Abstract
The aim of the present meta-analysis was to systematically assess the efficacy of the various treatments available for moderate to severe psoriasis. PubMed and Embase databases were systematically searched to select relevant studies up to February 2015. Odds ratios (ORs) and their 95% confidence intervals (CIs) were used as effect estimates. In addition, the Psoriasis Area and Severity Index (PASI) 50, PASI 75 and PASI 90 responses for the therapies were systematically assessed. A total of 33 randomized controlled trials were included in the present study. For the PASI 75 response rate, infliximab (5 mg) may be the most effective option for the treatment of moderate to severe psoriasis. Furthermore, the pooled results of the PASI 50 response rate demonstrated that infliximab (5 mg) and ustekinumab (90 mg) may be superior to other drugs for treating moderate to severe psoriasis. For the PASI 90 response rate, infliximab (5 mg), ustekinumab (90 mg) and briakinumab (weeks 0 and 4, 200 mg; week 8, 100 mg) exhibited improved results compared with other treatments. In conclusion, infliximab (5 mg) may be a superior option to treat moderate to severe psoriasis due to the relatively high PASI scores. However, despite the high PASI 90 responses, further studies are required to identify the efficacy of ustekinumab (90 mg) and briakinumab.
Keywords: biological therapies, psoriasis, Psoriasis Area and Severity Index, network meta-analysis
Introduction
Psoriasis is a common immune-mediated skin disease. The prevalence of psoriasis in adults ranges between 0.91 and 8.5% worldwide and the incidence of psoriasis is higher in adults than in children (1). Psoriasis is characterized by symptoms of plaque, pustular and other skin lesions. Chronic plaque psoriasis accounts for 90% of all psoriasis cases (2,3).
A number of biological therapies are used to treat moderate to severe psoriasis, including etanercept, briakinumab, ustekinumab, adalimumab and infliximab (4–8). Etanercept, adalimumab and infliximab are monoclonal antibodies against tumor necrosis factor (TNF), which function by neutralizing the biological activity of TNF for treating the TNF-mediated inflammation (5,9). By contrast, ustekinumab and briakinumab are human monoclonal antibodies against interleukin (IL)-12/23p40 (8). These biological therapies are used to treat psoriasis and improved clinical outcomes have been observed. However, the efficacy of these therapies has been not systematically reviewed.
In the present study, a network meta-analysis was performed to review and compare the efficacy of these aforementioned biological therapies of psoriasis. The Psoriasis Area and Severity Index (PASI) response (10) was used as an indicator for assessing the effect of treatment on the severity of psoriasis. PASI 50, PASI 75 and PASI 90 responses for the therapies were systematically assessed. The pooled results provide further information on selecting the most suitable treatments for moderate to severe psoriasis.
Materials and methods
Data sources
The PubMed (www.ncbi.nlm.nih.gov/pubmed) and Embase (www.elsevier.com/solutions/embase-biomedical-research) databases were systematically searched in order to select relevant studies up to February 2015. The search terms included the following: Psoriasis, methotrexate (MTX), cyclosporin A (CSA), ustekinumab, etanercept, infliximab, briakinumab and adalimumab.
Inclusion and exclusion criteria
Studies with the following characteristics were included in the current meta-analysis: i) Randomized controlled trials (RCTs) reporting the treatment of moderate to severe psoriasis with the aforementioned drugs. Moderate to severe psoriasis is defined as body surface area >10 or psoriasis area and severity index >10 and dermatology life quality index >10 (11); ii) studies including the adults as participants; and iii) studies reporting the PASI response rate (50, 75 and 90%). Any reviews, case reports and letters were excluded from the meta-analysis. Any studies investigating patients with mild psoriasis and those written in a language other than English were also excluded.
Data extraction and quality assessment
Two reviewers independently extracted the following data: The name of the first author, publication year, sample size, intervention, demographic characteristics of the included patients and PASI response rate. The controversies were discussed with a third reviewer to reach consensus. The methodological quality of the included studies was evaluated by the Cochrane Collaboration Risk of Bias Tool (12).
Statistical analysis
All analyses were performed using the ADDIS software version 1.16.5 (Drug Information and Monitoring Systems, Groningen, The Netherlands). Odds ratios (ORs) and their 95% confidence intervals (CIs) were pooled. The network analysis performed was based on the Bayesian framework. Data were evaluated by Markov chain Monte Carlo methods and all analyses were performed using the random effects model. The consistency of the RCTs was assessed by Node-splitting analysis, and the consistency model was used if P>0.05. Otherwise, the inconsistency model was used to pool the odd ratios (13).
Results
Study selection
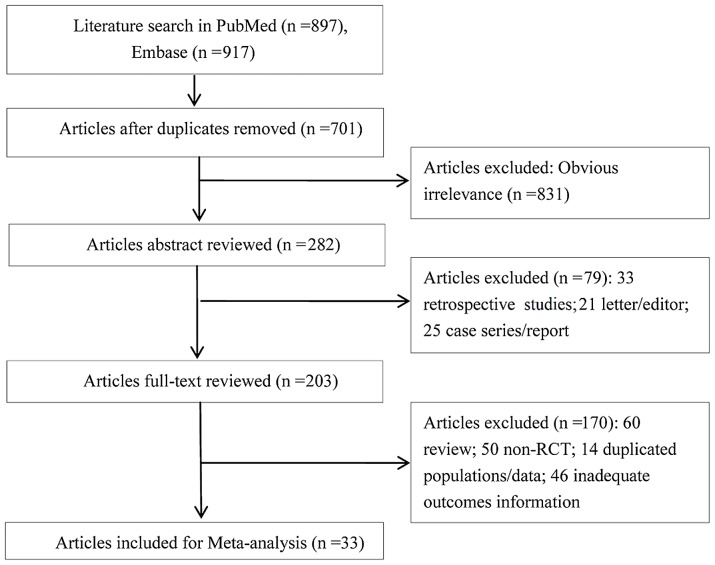
As presented in Fig. 1, a total of 897 studies were identified from PubMed and 917 studies from Embase by the initial search. Subsequent to excluding any duplicates, 1,113 studies remained. A total of 831 irrelevant studies were excluded by reviewing the titles and abstracts. In addition, 249 studies that did not meet the inclusion criteria were excluded. Finally, 33 RCTs were included in the present study (4–9,14–40).
Figure 1.
Process of the literature selection performed in the present meta-analysis. RCT, randomized controlled trial.
Characteristics of the included studies
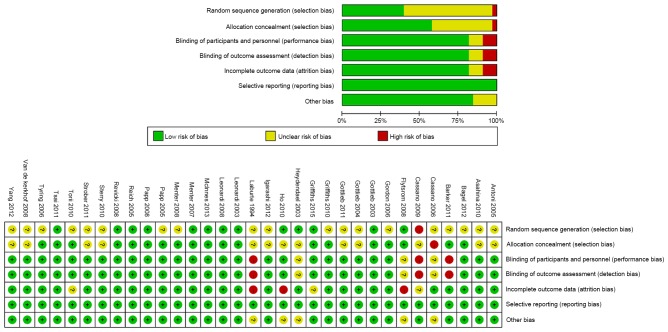
As presented in Table I, the demographic characteristics, including age, sex and weight of the patients in the included studies were similar. Included RCTs were published between 1994 and 2015. The mean duration of psoriasis of the included patients ranged between 11.1 and 21.5 years. Quality assessment demonstrated that the quality of the included RCTs was relatively high. With respect to random sequence generation (selection bias), a number of studies were assessed as having an unclear risk of bias. With regards to blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias) and incomplete outcome data (attrition bias), a small proportion of studies were assessed as having high risk of bias (Fig. 2) and were excluded from the current study. However, the studies by Laburte et al (25) and Cassano et al (15) were not excluded as they met with the inclusion criteria despite having quite a poor rating.
Table I.
Characteristics of the included studies.
| First author | Year | Follow-up | Treatment | N | Age (years) | M/F | Weight (kg) | Duration of psoriasis (years) | PASI score | PASI 75 | PASI 50 | PASI 90 | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laburte et al | 1994 | 0.2–18.3 | CSA 5 mg | 132 | 40.7±12.3 | 90/42 | 72.9±3.4 | 17.7±11.1 | 25.1±8.0 | 117 | NA | NA | (25) |
| months | CSA 2.5 mg | 119 | 42.0±12.6 | 86/33 | 77.4±15.5 | 18.4±11.1 | 24.9±7.0 | 57 | NA | NA | |||
| Gordon et al | 2006 | 60 weeks | Adalimumab | 45 | 46 (20–71) | 32/13 | 93 (63–159) | 21 (1.3–57.9) | 16.7 (5.4–39.0) | 24 | NA | NA | (17) |
| Placebo | 52 | 43 (20–70) | 34/18 | 94(50–147) | 19 (1.0–39.9) | 16.0(5.5–40.4) | 2 | NA | NA | ||||
| Menter et al | 2008 | 16 weeks | Adalimumab | 814 | 44.1±13.2 | 546/268 | 92.3±23.0 | 18.1±11.91 | 19.0±7.08 | 578 | NA | 366 | (2) |
| Placebo | 398 | 45.4±13.4 | 257/141 | 94.1±23.0 | 18.4±11.94 | 18.8±7.09 | 28 | NA | 8 | ||||
| Asahina et al | 2010 | 24 weeks | Adalimumab | 38 | 47.8±12.81 | 32/6 | 69.7±15.48 | 14.2±9.29 | 25.44±8.98 | 24 | 31 | 15 | (5) |
| Placebo | 46 | 43.9±10.75 | 41/5 | 71.3±15.28 | 15.5±8.83 | 29.10±11.77 | 2 | 9 | 0 | ||||
| Revicki et al | 2008 | 16 weeks | Adalimumab | 108 | 42.8±12.3 | 37/71 | NA | 17.6±10.0 | 20.1±7.4 | 86 | 95 | 55 | (34) |
| MTX | 110 | 41.9±11.9 | 36/74 | NA | 19.0±10.3 | 19.5±7.4 | 66 | 68 | 15 | ||||
| Placebo | 53 | 40.7±11.4 | 18/35 | NA | 18.9±8.7 | 19.2±6.9 | 10 | 16 | 6 | ||||
| Leonardi et al | 2003 | 12 weeks | Etanercept 50 mg BIW | 164 | 44.8±0.8 | 107/57 | NA | 18.6±0.9 | 18.4±0.7 | 81 | 121 | 36 | (27) |
| Etanercept 25 mg BIW | 162 | 45.4±1.0 | 109/53 | NA | 18.5±0.9 | 18.5±0.7 | 55 | 94 | 19 | ||||
| Etanercept 25 mg QW | 160 | 44.4±0.9 | 118/42 | NA | 19.3±0.9 | 18.2±0.7 | 23 | 65 | 5 | ||||
| Placebo | 166 | 45.6±1.0 | 105/61 | NA | 18.4±0.9 | 18.3±0.6 | 6 | 24 | 1 | ||||
| Papp et al | 2005 | 12 weeks | Etanercept 50 mg BIW | 194 | 44.5 (21.0–80.0) | 130/64 | NA | 18.1 (0.8–60.5) | 16.1 (7.0–57.3) | 96 | 150 | 40 | (32) |
| Etanercept 25 mg BIW | 196 | 46.0 (20.0–87.0) | 128/68 | NA | 21.5 (0.8–64.6) | 16.9 (4.0–51.2) | 67 | 126 | 21 | ||||
| Placebo | 193 | 44.0 (18.0–80.0) | 124/69 | NA | 17.5 (1.4–51.2) | 16.0 (7.0–62.4) | 6 | 18 | 1 | ||||
| Tyring et al | 2006 | 12 weeks | Etanercept 50 mg BIW | 311 | 45.8±12.8 | 203/108 | NA | 20.1±12.3 | 18.3±7.6 | 146 | 230 | 65 | (37) |
| Placebo | 307 | 45.6±12.1 | 216/91 | NA | 19.7±11.4 | 18.1±7.4 | 15 | 43 | 3 | ||||
| van de Kerkhof et al | 2008 | 12 weeks | Etanercept 25 mg BIW | 96 | 45.9±12.8 | 59/36 | 83.4±16.0 | 19.3±11.3 | 21.4±9.3 | 36 | 66 | 13 | (38) |
| Placebo | 46 | 43.6±12.6 | 25/21 | 79.1±20.2 | 17.3±8.2 | 21.0±8.7 | 1 | 4 | 1 | ||||
| Cassano et al | 2010 | 12 weeks | Etanercept 50 mg BIW | 36 | NA | NA | NA | NA | NA | 19 | 33 | NA | (40) |
| Etanercept 100 mg QW | 36 | NA | NA | NA | NA | NA | 13 | 27 | NA | ||||
| Strober et al | 2011 | 12 weeks | Etanercept 50 mg BIW | 139 | 45.2±14.8 | 85/54 | 96.9±24.9 | 15.2±12.1 | 18.5±6.0 | 55 | NA | 19 | (8) |
| Briakinumab | 139 | 44.9±12.9 | 93/46 | 96.1±24.5 | 16.3±12.0 | 19.4±7.9 | 112 | NA | 77 | ||||
| Placebo | 72 | 45.0±13.9 | 46/26 | 92.9±25.2 | 15.5±11.7 | 18.3±6.4 | 5 | NA | 3 | ||||
| Gottlieb et al | 2011 | 12 weeks | Etanercept 50 mg BIW | 141 | 43.1±12.5 | 98/43 | 94.5±20.4 | 17.0±12.7 | 19.4±8.0 | 78 | NA | 32 | (19) |
| Briakinumab | 138 | 43.6±14.3 | 89/49 | 93.2±22.9 | 16.1±12.5 | 18.4±7.2 | 113 | NA | 81 | ||||
| Placebo | 68 | 44.0±13.6 | 47/21 | 96.5±27.2 | 19.1±13.2 | 18.5±6.9 | 5 | NA | 1 | ||||
| Bagel et al | 2012 | 12 weeks | Etanercept 50 mg BIW | 62 | 39 (18.0–71.0) | 29/33 | 30.2 (18.2–44.2) | 17.5 (1–45) | 15.5 (8–46) | 37 | 53 | 16 | (8) |
| Placebo | 62 | 42 (18.0–70.0) | 26/36 | 30.2 (18.2–44.2) | 11.9 (1–49) | 15.2 (10–41) | 3 | 4 | 1 | ||||
| Gottlieb et al | 2003 | 10 weeks | Infliximab 5 mg | 99 | 44 (34, 53) | 73/26 | NA | 16 (10, 25) | 20 (14, 28) | 87 | 96 | 47 | (20) |
| Infliximab 3 mg | 99 | 45 (37, 55) | 70/29 | NA | 18 (12, 24) | 20 (15, 26) | 71 | 83 | 45 | ||||
| Placebo | 51 | 45 (30, 52) | 31/20 | NA | 16 (6, 22) | 18 (15, 27) | 3 | 11 | 1 | ||||
| Reich et al | 2005 | 24 weeks | Infliximab 5 mg | 301 | 42.6±11.7 | 207/94 | NA | 19.1±11.0 | 22.9±9.3 | 227 | 248 | 161 | (33) |
| Placebo | 77 | 43.8±12.6 | 61/16 | NA | 17.3±11.1 | 22.8±8.7 | 3 | 6 | 1 | ||||
| Menter et al | 2007 | 14 weeks | Infliximab 5 mg | 314 | 44.5±13.0 | 204/110 | 92.2±23.2 | 19.1±11.7 | 20.4±7.5 | 193 | 252 | 113 | (29) |
| Infliximab 3 mg | 313 | 43.4±12.6 | 206/107 | 92.0±22.5 | 18.1±11.8 | 20.1±7.9 | 149 | 213 | 75 | ||||
| Torii and Nakagawa | 2010 | 14 weeks | Infliximab 5 mg | 35 | 46.9±13.0 | 22/13 | 68.5±13.4 | 14.2±8.9 | 31.9±12.8 | 25 | 29 | 17 | (35) |
| Placebo | 19 | 43.3±12.3 | 14/5 | 69.7±8.9 | 11.1±6.5 | 33.1±15.6 | 2 | 2 | 1 | ||||
| Yang et al | 2012 | 10 weeks | Infliximab 5 mg | 84 | 39.4±12.3 | 60/24 | 68.2±9.2 | 16.0±10.8 | NA | 68 | 79 | 48 | (39) |
| Placebo | 45 | 40.1±11.1 | 35/10 | 67.4±9.9 | 16.0±8.9 | NA | 1 | 6 | 0 | ||||
| Barker et al | 2011 | 26 weeks | Infliximab 5 mg | 653 | 44.1 (±18–78) | 438/215 | 84.5±18.6 | 18.8±11.6 | 21.4±8.0 | 502 | 529 | 333 | (14) |
| MTX | 215 | 41.9 (±18–69) | 148/67 | 83.8±18.2 | 17.0±10.3 | 21.1±7.6 | 66 | 103 | 32 | ||||
| Leonardi et al | 2008 | 12 weeks | Ustekinumab 90 mg | 256 | 46.2±11.3 | 173/83 | 93.8±23.9 | 19.6±11.1 | 19.7±7.6 | 170 | 220 | 94 | (26) |
| Ustekinumab 45 mg | 255 | 44.8±12.5 | 175/80 | 93.7±23.8 | 19.7±11.7 | 20.5±8.6 | 171 | 213 | 106 | ||||
| Placebo | 255 | 44.8±11.3 | 183/72 | 94.2±23.5 | 20.4±11.7 | 20.4±8.6 | 8 | 26 | 5 | ||||
| Papp et al | 2008 | 12 weeks | Ustekinumab 90 mg | 411 | 46.6±12.1 | 274/137 | 91.5±21.3 | 20.3±12.3 | 20.1±7.5 | 311 | 367 | 209 | (31) |
| Ustekinumab 45 mg | 409 | 45.1±12.1 | 283/126 | 90.3±21.0 | 19.3±11.7 | 19.4±6.8 | 273 | 342 | 173 | ||||
| Placebo | 410 | 47.0±12.5 | 283/127 | 91.1±21.6 | 20.8±12.2 | 19.4±7.5 | 15 | 41 | 3 | ||||
| Griffiths | 2010 | 36 weeks | Ustekinumab 90 mg | 247 | NA | NA | NA | NA | NA | 183 | NA | 111 | (21) |
| Ustekinumab 45 mg | 209 | NA | NA | NA | NA | NA | 142 | NA | 75 | ||||
| Etanercept 50 mg BIW | 347 | NA | NA | NA | NA | NA | 198 | NA | 80 | ||||
| Tsai et al | 2011 | 12 weeks | Ustekinumab 45 mg | 61 | 40.9±12.7 | 50/11 | 73.1±12.7 | 11.9±7.5 | 25.2±11.9 | 41 | 51 | 30 | (36) |
| Placebo | 60 | 40.4±10.1 | 53/7 | 74.6±13.0 | 13.9±7.3 | 22.9±8.6 | 3 | 8 | 1 | ||||
| Igarashi et al | 2012 | 12 weeks | Ustekinumab 45 mg | 64 | M: 45.0 | 53/11 | 73.2±15.4 | 15.8±8.2 | 30.1±12.9 | 38 | 53 | 21 | (24) |
| Ustekinumab 90 mg | 62 | M: 44.0 | 47/15 | 71.1±14.0 | 17.3±10.7 | 28.7±11.2 | 42 | 52 | 27 | ||||
| Placebo | 32 | M: 49.0 | 26/6 | 71.2±10.9 | 16.0±11.2 | 30.3±11.8 | 2 | 4 | 1 | ||||
| Heydendael et al | 2003 | 17–52 weeks | CSA 2.5 mg | 42 | 41.6±13.0 | 29/13 | NA | NA | 14.0±6.6 | 30 | NA | NA | (22) |
| MTX | 43 | 38.3±12.4 | 28/15 | NA | NA | 13.4±3.6 | 26 | NA | NA | ||||
| Flytstrom et al | 2008 | CSA 5 mg | 31 | 45 (18–70) | 27/4 | 87 (61–130) | NA | 15.5±6.3 | 18 | 27 | 9 | (16) | |
| MTX | 37 | 48 (23–78) | 28/9 | 85 (56–132) | NA | 14.1±7.0 | 22 | 24 | 4 | ||||
| Ho et al | 2010 | 6 months | MTX | 20 | 38.45 (21–68) | 18/2 | NA | NA | NA | 13 | NA | 0 | (23) |
| Placebo | 20 | 43.45 (27–61) | 18/2 | NA | NA | NA | 16 | NA | 5 | ||||
| Gottlieb et al | 2003 | 24 weeks | Etanercept 25 mg BIW | 57 | 48.2 (25–72) | 33/24 | Mean: 91.8 | 23±1.6 | 17.8±1.1 | 17 | 40 | 6 | (20) |
| Placebo | 55 | 46.5 (18–77) | 37/18 | Mean: 90.7 | 20±1.7 | 19.5±1.3 | 1 | 6 | 0 | ||||
| Cassano et al | 2006 | 12 weeks | Etanercept 50 mg BIW | 53 | 42.3 (18–73) | 57/52 | NA | NA | 8.7 (5.4–11.6) | 29 | 39 | NA | (15) |
| Etanercept 100 mg QW | 55 | NA | NA | 28 | 43 | NA | |||||||
| Sterry et al | 2010 | 12 weeks | Etanercept 50 mg BIW | 379 | 46±11 | 243/136 | NA | 19±12 | 20±11 | 208 | NA | NA | (7) |
| Etanercept 50 mg QW | 373 | 47±11 | 230/143 | NA | 19±11 | 19±10 | 134 | NA | NA | ||||
| Antoni et al | 2005 | 16 weeks | Infliximab 5 mg | 52 | 45.7±11.1 | 30/22 | NA | 19.4±11.6 | 5.1±5.9 | 35 | NA | NA | (4) |
| Placebo | 52 | 45.2±9.7 | 30/22 | NA | 16.9±10.9 | 4.2±5.8 | 0 | NA | NA | ||||
| McInnes et al | 2013 | 12 weeks | Ustekinumab 45 mg | 205 | 48.0 (39.0–55.0) | 106/99 | NA | 12.0 (4.1–22.2) | 7.1 (3.3–15.3) | 83 | NA | NA | (28) |
| Ustekinumab 90 mg | 204 | 47.0 (38.5–54.0) | 116/88 | NA | 14.1 (5.4–22.4) | 8.4 (4.8–14.7) | 93 | NA | NA | ||||
| Placebo | 206 | 48.0 (39.0–57.0) | 108/98 | NA | 13.1 (5.3–23.5) | 8.8 (4.4–14.3) | 16 | NA | NA | ||||
| Griffiths et al | 2015 | 12 weeks | Etanercept 50 mg QW | 371 | 46.9±11.4 | 229 | NA | 18.6±11.4 | 19.0±9.8 | 148 | NA | NA | (6) |
| Etanercept 50 mg BIW | 377 | 46.1±11.4 | 241 | NA | 19.2±11.9 | 19.8±10.7 | 226 | NA | NA |
Data are presented as the mean ± standard deviation, or as the median (range). PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly; M/F, male/female; NA, not available; Ref, study reference number; M, mean value.
Figure 2.
Risk of bias of the included studies. +, low risk of bias; ?, unclear risk of bias; -, high risk of bias. The quality evaluation map indicates that, regarding random sequence generation (selection bias) and allocation concealment (selection bias), many references have an unclear risk of bias and few references have high risk of bias. However, regarding incomplete outcome data (attrition bias), blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias), ~10% references have high risk of bias and 10% references have an unclear risk of bias. The included references showed no evidence of selective reporting (reporting bias) in the included references-all references showed a low risk of bias.
Network meta-analysis
Based on the results of node-splitting analysis (Table II), the effect sizes were pooled using an inconsistency model. Regarding the PASI 75 response rate, infliximab (5 mg) was the most effective option for the treatment of moderate to severe psoriasis (Table III and Fig. 3). The pooled results of the PASI 50 response rate demonstrated that infliximab (5 mg) and ustekinumab (90 mg) may be superior to other drugs for treating moderate to severe psoriasis (Table IV). In addition, regarding the PASI 90 response rate, treatment with infliximab (5 mg), ustekinumab (90 mg) and briakinumab (weeks 0 and 4, 200 mg; week 8, 100 mg) indicated improved results compared with other agents (Table V). Finally, the drugs can be ranked in the following order according to their efficacy, defined as their PASI 90 response rate: Briakinumab > ustekinumab (90 mg) > infliximab (5 mg)> ustekinumab (45 mg) > adalimumab > infliximab (3 mg)> etanercept (50 mg BIW) > CSA (5 mg) > etanercept (25 mg BIW) > MTX > etanercept (25 mg QW) > placebo (Table VI). The odds ratio value of infliximab (5 mg) compared with the other drugs was >1, therefore, infliximab (5 mg) was regarded as the best treatment agent, although it ranked as third in terms of efficacy.
Table II.
Node-splitting analysis.
| Name | Direct effect | Indirect effect | Overall | P-value |
|---|---|---|---|---|
| PASI 75 | ||||
| Adalimumab, MTX | −1.03 (−2.13, 0.09) | −0.13 (−1.44, 1.31) | −0.81 (−1.64, 0.10) | 0.28 |
| CSA 2.5 mg, CSA 5 mg | 2.15 (1.15, 3.13) | −0.55 (−2.24, 1.08) | 1.47 (0.44, 2.41) | 0.01 |
| CSA 2.5 mg, MTX | −0.51 (−1.71, 0.64) | 2.24 (0.71, 3.77) | 0.46 (−0.62, 1.48) | 0.01 |
| CSA 5 mg, MTX | 0.02 (−1.18, 1.24) | −2.70 (−4.23, −1.12) | −1.01 (−2.06, 0.07) | 0.01 |
| Etanercept 25 mg BIW, Etanercept 50 mg BIW | 0.64 (−0.08, 1.35) | −0.05 (−1.03, 0.89) | 0.41 (−0.27, 1.01) | 0.23 |
| Etanercept 50 mg BIW, Placebo | −3.02 (−3.57, −2.49) | −3.18 (−4.11, −2.32) | −3.03 (−3.51, −2.58) | 0.74 |
| Etanercept 50 mg BIW, Ustekinumab 45 mg | 0.46 (−0.61, 1.51) | 0.51 (−0.19, 1.18) | 0.48 (−0.15, 1.08) | 0.9 |
| Etanercept 50 mg BIW, Ustekinumab 90 mg | 0.77 (−0.29, 1.80) | 0.54 (−0.20, 1.20) | 0.62 (−0.00, 1.23) | 0.69 |
| Infliximab 3 mg, Placebo | −3.86 (−5.56, −2.35) | −3.95 (−5.09, −2.86) | −3.95 (−4.95, −3.01) | 0.94 |
| Infliximab 5 mg, MTX | −2.01 (−3.06, −1.04) | −2.18 (−3.45, −0.84) | −2.08 (−2.86, −1.25) | 0.83 |
| Infliximab 5 mg, Placebo | −4.83 (−5.77, −4.02) | −4.65 (−6.16, −3.25) | −4.73 (−5.50, −4.08) | 0.81 |
| MTX, Placebo | −2.53 (−3.72, −1.49) | −2.70 (−3.69, −1.78) | −2.66 (−3.50, −1.92) | 0.81 |
| Placebo, Ustekinumab 45 mg | 3.54 (2.96, 4.11) | 3.44 (2.39, 4.44) | 3.52 (2.99, 4.02) | 0.83 |
| Placebo, Ustekinumab 90 mg PASI 50 | 3.60 (2.97, 4.18) | 3.83 (2.83, 4.83) | 3.65 (3.11, 4.17) | 0.65 |
| PASI 50 | ||||
| Adalimumab, MTX | −1.51 (−2.41, −0.70) | 0.04 (−1.06, 1.18) | −1.10 (−2.03, −0.21) | 0.02 |
| Etanercept 25 mg BIW, Etanercept 50 mg BIW | 0.64 (−0.05, 1.38) | 0.73 (−0.20, 1.76) | 0.61 (0.02, 1.21) | 0.88 |
| Etanercept 50 mg BIW, Placebo | −3.13 (−3.66, −2.70) | −3.82 (−4.93, −2.73) | −3.22 (−3.78, −2.75) | 0.24 |
| Infliximab 3 mg, Placebo | −3.11 (−4.31, −1.87) | −2.93 (−4.01, −1.89) | −3.04 (−3.92, −2.25) | 0.82 |
| Infliximab 5 mg, MTX | −1.54 (−2.19, −0.88) | −3.08 (−4.17, −2.09) | −2.02 (−2.88, −1.37) | 0.01 |
| Infliximab 5 mg, Placebo | −4.45 (−5.18, −3.92) | −2.93 (−3.90, −1.90) | −4.13 (−4.79, −3.55) | 0.01 |
| MTX, Placebo | −1.42 (−2.34, −0.45) | −2.57 (−3.39, −1.73) | −2.11 (−2.85, −1.31) | 0.06 |
| PASI 90 | ||||
| Adalimumab, MTX | −2.04 (−3.04, −0.99) | −0.49 (−1.91, 0.86) | −1.67 (−2.58, −0.81) | 0.08 |
| Etanercept 25 mg BIW, Etanercept 50 mg BIW | 0.86 (0.14, 1.62) | −0.03 (−1.47, 1.35) | 0.75 (0.04, 1.39) | 0.24 |
| Etanercept 50 mg BIW, Placebo | −3.15 (−3.90, −2.45) | −3.34 (−4.48, −2.42) | −3.16 (−3.78, −2.63) | 0.76 |
| Etanercept 50 mg BIW, Ustekinumab 45 mg | 0.63 (−0.36, 1.70) | 0.99 (0.12, 1.83) | 0.82 (0.19, 1.48) | 0.53 |
| Etanercept 50 mg BIW, Ustekinumab 90 mg | 1.00 (0.01, 2.02) | 0.73 (−0.10, 1.59) | 0.90 (0.22, 1.52) | 0.61 |
| Infliximab 3 mg, Placebo | −4.12 (−8.54, −2.14) | −3.54 (−4.91, −2.48) | −3.56 (−4.64, −2.64) | 0.69 |
| Infliximab 5 mg, MTX | −1.80 (−2.74, −0.87) | −2.98 (−4.73, −1.47) | −2.07 (−2.97, −1.38) | 0.18 |
| Infliximab 5 mg, Placebo | −4.46 (−6.00, −3.43) | −3.33 (−4.77, −2.10) | −3.93 (−4.80, −3.18) | 0.2 |
| MTX, Placebo | −1.24 (−2.38, −0.25) | −2.30 (−3.43, −1.40) | −1.85 (−2.60, −1.07) | 0.12 |
| Placebo, Ustekinumab 45 mg | 4.15 (3.45, 4.86) | 3.78 (2.83, 4.68) | 3.99 (3.37, 4.61) | 0.37 |
| Placebo, Ustekinumab 90 mg | 3.99 (3.22, 4.77) | 4.32 (3.40, 5.28) | 4.07 (3.40, 4.70) | 0.44 |
PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly.
Table III.
Network meta-analysis of PASI 75 response rate between drugs for treating psoriasis.
| CSA | Etanercept | Infliximab | Ustekinumab | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Adalimumab | Briakinumab | 2.5 mg | 5 mg | 100 mg QW | 25 mg BIW | 25 mg QW | 50 mg BIW | 50 mg QW | 3 mg | 5 mg | MTX | Placebo | 45 mg | 90 mg |
| Adalimumab | – | 2.25 | 0.31 | 1.50 | 0.37 | 0.43 | 0.10 | 0.55 | 0.25 | 1.39 | 3.04 | 0.39 | 0.03 | 0.91 | 1.04 |
| (0.49,7.32) | (0.08,1.34) | (0.34,8.64) | (0.06,1.53) | (0.11,1.59) | (0.02,0.45) | (0.11,1.67) | (0.04,0.94) | (0.37,4.75) | (0.99,8.77) | (0.16,1.02) | (0.02,0.06) | (0.22,2.54) | (0.25,2.91) | ||
| Briakinumab | 0.44 | – | 0.14 | 0.67 | 0.16 | 0.20 | 0.04 | 0.23 | 0.11 | 0.62 | 1.37 | 0.18 | 0.01 | 0.40 | 0.46 |
| (0.14,2.03) | (0.03,1.01) | (0.12,6.22) | (0.04,0.61) | (0.07,0.69) | (0.01,0.19) | (0.05,1.03) | (0.03,0.37) | (0.18,2.46) | (0.44,4.88) | (0.05,0.70) | (0.01,0.03) | (0.15,1.02) | (0.18,1.17) | ||
| CSA 2.5 mg | 3.22 | 7.11 | – | 4.98 | 1.16 | 1.43 | 0.34 | 1.74 | 0.78 | 4.42 | 9.89 | 1.29 | 0.09 | 2.88 | 3.32 |
| (0.74,12.82) | (0.99,36.73) | (1.75,13.54) | (0.13,7.39) | (0.24,7.30) | (0.04,2.07) | (0.23,8.30) | (0.10,4.39) | (0.85,20.65) | (2.18,39.43) | (0.37,3.97) | (0.01,0.35) | (0.46,12.78) | (0.51,14.83) | ||
| CSA 5 mg | 0.67 | 1.49 | 0.20 | – | 0.24 | 0.29 | 0.07 | 0.36 | 0.16 | 0.91 | 2.06 | 0.44 | 0.02 | 0.60 | 0.69 |
| (0.12,2.95) | (0.16,8.29) | (0.07,0.57) | (0.02,1.67) | (0.04,1.67) | (0.01,0.47) | (0.03,1.95) | (0.01,1.02) | (0.12,4.84) | (0.30,9.18) | (0.14,1.57) | (0.00,0.08) | (0.07,2.96) | (0.08,3.38) | ||
| Etanercept | 2.73 | 6.15 | 0.86 | 4.15 | – | 1.23 | 0.28 | 1.46 | 0.66 | 3.85 | 8.51 | 1.11 | 0.07 | 2.48 | 2.85 |
| 100 mg QW | (0.65,17.41) | (1.63,23.99) | (0.14,7.79) | (0.60,51.05) | (0.35,5.34) | (0.08,1.07) | (0.62,3.62) | (0.22,2.09) | (0.86,19.38) | (2.13,40.28) | (0.26,5.97) | (0.02,0.24) | (0.80,7.72) | (0.91,8.85) | |
| Etanercept | 2.32 | 5.07 | 0.70 | 3.40 | 0.82 | – | 0.28 | 1.43 | 0.55 | 3.19 | 7.09 | 0.90 | 0.06 | 2.02 | 2.32 |
| 25 mg BIW | (0.63,8.84) | (1.44,14.66) | (0.14,4.22) | (0.60,27.08) | (0.19,2.89) | (0.06,1.16) | (0.39,4.95) | (0.15,1.65) | (0.77,11.49) | (1.92,22.79) | (0.24,3.19) | (0.02,0.13) | (0.72,4.84) | (0.81,5.63) | |
| Etanercept | 9.73 | 22.27 | 2.97 | 14.40 | 3.55 | 3.60 | – | 5.25 | 2.37 | 13.91 | 29.78 | 3.86 | 0.26 | 8.81 | 10.19 |
| 25 mg QW | (2.22,58.96) | (5.23,85.70) | (0.48,27.13) | (2.13,179.59) | (0.94,12.49) | (0.86,16.14) | (1.99,13.30) | (0.73,7.27) | (2.92,65.68) | (7.31,139.88) | (0.91,20.80) | (0.05,1.37) | (2.69,28.14) | (3.01,31.84) | |
| Etanercept | 1.83 | 4.29 | 0.58 | 2.75 | 0.69 | 0.70 | 0.19 | – | 0.45 | 2.65 | 5.70 | 0.74 | 0.05 | 1.68 | 1.85 |
| 50 mg BIW | (0.60,9.27) | (0.97,18.85) | (0.12,4.32) | (0.51,30.05) | (0.28,1.62) | (0.20,2.55) | (0.08,0.50) | (0.23,0.89) | (0.76,10.26) | (1.94,21.09) | (0.24,3.10) | (0.01,0.21) | (0.81,3.59) | (0.48,7.46) | |
| Etanercept | 4.05 | 9.31 | 1.28 | 6.17 | 1.51 | 1.81 | 0.42 | 2.21 | – | 5.75 | 12.64 | 1.65 | 0.11 | 3.75 | 4.28 |
| 50 mg QW | (1.07,23.01) | (2.69,31.37) | (0.23,10.48) | (0.98,71.10) | (0.48,4.51) | (0.61,6.84) | (0.14,1.36) | (1.12,4.33) | (1.43,27.06) | (3.64,54.57) | (0.44,7.93) | (0.04,0.30) | (1.36,9.87) | (1.56,11.25) | |
| Infliximab | 0.72 | 1.61 | 0.23 | 1.10 | 0.26 | 0.31 | 0.07 | 0.38 | 0.17 | – | 2.18 | 0.29 | 0.02 | 0.65 | 0.74 |
| 3 mg | (0.21,2.67) | (0.41,5.67) | (0.05,1.17) | (0.21,8.04) | (0.05,1.16) | (0.09,1.30) | (0.02,0.34) | (0.10,1.31) | (0.04,0.70) | (1.10,4.75) | (0.10,0.87) | (0.01,0.05) | (0.19,1.97) | (0.21,2.29) | |
| Infliximab | 0.33 | 0.73 | 0.10 | 0.49 | 0.12 | 0.14 | 0.03 | 0.18 | 0.08 | 0.46 | – | 0.13 | 0.01 | 0.29 | 0.34 |
| 5 mg | (0.11,1.01) | (0.20,2.28) | (0.03,0.46) | (0.11,3.34) | (0.02,0.47) | (0.04,0.52) | (0.01,0.14) | (0.05,0.51) | (0.02,0.27) | (0.21,0.91) | (0.06,0.31) | (0.00,0.02) | (0.09,0.78) | (0.11,0.90) | |
| MTX | 2.55 | 5.57 | 0.78 | 2.25 | 0.90 | 1.11 | 0.26 | 1.35 | 0.61 | 3.49 | 7.69 | – | 0.05 | 2.26 | 2.59 |
| (0.99,6.18) | (1.42,18.42) | (0.25,2.68) | (0.64,6.94) | (0.17,3.89) | (0.31,4.20) | (0.05,1.10) | (0.32,4.20) | (0.13,2.26) | (1.15,9.75) | (3.28,17.36) | (0.00,0.15) | (0.63,6.30) | (0.72,7.30) | ||
| Placebo | 30.93 | 84.29 | 11.71 | 55.90 | 13.57 | 16.61 | 3.83 | 20.24 | 9.10 | 53.49 | 117.81 | 19.04 | – | 33.57 | 38.81 |
| (16.26,58.97) | (37.74,187.34) | (2.86,69.09) | (12.23,452.23) | (4.22,41.45) | (7.77,43.27) | (0.73,18.81) | (4.73,75.32) | (3.34,24.47) | (19.24,158.08) | (54.46,286.70) | (6.83,242.20) | (20.28,55.73) | (22.22,64.79) | ||
| Ustekinumab | 1.09 | 2.48 | 0.35 | 1.66 | 0.40 | 0.49 | 0.11 | 0.60 | 0.27 | 1.54 | 3.43 | 0.44 | 0.03 | – | 1.19 |
| 45 mg | (0.39,4.55) | (0.98,6.47) | (0.08,2.18) | (0.34,14.71) | (0.13,1.26) | (0.21,1.38) | (0.04,0.37) | (0.28,1.23) | (0.10,0.74) | (0.51,5.35) | (1.28,10.57) | (0.16,1.58) | (0.02,0.05) | (0.34,4.29) | |
| Ustekinumab | 0.96 | 2.17 | 0.30 | 1.44 | 0.35 | 0.43 | 0.10 | 0.54 | 0.23 | 1.36 | 2.97 | 0.39 | 0.03 | 0.84 | – |
| 90 mg | (0.34,3.97) | (0.85,5.68) | (0.07,1.95) | (0.30,13.07) | (0.11,1.10) | (0.18,1.23) | (0.03,0.33) | (0.13,2.07) | (0.09,0.64) | (0.44,4.82) | (1.11,9.46) | (0.14,1.40) | (0.02,0.05) | (0.23,2.94) | |
PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly.
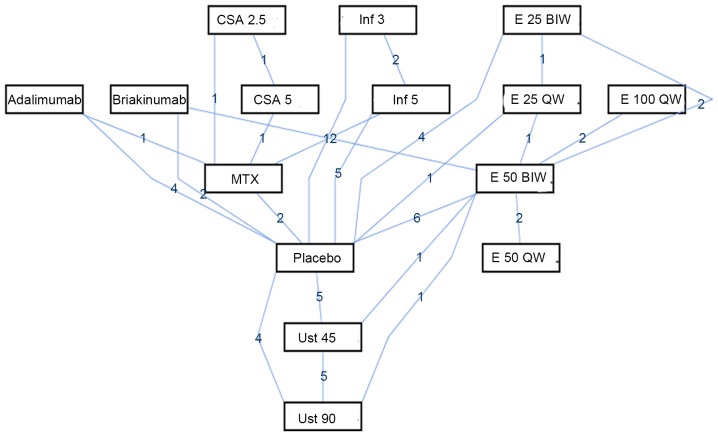
Figure 3.
Network of PASI 75 response rate. The figures on the blue edges refer to the comparison times. PASI, Psoriasis Area and Severity Index. Image generated using ADDIS software 1.16.5.
Table IV.
Network meta-analysis of PASI 50 response rate between drugs for treating psoriasis.
| Etanercept | Infliximab | Ustekinumab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Adalimumab | CSA 5 mg | 100 mg QW | 25 mg BIW | 25 mg QW | 50 mg BIW | 3 mg | 5 mg | MTX | Placebo | 45 mg | 90 mg |
| Adalimumab | – | 1.12 | 0.59 | 0.41 | 0.17 | 0.79 | 0.61 | 1.75 | 0.29 | 0.04 | 1.40 | 1.85 |
| (0.22,7.80) | (0.12,2.68) | (0.11,1.38) | (0.04,0.65) | (0.21,2.82) | (0.18,2.20) | (0.59,5.64) | (0.12,0.72) | (0.01,0.11) | (0.36,4.25) | (0.47,5.75) | ||
| CSA 5 mg | 0.89 | – | 0.51 | 0.35 | 0.15 | 0.67 | 0.55 | 1.57 | 0.26 | 0.03 | 1.21 | 1.59 |
| (0.13,4.63) | (0.06,3.81) | (0.05,2.02) | (0.02,0.94) | (0.10,4.15) | (0.08,3.08) | (0.27,8.03) | (0.05,1.01) | (0.00,0.13) | (0.17,6.72) | (0.22,8.98) | ||
| Etanercept | 1.71 | 1.97 | – | 0.70 | 0.29 | 1.33 | 1.06 | 3.04 | 0.50 | 0.05 | 2.36 | 3.14 |
| 100 mg QW | (0.37,8.01) | (0.26,16.88) | (0.22,2.31) | (0.08,1.10) | (0.58,3.37) | (0.27,4.26) | (0.85,11.44) | (0.12,2.03) | (0.02,0.15) | (0.70,7.67) | (0.90,10.08) | |
| Etanercept | 2.44 | 2.83 | 1.43 | – | 0.42 | 1.88 | 1.54 | 4.35 | 0.71 | 0.08 | 3.36 | 4.51 |
| 25 mg BIW | (0.72,9.29) | (0.49,18.45) | (0.43,4.52) | (0.19,0.88) | (0.60,7.67) | (0.56,3.92) | (1.84,10.84) | (0.25,1.96) | (0.04,0.12) | (1.56,6.53) | (1.92,8.78) | |
| Etanercept | 5.82 | 6.76 | 3.47 | 2.38 | – | 4.48 | 3.67 | 10.43 | 1.70 | 0.18 | 8.06 | 10.77 |
| 25 mg QW | (1.55,24.11) | (1.06,50.81) | (0.91,12.67) | (1.14,5.26) | (1.25,21.93) | (1.18,11.20) | (3.89,31.14) | (0.52,5.66) | (0.08,0.39) | (3.09,19.57) | (3.84,25.87) | |
| Etanercept | 1.27 | 1.48 | 0.75 | 0.53 | 0.22 | – | 0.80 | 2.28 | 0.37 | 0.04 | 1.76 | 2.36 |
| 50 mg BIW | (0.36,4.71) | (0.24,9.91) | (0.30,1.74) | (0.13,1.66) | (0.05,0.80) | (0.26,2.15) | (0.88,5.66) | (0.12,1.08) | (0.02,0.07) | (0.72,3.61) | (0.90,4.92) | |
| Infliximab | 1.63 | 1.83 | 0.94 | 0.65 | 0.27 | 1.25 | – | 2.87 | 0.47 | 0.05 | 2.18 | 2.91 |
| 3 mg | (0.45,5.59) | (0.33,11.99) | (0.23,3.72) | (0.26,1.79) | (0.09,0.85) | (0.47,3.79) | (1.49,5.97) | (0.17,1.18) | (0.02,0.11) | (0.79,5.79) | (1.00,7.85) | |
| Infliximab | 0.57 | 0.64 | 0.33 | 0.23 | 0.10 | 0.44 | 0.35 | – | 0.16 | 0.01 | 0.77 | 1.01 |
| 5 mg | (0.18,1.69) | (0.12,3.76) | (0.09,1.18) | (0.09,0.54) | (0.03,0.26) | (0.18,1.14) | (0.17,0.67) | (0.07,0.32) | (0.01,0.03) | (0.30,1.81) | (0.38,2.38) | |
| MTX | 3.47 | 3.90 | 2.01 | 1.41 | 0.59 | 2.68 | 2.13 | 6.07 | – | 0.16 | 4.73 | 6.32 |
| (1.40,8.48) | (0.99,20.60) | (0.49,8.47) | (0.51,4.03) | (0.18,1.93) | (0.92,8.59) | (0.85,5.91) | (3.09,14.56) | (0.07,0.42) | (1.65,12.90) | (2.13,17.45) | ||
| Placebo | 25.54 | 37.03 | 19.15 | 13.18 | 5.56 | 25.18 | 20.48 | 75.64 | 6.11 | – | 44.24 | 59.44 |
| (8.86,70.76) | (7.41,242.17) | (6.52,56.34) | (8.43,23.22) | (2.58,12.19) | (14.53,50.34) | (8.87,47.46) | (37.84,166.80) | (2.36,14.83) | (26.51,73.29) | (32.83,99.32) | ||
| Ustekinumab | 0.72 | 0.83 | 0.42 | 0.30 | 0.12 | 0.57 | 0.46 | 1.30 | 0.21 | 0.02 | – | 1.34 |
| 45 mg | (0.24,2.79) | (0.15,5.84) | (0.13,1.43) | (0.15,0.64) | (0.05,0.32) | (0.28,1.38) | (0.17,1.26) | (0.55,3.33) | (0.08,0.61) | (0.01,0.04) | (0.77,2.22) | |
| Ustekinumab | 0.54 | 0.63 | 0.32 | 0.22 | 0.09 | 0.42 | 0.34 | 0.99 | 0.16 | 0.02 | 0.75 | – |
| 90 mg | (0.17,2.12) | (0.11,4.46) | (0.10,1.12) | (0.11,0.52) | (0.04,0.26) | (0.20,1.11) | (0.13,1.00) | (0.42,2.64) | (0.06,0.47) | (0.01,0.03) | (0.45,1.30) | |
PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly.
Table V.
Network meta-analysis of PASI 90 response rate between different drugs used to treat psoriasis.
| Etanercept | Infliximab | Ustekinumab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Adalimumab | Briakinumab | CSA 5 mg | 25 mg BIW | 25 mg QW | 50 mg BIW | 3 mg | 5 mg | MTX | Placebo | 45 mg | 90 mg |
| Adalimumab | – | 3.84 (1.16,11.56) | 0.66 (0.11,4.09) | 0.33 (0.12,1.04) | 0.08 (0.02,0.33) | 0.70 (0.28,1.85) | 1.03 (0.35,3.58) | 1.50 (0.58,4.27) | 0.19 (0.08,0.44) | 0.03 (0.01,0.06) | 1.60 (0.63,4.31) | 1.71 (0.64,4.60) |
| Briakinumab | 0.26 (0.09,0.86) | – | 0.17 (0.03,1.36) | 0.09 (0.04,0.25) | 0.02 (0.00,0.08) | 0.18 (0.10,0.38) | 0.27 (0.08,1.14) | 0.39 (0.13,1.44) | 0.05 (0.02,0.16) | 0.01 (0.00,0.02) | 0.42 (0.18,1.11) | 0.45 (0.19,1.15) |
| CSA 5 mg | 1.51 (0.24,9.19) | 5.76 (0.73,39.77) | – | 0.50 (0.07,3.26) | 0.12 (0.01,0.91) | 1.09 (0.16,6.79) | 1.58 (0.25,9.60) | 2.27 (0.38,12.56) | 0.28 (0.06,1.23) | 0.05 (0.01,0.27) | 2.48 (0.34,15.66) | 2.65 (0.37,17.51) |
| Etanercept | 3.03 | 11.47 | 2.00 | – | 0.23 | 2.12 | 3.13 | 4.52 | 0.56 | 0.09 | 4.79 | 5.20 |
| 25 mg BIW | (0.96,8.27) | (4.06,27.25) | (0.31,14.20) | (0.06,0.74) | (1.04,4.02) | (0.90,11.65) | (1.51,13.74) | (0.17,1.64) | (0.04,0.18) | (1.92,11.25) | (2.01,11.76) | |
| Etanercept | 13.17 | 50.02 | 8.64 | 4.34 | – | 9.08 | 13.87 | 19.96 | 2.54 | 0.39 | 20.56 | 22.36 |
| 25 mg QW | (3.06,58.45) | (12.01,212.82) | (1.10,79.40) | (1.36,17.31) | (2.86,33.94) | (2.99,74.25) | (4.65,97.31) | (0.53,10.70) | (0.11,1.48) | (5.65,85.77) | (5.77,93.02) | |
| Etanercept | 1.42 | 5.43 | 0.92 | 0.47 | 0.11 | – | 1.46 | 2.12 | 0.27 | 0.04 | 2.27 | 2.46 |
| 50 mg BIW | (0.54,3.57) | (2.62,10.08) | (0.15,6.31) | (0.25,0.96) | (0.03,0.35) | (0.51,5.15) | (0.85,6.07) | (0.10,0.70) | (0.02,0.07) | (1.21,4.37) | (1.25,4.56) | |
| Infliximab | 0.97 | 3.71 | 0.63 | 0.32 | 0.07 | 0.68 | – | 1.45 | 0.18 | 0.03 | 1.55 | 1.68 (0.45,4.92) |
| 3 mg | (0.28,2.87) | (0.88,12.40) | (0.10,4.03) | (0.09,1.11) | (0.01,0.33) | (0.19,1.97) | (0.74,2.71) | (0.06,0.43) | (0.01,0.07) | (0.43,4.64) | ||
| Infliximab | 0.67 | 2.56 | 0.44 | 0.22 | 0.05 | 0.47 | 0.69 | – | 0.13 | 0.02 | 1.07 | 1.16 |
| 5 mg | (0.23,1.71) | (0.70,7.58) | (0.08,2.63) | (0.07,0.66) | (0.01,0.21) | (0.16,1.18) | (0.37,1.36) | (0.05,0.25) | (0.01,0.04) | (0.36,2.79) | (0.37,3.04) | |
| MTX | 5.31 (2.25,13.20) | 20.30 (6.20,62.98) | 3.55 (0.81,17.74) | 1.79 (0.61,5.77) | 0.39 (0.09,1.89) | 3.77 (1.43,10.05) | 5.50 (2.32,16.93) | 7.96 (3.98,19.45) | – | 0.16 (0.07,0.34) | 8.58 (3.19,23.75) | 9.20 (3.33,25.36) |
| Placebo | 33.59 | 130.12 | 22.14 | 11.08 | 2.57 | 23.55 | 35.13 | 51.03 | 6.34 | – | 53.93 | 58.50 |
| (16.46,70.67) | (52.97,292.89) | (3.73,137.96) | (5.43,26.18) | (0.67,8.86) | (13.83,43.77) | (13.99,103.31) | (23.99,121.47) | (2.92,13.46) | (29.01,100.56) | (29.98,110.26) | ||
| Ustekinumab | 0.63 | 2.41 | 0.40 | 0.21 | 0.05 | 0.44 | 0.65 | 0.93 | 0.12 | 0.02 | – | 1.09 |
| 45 mg | (0.23,1.58) | (0.90,5.51) | (0.06,2.91) | (0.09,0.52) | (0.01,0.18) | (0.23,0.82) | (0.22,2.31) | (0.36,2.81) | (0.04,0.31) | (0.01,0.03) | (0.66,1.67) | |
| Ustekinumab | 0.58 | 2.21 | 0.38 | 0.19 | 0.04 | 0.41 | 0.60 | 0.86 | 0.11 | 0.02 | 0.92 | – |
| 90 mg | (0.22,1.57) | (0.87,5.33) | (0.06,2.69) | (0.09,0.50) | (0.01,0.17) | (0.22,0.80) | (0.20,2.24) | (0.33,2.74) | (0.04,0.30) | (0.01,0.03) | (0.60,1.52) | |
PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly.
Table VI.
Rank analysis of PASI 90 response rate of the drugs for treating psoriasis.
| Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 | Rank 9 | Rank 10 | Rank 11 | Rank 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | 0.01 | 0.03 | 0.05 | 0.13 | 0.21 | 0.28 | 0.22 | 0.07 | 0.01 | 0.00 | 0.00 | 0.00 |
| Briakinumab | 0.86 | 0.08 | 0.03 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CSA 5 mg | 0.03 | 0.07 | 0.04 | 0.06 | 0.07 | 0.09 | 0.14 | 0.25 | 0.17 | 0.05 | 0.01 | 0.00 |
| Etanercept 25 mg BIW | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.23 | 0.59 | 0.14 | 0.00 | 0.00 |
| Etanercept 25 mg QW | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.09 | 0.81 | 0.07 |
| Etanercept 50 mg BIW | 0.00 | 0.00 | 0.00 | 0.03 | 0.07 | 0.18 | 0.35 | 0.35 | 0.01 | 0.00 | 0.00 | 0.00 |
| Infliximab 3 mg | 0.02 | 0.04 | 0.09 | 0.09 | 0.22 | 0.24 | 0.20 | 0.09 | 0.02 | 0.00 | 0.00 | 0.00 |
| Infliximab 5 mg | 0.05 | 0.23 | 0.16 | 0.30 | 0.18 | 0.08 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MTX | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.18 | 0.70 | 0.10 | 0.00 |
| Placebo | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.93 |
| Ustekinumab 45 mg | 0.01 | 0.18 | 0.35 | 0.21 | 0.14 | 0.08 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ustekinumab 90 mg | 0.02 | 0.37 | 0.28 | 0.16 | 0.10 | 0.05 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Rank 1 indicates the best rating, while Rank 12 indicates the worst rating. PASI, Psoriasis Area and Severity Index; MTX, methotrexate; CSA, cyclosporin A; BIW, twice weekly; QW, once weekly.
Discussion
In the present study, a network meta-analysis was performed to systematically review and compare the efficacy of seven drugs used at different doses for treating moderate to severe psoriasis. Based on the results of the network analysis, infliximab (5 mg) may be an appropriate option to treat moderate to severe psoriasis.
Psoriasis has been reported to be associated with a high concentration of TNF-α (41) and infliximab treatment can neutralize the biological activity of TNF-α (42). However, the role of the TNF-α in the pathogenesis of psoriasis remains unclear. Previous studies have reported that TNF-α may serve an important role in the upstream of the inflammatory responses of psoriasis (43,44). An in vitro study determined that infliximab was able to inhibit the activation of skin-homing T cells and impair the antigen-presenting capacity of immature dendritic cells in psoriasis patients (43). However, another TNF-α inhibitor, etanercept, has been found to be effective in the treatment of psoriasis by reducing the Th17 cell products, as well as the production of IL-17, IL-22, IL-23 and inducible NO synthase from dendritic cells (44). Thus, it has been suggested that the infliximab may serve a different role with other treatments on moderate to severe psoriasis.
Although the present meta-analysis indicated that infliximab treatment had a high PASI score, a higher percentage of adverse events were observed in infliximab-treated patients compared with those in the placebo group (18), indicating that infliximab treatment induces adverse effects. In addition, infliximab treatment increases the incidence of infusion reactions (45). However, these outcomes were not considered to be important due to the small sample size of each study or the fact that the data were unavailable. Thus, the therapeutic effect of the infliximab should be systematically assessed in further studies. Besides, the dosage and treatment duration of infliximab should be optimized according to the disease severity of psoriasis.
In the present study, briakinumab and ustekinumab (90 mg) treatments were superior to other treatments for PASI 90 response. Thus, anti-IL-12/23 monoclonal antibodies appear to be more appropriate compared with anti-TNF-α treatment for treating moderate to severe psoriasis. However, briakinumab and ustekinumab showed no significantly improved therapeutic effect in PASI 75 and PASI 50 responses when compared with the anti-TNF-α treatments. In addition, the long-term safety profile, including severe infections and cardiac disorders, should be evaluated in further studies with large sample sizes and strict study design.
To the best of our knowledge, the present study is the first network meta-analysis for evaluating the efficacy of various treatments for moderate to severe psoriasis. The current results may provide information for clinician and patients on the selection of the suitable treatment for moderate to severe psoriasis. However, there were also several limitations in the present meta-analysis. Firstly, due to unavailable data in certain included studies, confounding variables could not be adjusted and subgroup analysis was not performed to reduce the effect of the confounding variables. Secondly, due to unknown bias, the network analyses of PASI 75 and PASI 50 responses were performed using an inconsistency model. Finally, the results of the network meta-analysis should be pooled only by a random effects model. Thus, the pooled results may be conservative and certain borderline significant effects may have been ignored (46).
In conclusion, the present meta-analysis results suggested that infliximab (5 mg) may be a superior option compared with other drugs for treating moderate to severe psoriasis due to the relatively high PASI scores of patients. However, despite the high PASI 90 responses, the efficacy of ustekinumab (90 mg) and briakinumab were also high and therefore should be investigated in further studies.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team, corp-author. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 4.Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 5.Asahina A, Nakagawa H, Etoh T, Ohtsuki M Adalimumab M04-688 Study Group, corp-author. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: Efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37:299–310. doi: 10.1111/j.1346-8138.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths C, Sterry W, Brock F, Dilleen M, Stefanidis D, Germain JM, Mallbris L. Pattern of response in patients with moderate-to-severe psoriasis treated with etanercept. Br J Dermatol. 2015;172:230–238. doi: 10.1111/bjd.13139. [DOI] [PubMed] [Google Scholar]
- 7.Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, Estojak J, Molta CT, Freundlich B. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340:c147. doi: 10.1136/bmj.c147. [DOI] [PubMed] [Google Scholar]
- 8.Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661–668. doi: 10.1111/j.1365-2133.2011.10419.x. [DOI] [PubMed] [Google Scholar]
- 9.Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: A randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86–92. doi: 10.1016/j.jaad.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer I, Hacker J, Rustenbach SJ, Radtke M, Franzke N, Augustin M. Concordance of the psoriasis area and severity index (PASI) and patient-reported outcomes in psoriasis treatment. Eur J Dermatol. 2010;20:62–67. doi: 10.1684/ejd.2010.0815. [DOI] [PubMed] [Google Scholar]
- 11.Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, Nast A, Franke J, Antoniou C, Arenberger P, Balieva F, et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch Dermatol Res. 2011;303:1–10. doi: 10.1007/s00403-010-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 14.Barker J, Hoffmann M, Wozel G, Ortonne JP, Zheng H, van Hoogstraten H, Reich K. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: Results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165:1109–1117. doi: 10.1111/j.1365-2133.2011.10615.x. [DOI] [PubMed] [Google Scholar]
- 15.Cassano N, Miracapillo A, Coviello C, Loconsole F, Bellino M, Vena GA. Treatment of psoriasis vulgaris with the two-compound product calcipotriol/betamethasone dipropionate followed by different formulations of calcipotriol. Clin Drug Investig. 2006;26:227–233. doi: 10.2165/00044011-200626040-00008. [DOI] [PubMed] [Google Scholar]
- 16.Flytstrom I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158:116–121. doi: 10.1111/j.1365-2133.2007.08284.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, Heffernan M, Miller B, Hamlin R, Lim L, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: Double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, Bala M, Marano CW, Menter A. Infliximab induction therapy for patients with severe plaque-type psoriasis: A randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:652–660. doi: 10.1111/j.1365-2133.2011.10418.x. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, Gaspari AA, Ling M, Weinstein GD, Nayak A, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–1632. doi: 10.1001/archderm.139.12.1627. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths CE. Comparing biological therapies in psoriasis: Implications for clinical practice. J Eur Acad Dermatol Venereol. 2010;24(Suppl 6):S10–S14. doi: 10.1111/j.1468-3083.2010.03831.x. [DOI] [PubMed] [Google Scholar]
- 22.Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, Bossuyt PM, Bos JD, de Rie MA. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–665. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 23.Ho SG, Yeung CK, Chan HH. Methotrexate versus traditional Chinese medicine in psoriasis: A randomized, placebo-controlled trial to determine efficacy, safety and quality of life. Clin Exp Dermatol. 2010;35:717–722. doi: 10.1111/j.1365-2230.2009.03693.x. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi A, Kato T, Kato M, Song M, Nakagawa H Japanese Ustekinumab Study Group, corp-author. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: Long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252. doi: 10.1111/j.1346-8138.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- 25.Laburte C, Grossman R, Abi-Rached J, Abeywickrama K, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis. Br J Dermatol. 1994;130:366–375. doi: 10.1111/j.1365-2133.1994.tb02935.x. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB PHOENIX 1 study investigators, corp-author. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB Etanercept Psoriasis Study Group, corp-author. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 28.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 29.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C, Gottlieb AB. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(31):e1–e15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M, Papp K. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 32.Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, Zitnik R, van de Kerkhof PC, Melvin L Etanercept Psoriasis Study Group, corp-author. A global phase III randomized controlled trial of etanercept in psoriasis: Safety, efficacy and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 33.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE. EXPRESS study investigators: Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 34.Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, Camez A. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: Results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158:549–557. doi: 10.1111/j.1365-2133.2007.08236.x. [DOI] [PubMed] [Google Scholar]
- 35.Torii H, Nakagawa H Japanese Infliximab Study investigators, corp-author. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59:40–49. doi: 10.1016/j.jdermsci.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, Li S, Kim KJ, Kim TY, Choi JH, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: A phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL) J Dermatol Sci. 2011;63:154–163. doi: 10.1016/j.jdermsci.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 38.van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, Leigheb G, Camacho FM, Forsea D, Zang C, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: A randomized controlled trial with open-label extension. Br J Dermatol. 2008;159:1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, Wang BX, Zhang FR, Li CY, Liu XM, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: A randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl) 2012;125:1845–1851. [PubMed] [Google Scholar]
- 40.Cassano N, Loconsole F, Miracapillo A, Travaglini M, Digiuseppe MD, Congedo M, Galluccio A, Buquicchio R, Mastrandrea V, Filieri M, et al. Treatment of psoriasis with different dosage regimens of etanercept: Preliminary results from the Tαranta plastic study group. Int J Immunopathol Pharmacol. 2010;23:797–802. doi: 10.1177/039463201002300314. [DOI] [PubMed] [Google Scholar]
- 41.Mussi A, Bonifati C, Carducci M, D'Agosto G, Pimpinelli F, D'Urso D, D'Auria L, Fazio M, Ameglio F. Serum TNF-alpha levels correlate with disease severity and are reduced by effective therapy in plaque-type psoriasis. J Biol Regul Homeost Agents. 1997;11:115–118. [PubMed] [Google Scholar]
- 42.Cooper C, Shafran S, Greenbloom S, Enns R, Farley J, Hilzenrat N, Williams K, Elkashab M, Abadir N, Neuman M. Single-dose infliximab in hepatitis C genotype 1 treatment-naive patients with high serum tumour necrosis factor-alpha does not influence the efficacy of pegylated interferon alpha-2b/ribavirin therapy. Can J Gastroenterol Hepatol. 2014;28:35–40. doi: 10.1155/2014/367131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedini C, Nasorri F, Girolomoni G, Pità Od, Cavani A. Antitumour necrosis factor-alpha chimeric antibody (infliximab) inhibits activation of skin-homing CD4+ and CD8+ T lymphocytes and impairs dendritic cell function. Br J Dermatol. 2007;157:249–258. doi: 10.1111/j.1365-2133.2007.07945.x. [DOI] [PubMed] [Google Scholar]
- 44.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 45.Steenholdt C, Svenson M, Bendtzen K, Thomsen OØ, Brynskov J, Ainsworth MA. Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:51–58. doi: 10.1111/j.1365-2036.2011.04682.x. [DOI] [PubMed] [Google Scholar]
- 46.Van Valkenhoef G, Tervonen T, Zwinkels T, de Brock B, Hillege H. ADDIS: A decision support system for evidence-based medicine. Decis Support Syst. 2013;55:459–475. doi: 10.1016/j.dss.2012.10.005. [DOI] [Google Scholar]