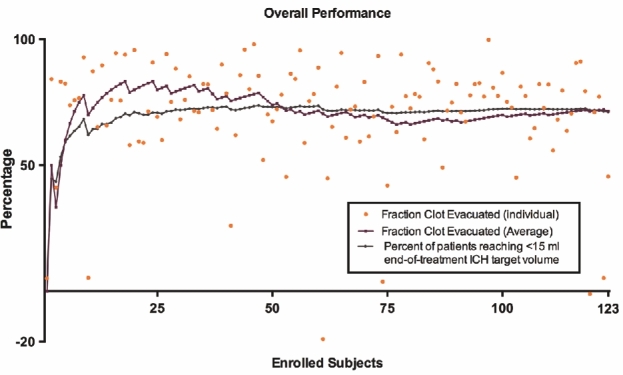
Efficacy of hematoma evacuation over time. A graph illustrating the distribution of clot evacuation rates in subjects randomized to the surgical arm in the first half of the trial. The plot also shows the overall consistency of ICH evacuation efficacy over time as demonstrated by average clot evacuation rate and percentage of subjects reaching target end-of-treatment volume <15 mL. Two cases experienced hematoma size expansion by the end of treatment resulting in a negative clot evacuation rate.
DISCUSSION
Perseverance in the refinement and modification of novel surgical techniques continues to be driven by the unsatisfactory outcome of best medical treatment or conventional surgery by open craniotomy for ICH.15,16 Several techniques have been proposed that share some similarities in the principles of approaching and evacuating the hematoma with minimal invasiveness but differ in physical and technical means of evacuation.17-20 Current guidelines on ICH management pronounce uncertainty of the effectiveness of minimally invasive hematoma evacuation with stereotactic or endoscopic aspiration with or without thrombolytic usage (class IIb; level of evidence B).16
These various techniques of minimally invasive ICH evacuation are undergoing preliminary evaluation of safety and efficacy, with the MISTIE technique having undergone the most rigorous optimization to date in phase II trial and currently being assessed in an ongoing phase III prospective randomized trial. The MISTIE protocol comprises a mechanical component (cannula aspiration) and pharmacological component (rtPA instillation though implanted catheter) to achieve a rather gentle hematoma evacuation over a period of 72 h.6 Several convenience sample reports have demonstrated the safety and feasibility of this approach.3,4,17,21-26 More importantly, results from 96 subjects enrolled in MISTIE II have shown potential therapeutic benefit in short- and long-term functional outcomes and need for long-term care.10
The Surgical Center-monitored experience from the phase II trial taught us that efficacy of the MISTIE surgical task is directly related to satisfactory catheter placement.10 This allowed the optimization of catheter trajectory selection and the training of surgeons to help optimize the surgical task.5 The ongoing phase III trial has tasked a Surgical Center team with qualifying, mentoring, and monitoring surgical performance. However, it was not known until now how often catheters can be placed satisfactorily by surgeons at different levels of experience and how consistently ICH evacuation can be executed. Ongoing results from MISTIE III allowed us to query these performance issues at the midpoint of the trial.
Our findings suggest that placement accuracy varies depending on the technical difficulty of the surgical trajectory. Poor placement was more commonly encountered with the posterior approach (trajectory B) regardless of the surgeon's experience. The relative ease in targeting the hematoma in the anterior and lobar approaches could be due to a more accurate neuronavigation using the facial anatomic landmarks or the proximity of the hematoma to the vertex, respectively. The posterior approach often requires angling of the trajectory through the burr hole, rather than a more direct route perpendicular to the burr hole with anterior and lobar approaches. These challenges, particularly with the posterior approach, will motivate technical innovations, aimed at enhancing catheter placement accuracy. Techniques to improve catheter positioning, such as ultrasound,27-29 endoscopy,30 improved neuronavigation,31,32 or even smartphone-based solutions,33 have been proposed in the literature. Some simulation modules for external ventricular drain insertion may also be applied.34 Analysis also showed that rates of poor placement were inversely related to surgeons’ experience, a relationship that approached statistical significance. This impact of surgeon experience on placement accuracy was maintained when correcting for the surgical approach, denoting a better performance by fully qualified surgeons even with the technically challenging posterior approach.
Efficacy of hematoma evacuation reflects both the surgical performance of the operator and ongoing education and oversight at the Surgical Center. Monthly safety webinars are a component of the ongoing performance improvement plan in the trial. Lessons learned herein have been shared with surgeons at all levels of experience. We note that the initial catheter placement accuracy reported herein did not reflect the final placement after adjustment or catheter replacement, and many cases with pragmatically assessed suboptimal catheter placements still achieved good rates of hematoma evacuation. Nevertheless, important lessons were learned that will motivate improvement in surgical technique.
In this analysis, we used the 3 volumetric parameters to assess the immediate efficacy of the MISTIE procedure in evacuating the hematoma. All parameters showed consistent rates of evacuation among different surgical approaches and in the hands of surgeons of different experience levels with the procedure. The equivalent efficacy of hematoma evacuation indicated that catheters were replaced when needed, ultimately achieving the desired hematoma evacuation. This adds confidence regarding the internal consistency of the surgical task within the trial and its ultimate generalizability to the surgical community at large. This information will be useful to surgeons who are integrating the MISTIE procedure in their practice and will help in the ultimate interpretation of trial results.
Results from this analysis also do not clarify technical challenges faced and learned in each case. An example is the observation of higher poor placement rates in the posterior approach (B) with a tendency for medial deviation of the trajectory. Common technical pitfalls encountered in these cases included failing to use posterior fiducials for image-guided neuronavigation and using smaller and/or too medial burr holes, with limited angles of freedom, forcing the cannula along a more medial trajectory than intended. The amount of blood suctioned by cannula prior to catheter placement is also highly variable, depending on clot consistency and possibly surgeon experience. We have noted cases in which thorough aspiration resulted in suboptimal catheter location that had targeted the original clot. Often, blood is mixed with necrotic brain at initial aspiration regardless of catheter placement accuracy. We do not have specific information about the consistency of blood evacuated in good vs poor placement cases.
Differences in adverse events or surgical complications in relation to surgical performance were not addressed in this report. The latter are being monitored closely and the rates have been very low, not allowing any meaningful comparison at this stage of the trial. Finally, it is worth emphasizing that these results on ICH evacuation efficacy (surgical task efficacy) do not imply a long-term outcome benefit of MISTIE, which must await the results of the trial.
CONCLUSION
We have identified challenges with different surgical approaches in the MISTIE task at various levels of surgical experience. Ongoing education and mentoring allowed consistent rates of hematoma evacuation efficacy despite these challenges.
Disclosures
MISTIE Phase III trial was funded by grant 5U01NS062851 from the National Institutes of Health. Alteplase was donated by Genentech, Inc. D. Hanley reports grants from NIH/NINDS, during the conduct of the study; grants and personal fees from EKOS Corp, grants and personal fees from Brainscope, personal fees from Rand Corp, personal fees from Medico-Legal, outside the submitted work. A. Stadnik, M. Jesselson, L. Money, N. McBee, A.J. Bistran-Hall, W.A. Mould, K. Lane, P.J. Camarata, M. Zuccarello, and I.A. Awad received salary support from grants from NINDS during the conduct of the study. Intracranial alteplase is not approved by the US Food and Drug Administration as a labeled indication. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENTS
The optimal treatment for intracerebral hemorrhage (ICH) remains one of the most controversial topics in neurosurgery. It remains the deadliest and most disabling stroke, with a high economic burden worldwide. Recent literature showed promising result with minimally-invasive treatments. These include minimally invasive clot evacuation with stereotactic or endoscopic aspiration with or without thrombolytic usage. However, these results deserve careful interpretation.
First of all, most of these trials selected patients with stable clots demonstrated by follow-up CT scan after 6 hours. This is true for the MISTIE trial here, and also the recently published INVEST and ICES trials.1,2 These minimally invasive treatments have not only decreased the mortality, they also demonstrated the potential to enhance neurological recovery.2 This is rarely seen in the treatment of ICH and is very encouraging. However, we must be aware that a majority (25%) of ICH patients deteriorate within the first few hours of ictus (especially those patients with spot sign or black hole sign3) and may require clot evacuation. The interesting questions is will these minimally invasive treatments decrease mortality and enhance neurological recovery in this group of patients as well?
The main reason why most of these trials require demonstration of a stability scan is that a previous study showed ultra-early surgery within 4 hours after ictus is associated with high rebleeding rate. However, this data was generated from a study using ultra-early craniotomy (as opposed to minimally invasive treatment) that involved 24 patients and the rebleeding rate was 40%.4 However, minimally invasive treatments has been applied to a many patients in early stage with low rebleeding rate in the range of 0-3.3%.5,6,7,8 It would be great to see if we can further expand minimally invasive treatment like MISTIE to benefit this group of patients in the future.
One of the most important criteria for the success and good outcome of patients enrolled in the surgical arm of the MISTIE trial is catheter location. This lead to the creation of the Surgical Trial Center which have a crucial role in this trial. We do think the high rate of misplacement of the catheter in the posterior trajectory can be improved with training and also new technologies such as virtual reality and augmented reality which has been applied successfully to many emergency cases before.9 Finally, we are very excited about the progress in the MISTIE trial and look forward to applying this technique to our selected patients.
Abel Po-Hao Huang
Taipei, Taiwan
- 1. Fiorella D, Arthur AS, Mocco JD. 305 The INVEST Trial: A Randomized, Controlled Trial to Investigate the Safety and Efficacy of Image-Guided Minimally Invasive Endoscopic Surgery With Apollo vs Best Medical Management for Supratentorial Intracerebral Hemorrhage. Neurosurgery. 2016 Aug;63 Suppl 1:187. doi: 10.1227/01.neu.0000489793.60158.20. PubMed PMID: 27399503. [Google Scholar]
- 2. doi: 10.1161/STROKEAHA.116.013837. Vespa P, Hanley D, Betz J, Hoffer A, Engh J, Carter R, Nakaji P, Ogilvy C, Jallo J, Selman W, Bistran-Hall A, Lane K, McBee N, Saver J, Thompson RE, Martin N; ICES Investigators.. ICES (Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery) for Brain Hemorrhage: A Multicenter Randomized Controlled Trial. Stroke. 2016 Nov;47(11):2749-2755. PubMed PMID: 27758940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. doi: 10.1161/STROKEAHA.116.013186. Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, Wei X, Xie P. Black Hole Sign: Novel Imaging Marker That Predicts Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke. 2016 Jul;47(7):1777-81. doi: 10.1161/STROKEAHA.116.013186. PubMed PMID: 27174523. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1212/wnl.56.10.1294. Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001 May 22;56(10):1294-9. PubMed PMID: 11376176. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1385/NCC:2:1:067. Nishihara T, Nagata K, Tanaka S, Suzuki Y, Izumi M, Mochizuki Y, Akabane A, Ochiai C. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. 2005;2(1):67-74. PubMed PMID: 16174973. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1016/j.jocn.2005.04.006. Chen CC, Cho DY, Chang CS, Chen JT, Lee WY, Lee HC. A stainless steel sheath for endoscopic surgery and its application in surgical evacuation of putaminal haemorrhage. J Clin Neurosci. 2005 Nov;12(8):937-40. PubMed PMID: 16275100. [DOI] [PubMed] [Google Scholar]
- 7. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.021. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011 May-Jun;20(3):208-13. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.021. PubMed PMID: 20621516. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.3171/2011.2.FOCUS10313. Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC, Liu LC, Tu YK, Huang AP. Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: case selection, surgical technique, and long-term results. Neurosurg Focus. 2011 Apr;30(4):E9. doi: 10.3171/2011.2.FOCUS10313. PubMed PMID: 21456936. [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.3390/s130506477. Mandel M, Amorim R, Paiva W, Prudente M, Teixeira MJ, Andrade AF. 3D preoperative planning in the ER with OsiriX®: when there is no time for neuronavigation. Sensors (Basel). 2013 May 16;13(5):6477-91. doi: 10.3390/s130506477. PubMed PMID: 23681091; PubMed Central PMCID: PMC3690066. [DOI] [PMC free article] [PubMed] [Google Scholar]
As the authors state, minimally invasive surgical evacuation of intracerebral hematoma with aspiration and subsequent thrombolysis is investigated in the ongoing phase III clinical trial (MISTIE III). This research was done to assess the accuracy of catheter placement and efficacy of hematoma evacuation in relation to surgical approach and surgeon experience. They performed a trial midpoint interim assessment of 123 cases who underwent the surgical procedure. Accuracy of catheter placement was prospectively assessed by the trial. The found that the average hematoma volume was 49.7 mL (range 20.0-124), and the mean evacuation rate was 71% (range 18.4-99.8). First placed catheters were 58% in good position, 28% suboptimal (but suitable to dose), and 14% poor (requiring repositioning). Posterior trajectory (B) was associated with significantly higher rates of poor placement (35%, P = .01). There was no significant difference in catheter placement accuracy among surgeons of varying experience. Hematoma evacuation efficacy was not significantly different among the three surgical approaches, or different surgeons' experience.
This is a well-written paper and, as the authors state, it is important to have of rigorous surgical oversight for surgeons new to the technique. To me the new interest in the surgical evacuation is encouraging as the STICH trial may have not accurately reflected the results of surgical evacuation using modern techniques such aspiration and subsequent thrombolysis as well as the newer techniques such as using a tube or cannula to remove the hematoma.
Gavin W. Britz
Houston, Texas
REFERENCES
- 1. Mendelow AD, Gregson BA, Fernandes HM et al. . Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387-397. [DOI] [PubMed] [Google Scholar]
- 2. Mendelow AD, Gregson BA, Rowan EN et al. . Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56(10):1294-1299. [DOI] [PubMed] [Google Scholar]
- 4. Zuccarello M, Brott T, Derex L et al. . Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30(9):1833-1839. [DOI] [PubMed] [Google Scholar]
- 5. Dey M, Stadnik A, Awad IA. Spontaneous intracerebral and intraventricular hemorrhage: advances in minimally invasive surgery and thrombolytic evacuation, and lessons learned in recent trials. Neurosurgery. 2014;74(suppl 1):S142-S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdu E, Hanley DF, Newell DW. Minimally invasive treatment for intracerebral hemorrhage. Neurosurg Focus. 2012;32(4):E3. [DOI] [PubMed] [Google Scholar]
- 7. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008;105:147-151. [DOI] [PubMed] [Google Scholar]
- 8. Jackson DA, Patel AV, Darracott RM, Hanel RA, Freeman WD, Hanley DF. Safety of intraventricular hemorrhage (IVH) thrombolysis based on CT localization of external ventricular drain (EVD) fenestrations and analysis of EVD tract hemorrhage. Neurocritic Care. 2013;19(1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer SA, Brun NC, Begtrup K et al. . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. [DOI] [PubMed] [Google Scholar]
- 10. Hanley DF, Thompson RE, Muschelli J et al. . Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15(12):1228-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mould WA, Carhuapoma JR, Muschelli J et al. . Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013;44(3):627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnes B, Hanley DF, Carhuapoma JR. Minimally invasive surgery for intracerebral haemorrhage. Curr Opin Crit Care. 2014;20(2):148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1(2):113-119. [DOI] [PubMed] [Google Scholar]
- 14. Brain Injury Outcomes (BIOS), Johns Hopkins University MISTIE Surgical Training Module. Available at: http://braininjuryoutcomes.com/emissary-college/mistie-training/141. Accessed March 6, 2017. [Google Scholar]
- 15. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hemphill JC 3rd, Greenberg SM, Anderson CS et al. . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for Healthcare Professionals from the American Heart Association/American stroke association. Stroke. 2015;46(7):2032-2060. [DOI] [PubMed] [Google Scholar]
- 17. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006;65(6):547-555; discussion 555-546. [DOI] [PubMed] [Google Scholar]
- 18. Auer LM, Deinsberger W, Niederkorn K et al. . Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70(4):530-535. [DOI] [PubMed] [Google Scholar]
- 19. Newell DW, Shah MM, Wilcox R et al. . Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. J Neurosurg. 2011;115(3):592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. 2012;117(4):767-773. [DOI] [PubMed] [Google Scholar]
- 21. Miller CM, Vespa PM, McArthur DL, Hirt D, Etchepare M. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocritic Care. 2007;6(1):22-29. [DOI] [PubMed] [Google Scholar]
- 22. Niizuma H, Otsuki T, Johkura H, Nakazato N, Suzuki J. CT-guided stereotactic aspiration of intracerebral hematoma–result of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985;48(1-6):427-430. [DOI] [PubMed] [Google Scholar]
- 23. Schaller C, Rohde V, Meyer B, Hassler W. Stereotactic puncture and lysis of spontaneous intracerebral hemorrhage using recombinant tissue-plasminogen activator. Neurosurgery. 1995;36(2):328-333; discussion 333-325. [DOI] [PubMed] [Google Scholar]
- 24. Rohde V, Rohde I, Thiex R et al. . Fibrinolysis therapy achieved with tissue plasminogen activator and aspiration of the liquefied clot after experimental intracerebral hemorrhage: rapid reduction in hematoma volume but intensification of delayed edema formation. J Neurosurg. 2002;97(4):954-962. [DOI] [PubMed] [Google Scholar]
- 25. Vespa P, McArthur D, Miller C et al. . Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocritic Care. 2005;2(3):274-281. [DOI] [PubMed] [Google Scholar]
- 26. Barrett RJ, Hussain R, Coplin WM et al. . Frameless stereotactic aspiration and thrombolysis of spontaneous intracerebral hemorrhage. Neurocritic Care. 2005;3(3):237-245. [DOI] [PubMed] [Google Scholar]
- 27. Phillips SB, Gates M, Krishnamurthy S. Strategic placement of bedside ventriculostomies using ultrasound image guidance: report of three cases. Neurocritic Care. 2012;17(2):255-259. [DOI] [PubMed] [Google Scholar]
- 28. Strowitzki M, Komenda Y, Eymann R, Steudel WI. Accuracy of ultrasound-guided puncture of the ventricular system. Childs Nerv Syst. 2008;24(1):65-69. [DOI] [PubMed] [Google Scholar]
- 29. Whitehead WE, Jea A, Vachhrajani S, Kulkarni AV, Drake JM. Accurate placement of cerebrospinal fluid shunt ventricular catheters with real-time ultrasound guidance in older children without patent fontanelles. J Neurosurg. 2007;107(5 suppl):406-410. [DOI] [PubMed] [Google Scholar]
- 30. Roth J, Constantini S. Selective use of intra-catheter endoscopic-assisted ventricular catheter placement: indications and outcome. Childs Nerv Syst. 2012;28(8):1163-1169. [DOI] [PubMed] [Google Scholar]
- 31. Hayhurst C, Beems T, Jenkinson MD et al. . Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg. 2010;113(6):1273-1278. [DOI] [PubMed] [Google Scholar]
- 32. Hermann EJ, Capelle HH, Tschan CA, Krauss JK. Electromagnetic-guided neuronavigation for safe placement of intraventricular catheters in pediatric neurosurgery. J Neurosurg Pediatr. 2012;10(4):327-333. [DOI] [PubMed] [Google Scholar]
- 33. Thomale UW, Knitter T, Schaumann A et al. . Smartphone-assisted guide for the placement of ventricular catheters. Childs Nerv Syst. 2013;29(1):131-139. [DOI] [PubMed] [Google Scholar]
- 34. Schirmer CM, Elder JB, Roitberg B, Lobel DA. Virtual reality-based simulation training for ventriculostomy: an evidence-based approach. Neurosurgery. 2013;73(suppl 1):66-73. [DOI] [PubMed] [Google Scholar]