Abstract
Background
Half of all patients with diabetes develop diabetic peripheral neuropathy (DPN), a complication leading to reduced mobility and quality of life. Although there are no proven pharmacologic approaches to reduce DPN risk or slow its progression, evidence suggests that physical activity may improve symptoms and enhance peripheral nerve regeneration.
Objective
The aim of the study will be to determine the impact of an intense lifestyle intervention on neuropathy progression and quality of life in individuals with DPN.
Design
The study will be a randomized controlled trial.
Setting
The study will be conducted at 2 academic medical centers.
Participants
The participants will be 140 individuals with type 2 diabetes and mild to moderate DPN.
Intervention
The intervention group will receive 18 months of supervised exercise training, actigraphy-based counseling to reduce sedentary behavior, and individualized dietary counseling. Control group participants will receive diet and activity counseling at baseline and at 9 months.
Measurements
The primary outcomes are neuropathy progression as measured by intraepidermal nerve fiber density in a distal thigh skin biopsy and the Norfolk Quality of Life–Diabetic Neuropathy score. Secondary outcomes include pain, gait, balance, and mobility measures.
Limitations
Due to the combined intervention approach, this protocol will not be able to determine which intervention components influence outcomes. There also may be difficulty with participant attrition during the 18-month study intervention.
Conclusions
The Activity for Diabetic Polyneuropathy (ADAPT) protocol resulted from a collaboration between physical therapists and neurologist researchers that includes as primary outcomes both a quality-of-life measure (NQOL-DN) and a physiologic biomarker (IENFD). It has the potential to demonstrate that an intensive lifestyle intervention may be a sustainable, clinically effective approach for people with DPN that improves patient outcomes and can have an immediate impact on patient care and future clinical trials.
Approximately 30 million Americans have diabetes, half of whom will develop diabetic peripheral neuropathy (DPN).1 Diabetic peripheral neuropathy causes reduced mobility and quality of life due to pain, sensory loss, impaired balance, foot ulcers, and fall-related injury.2–4 There are no proven pharmacologic approaches to reduce DPN risk or slow its progression.5 Treatment for DPN primarily focuses on symptom management and improved glycemic control,5 although several large studies have failed to demonstrate an effect of intensive glycemic control on neuropathy.6 A growing body of evidence suggests that physical activity is a promising therapeutic approach that may improve symptoms and enhance peripheral nerve regeneration capacity.7–9
Sedentary behavior, defined as seated or recumbent postures without increased energy expenditure above the resting level, is associated with multiple metabolic risk factors, including increased waist circumference, high lipid and cholesterol levels, decreased glycemic control, and increased blood pressure.10–12 Recent small trials suggest that behavioral counseling designed to reduce sedentary behavior can have reduce sedentary time in both healthy adults and patients with diabetes.13,14
The Activity for Diabetic Polyneuropathy (ADAPT) study proposes to combine exercise with approaches to reduce sedentary behavior. The ADAPT study will randomize people with DPN to either an intensive lifestyle intervention with moderate-intensity supervised exercise, sedentary behavior counseling based on actigraphy data, and dietary counseling or a standard of care counseling group to examine the impact on DPN progression and quality of life. This single-blinded study will randomize 140 participants with mild to moderate DPN at 1 of 2 sites, the University of Utah and the University of Kansas Medical Center, and follow them for 18 months. We hypothesize that improvements in both neuropathy progression and quality of life will be observed in the intensive intervention group compared with the standard group.
The progression of neuropathy will be determined via skin biopsy with measurement of intraepidermal nerve fiber density (IENFD) at the distal thigh, which is a reliable and validated direct measure of small nociceptive fibers in the skin.9,15 Quality of life will be assessed via the Norfolk Quality of Life–Diabetic Neuropathy (NQOL-DN) scale.16 A secondary aim of the study will be to evaluate the minimal clinically important difference in IENFD by evaluating the relationship between change in IENFD and change in NQOL-DN scores, as well as change in the participant-reported measures of pain, sleep, and general health. In addition to the primary outcomes, multiple other secondary outcomes will be assessed for potential change during the intervention. These secondary outcomes include neuropathy signs and symptoms (including pain), measures of physical function, sedentary behavior patterns, and other relevant health measures. Outcome measures will be assessed at baseline, 9 months, and 18 months.
The ADAPT trial protocol presents a unique opportunity to examine sustainable, meaningful improvement in participant-centered outcomes with a comprehensive intervention. These results could have an immediate impact on the physical therapy treatment approach and selection of outcomes for people with DPN.
Method
Study Sites
The ADAPT study will be conducted at 2 academic medical centers: the University of Utah (Salt Lake City, Utah; lead site) and the University of Kansas Medical Center (KUMC) (Kansas City, Kansas).
Study Design
ADAPT is a single-blinded randomized controlled trial comparing the effect of an 18-month intensive lifestyle intervention with that of standard of care. Randomization will occur using a 1:1 allocation ratio following confirmation of eligibility and completion of all required baseline procedures. Randomization codes were constructed using an interactive Web response system created in a Research Electronic Data Capture (REDCap) project.17 Random permuted blocks were established to ensure balanced randomization over time with stratification by study site (Kansas and Utah), age (30–54 years and 55–70 years), and body mass index (BMI) (<30 and >30 kg/m2). The stratification cutoffs for age and BMI reflect anticipated median values.
Recruitment Methods
Both study sites will recruit directly from neurology clinics, through referral at other clinics, and through study information disseminated to the community. The specific recruitment strategies will differ at each site to take advantage of local recruitment resources, including research participant registry programs.
Enrollment Criteria
A total of 140 participants will be enrolled and followed for 18 months. Participants must be between 30 and 70 years of age, have a confirmed diagnosis of type 2 diabetes (defined by American Diabetes Association [ADA] criteria5), and have confirmed DPN based on the Toronto Diabetic Neuropathy Expert Group consensus criteria.18 Diabetic peripheral neuropathy severity must be moderate (Utah Early Neuropathy Scale [UENS] score=5–18).19 Participants also must identify a primary care physician or provider who will be responsible for managing changes to medication or other medical concerns that might arise during the study.
Exclusion criteria are: (1) any alternative cause for peripheral neuropathy, including abnormal serum vitamin B12 or serum protein electrophoresis and immunofixation, or family history of nondiabetic neuropathy; (2) history of foot ulceration or amputation; (3) contraindications for skin biopsy, such as use of anticoagulant medications, severe lower extremity edema, or other condition that would limit healing; (4) any serious medical condition that might shorten the life span, prevent completion of a graded maximal exercise test, or hinder adherence to exercise; (5) obesity of sufficient severity (BMI >45 kg/m2) to limit exercise equipment use; (6) premenopausal women who are pregnant or who plan to become pregnant during the study period; (7) diagnosis of hypothyroidism without adequate thyroid hormone supplementation; and (8) unwilling or unable to adhere to study procedures.
A standardized, graded maximal exercise test will be performed prior to randomization to ensure sufficient exercise tolerance to safely adhere to the study intervention, to obtain baseline peak volume of oxygen update as an outcome measure for aerobic fitness, and to individually prescribe exercise intensity during the exercise intervention. The exercise test will use a recumbent stepper (T5XR, NuStep Inc, Ann Arbor, Michigan) with a metabolic cart (TrueOne 2400, ParvoMedics, Sandy, Utah) and integrated electrocardiography under physician supervision. This test has been used previously and validated in a similar population.8,20–22
Intervention Procedures
Eligible study participants will be randomized to the standard of care control group or the intensive intervention group using the allocation methods previously described. There will be no prohibited interventions (eg, concomitant activity, medication changes) in either group during the study aside from enrollment in another investigational clinical trial.
Standard of care counseling intervention.
Participants in this group will receive diet and activity counseling at baseline and at 9 months. A 20-minute standardized nutritional counseling session will be provided by a registered dietitian following ADA guidelines.5 During this session, the results of body composition, blood tests, and 3-day food record will be reviewed, and standard handouts will be provided.
Participants also will receive a 20-minute standardized activity counseling session with a physical therapist. During this session, current activity levels will be reviewed and information about the ADA exercise goal will be shared (150 minutes of light aerobic exercise each week).5 The Be Active Your Way: A Guide for Adults booklet23 will be provided, with guidance and recommendations for the appropriate activity stage for each participant. At the end of the study (ie, after the 18-month assessment), standard counseling intervention participants will receive a personal activity tracker wristband (Vivofit, Garmin International, Olathe, Kansas) for their personal use and to keep after the study.
Intensive intervention.
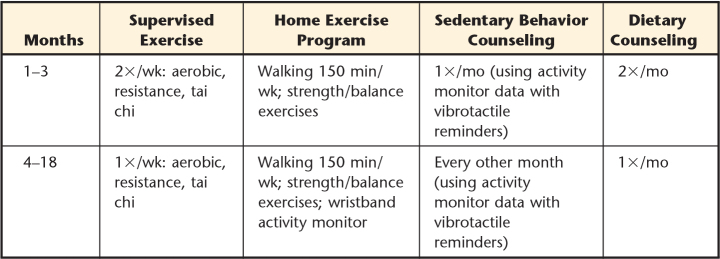
Participants in this group will receive supervised exercise training, actigraphy-based behavioral change counseling directed toward reducing sedentary behavior, and individualized dietary counseling. Table 1 provides an overview and timing of each component in the intensive intervention over the 18-month study period. The study teams will have the flexibility to customize the intervention to increase adherence and maximize safety based on individual participant needs.
Table 1.
Intensive Intervention Components
|
Supervised exercise training sessions will include aerobic exercise, resistance exercise, and balance exercises. At the beginning of each session, resting blood pressure, heart rate, and blood glucose will be assessed. At least once per week, a visual foot examination will be performed by the study team to ensure the absence of developing foot ulcers or other skin conditions.
The intensity of the aerobic exercise will be individually prescribed based on the results of the graded peak exercise test. Heart rate reserve will be calculated as the difference between peak heart rate during the test and resting heart rate. The aerobic exercise goal will start at 30 minutes of a heart rate representing 50% effort using the following formula:
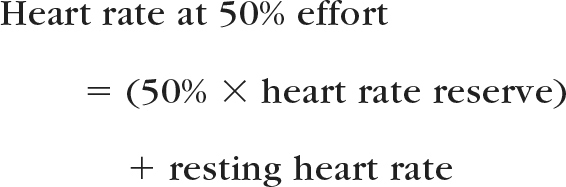
The supervised aerobic intervention will be gradually progressed in duration from 30 to 50 minutes and in intensity from 50% to 70% effort over the first 7 weeks, then maintained for the remainder of the study.
Resistance exercise will include 3 strengthening exercises for the upper extremities (biceps curl, triceps extension, seated row) and 3 strengthening exercises for the lower extremities (partial squats, standing abduction, and heel raises). An estimate of 1-repetition-maximum (1RM) for each exercise will be determined during weeks 1, 6, and 10 and as indicated through the remainder of the study. The resistance will be gradually progressed from 2 sets of 15 repetitions at 40% to 50% of 1RM to 3 sets of 15 repetitions at 60% to 70% 1RM.
Tai chi exercise will be used as a warm-up/cool-down activity during each session to promote improved balance responses. A poster illustrating several tai chi movements will be provided for reference, with supervision provided by the study team for safety and guidance about correct form. The duration of tai chi will be 10 minutes each session.
A customized home exercise program will be provided starting at week 2 to increase physical activity at home. In general, this program will be progressed from an initial walking program at 150 minutes per week to gradually introduce strength and balance exercises. At week 12, when supervised exercise sessions are decreased to one time per week, participants will receive a personal activity tracker wristband (Vivofit, Garmin International) for their personal use during the study and to keep after the study. This tracker will be used as a motivational tool to set and monitor individual activity goals during the remainder of the study.
Initial counseling to reduce sedentary behavior will be based on 7-day actigraphy data. Each participant will wear a small inclinometer (activPAL VT, PAL Technologies Ltd, Glasgow, United Kingdom) taped to the skin of the anterior thigh to monitor activity. A report on daily activity and sedentary behavior patterns will be reviewed during the counseling sessions using an interview guide (Appendix) developed through a review of literature24–26 and beta tested with several older adults. The intent of the counseling sessions is to integrate information about quantity and timing of sedentary activity with individual contextual determinants of sedentary behavior.
After the initial counseling session, participants will wear the activity monitor with an additional feature that is not part of the initial data collection period, a vibrotactile reminder that will activate after a set period (eg, 20 minutes) of sustained seated or lying time, to prompt them to stand or walk. These vibrotactile reminders will be worn for 7 days once each month or every other month (Tab. 1); data from each of these 7-day periods will be reviewed with participants during subsequent counseling sessions to develop goals to encourage additional reduced sedentary behavior.
Individualized dietary counseling sessions with a registered dietitian will be provided twice each month for the first 12 weeks and monthly thereafter. The objectives of these sessions are to recommend dietary changes based on the personalized diet analysis; encourage daily food tracking; and work toward goals of improved blood glucose, improved body composition, and nutritional support for physical activity through a well-balanced, healthy diet.
Outcome Measures
Study team members at each study site who are performing clinical examinations and assessing primary outcomes will be blinded to treatment group and baseline data. Only nonblinded study personnel have access to the REDCap data system, where the group assignment is determined as described previously. Prior to each return visit, participants will be reminded about the importance of blinding the assessors to group assignment. Unblinding may occur in case of medical emergency after discussion with the medical monitor.
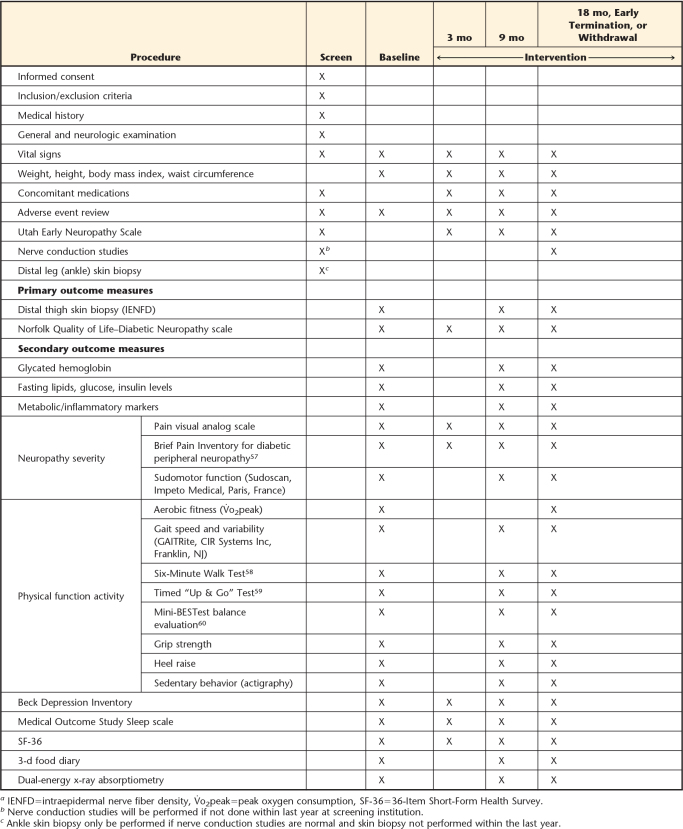
A schedule of study events, with a comprehensive list of outcome measures, is provided in Table 2. Several steps have been taken by the study team to promote consistency of data collection, including the development of an extensive assessment protocol guide shared by the 2 sites with identical forms and instructions for all tests and practice and competency assessment training of assessors at both sites.
Table 2.
Activity for Diabetic Polyneuropathy (ADAPT) Study Schedule of Eventsa
|
The ADAPT study has 2 co-primary outcome measures. Intraepidermal nerve fiber density at the distal thigh will be used to objectively assess neuropathy severity, and the NQOL-DN scale will be used to evaluate the effect of treatment on quality of life. Intraepidermal nerve fiber density via skin biopsy is a direct measure of small nociceptive fibers in the skin, which are preferentially affected by early DPN.27 Although these small unmyelinated axons may be particularly vulnerable to pathology, they also have greater regenerative capacity than large myelinated axons.28,29 Commonly used clinical scales and surrogate measures (eg, nerve conduction studies) primarily assess large axons and do not change appreciably in early DPN.30 Our previous studies9,31–33 have indicated that IENFD is reliable and responsive to change with lifestyle interventions in people with diabetes.
A 3-mm punch skin biopsy will be performed at the distal lateral thigh, 10 cm proximal to the superior margin of the patella. Tissue will be fixed, processed, and stained with protein gene product 9.5, and IENFD will be determined at the University of Utah Cutaneous Nerve Laboratory using established methods and criteria for counting fibers.15
The NQOL-DN scale16 is the other co-primary outcome measure. The NQOL-DN was developed specifically for people with DPN, and this participant report measure contains small- and large-fiber subscores that are individually responsive to symptomatic pharmacologic treatment. Participants will be asked to complete this questionnaire during the assessment sessions.16
Data Analysis
Data management.
Study data will be collected and managed using REDCap electronic data capture tools hosted at the University of Utah.17 REDCap is a secure, Web-based application designed to support data capture for research studies. The REDCap system provides an interface for validated data entry, audit trails for tracking data manipulation and export procedures, and automated export procedures for data downloads for statistical analysis.
Sample size calculations.
The preliminary analyses of longitudinal IENFD measurements among participants with DPN in our previous studies (unpublished data) indicated a mean IENFD slope of 0.98 per year, with an adjusted standard deviation of 1.03 per year after controlling for baseline IENFD. Using these estimates, 112 participants will provide 80% power with a 2-sided α of .05 to detect a 50% reduction in the mean change in the IENFD over 18 months. Allowing for a 20% attrition rate at 18 months, 140 participants will be randomized.
Assuming a 14-point standard deviation for the change in the NQOL-DN total score based on previous research,34,35 112 participants will provide at least 80% power with a 2-sided α of .05 to detect a difference in the mean change of the total score of 7.5 points between the treatment groups. This power calculation is conservative, as adjustment for the baseline NQOL-DN total score in the statistical analysis can be expected to lead to a smaller detectable effect.
Statistical analysis.
Longitudinal mixed-effects models will be applied to continuous variables (eg, IENFD) to compare mean change from baseline to 9- and 18-month assessments between intervention and control groups.36 Univariate descriptive summaries will be provided as a preliminary analysis, and transformations will be sought for variables failing to meet distributional assumptions prior to subsequent analyses. An unstructured covariance matrix will account for serial correlations in outcome measurements within participants.37 The model will constrain baseline means of the outcome to be equal in the treatment groups. Adjustment for the baseline level accounts for regression to the mean, thus increasing statistical power for estimating treatment effects. Linear contrasts will be constructed to estimate: (1) the mean difference in each outcome between the treatment groups at month 18, (2) the mean difference in each outcome between the treatment groups at month 9, and (3) the difference in the estimated treatment effects between months 9 and 18, and (4) the effect of the treatment on mean slope from baseline through month 18. The first comparison will be the primary assessment of treatment effect. The second comparison will evaluate early treatment effect, The third comparison will evaluate the persistence of the early effect to 18 months. The last comparison will provide an overall assessment of the treatment effect incorporating both follow-up visits.
Intraepidermal nerve fiber density will be a co-primary outcome and the primary physiologic measure for evaluating efficacy. The global NQOL-DN will be the other co-primary outcome. Because these 2 outcomes address fundamentally distinct questions, the statistical hypothesis tests will be performed on a comparison-wise basis using a 2-sided α of .05 without adjustment for multiple comparisons.38 Secondary analyses also will be performed on a comparison-wise basis, but study-wise type 1 error will be estimated using bootstrap resampling,39 and interpretation of results will account for both comparison-wise and study-wise type 1 error rates.
The potential for bias due to attrition will be addressed through the mixed-effects modeling approach. This approach will mitigate the effects of attrition after the 9-month visit by incorporating data from baseline and 9 months for participants who subsequently drop out when estimating treatment effects at 18 months. Multiple imputation will be used to further limit the effects of missing outcome scores.40 The imputation model will incorporate outcome measurements at the 3-month visit to reduce the potential for bias resulting from attrition after 3 months.
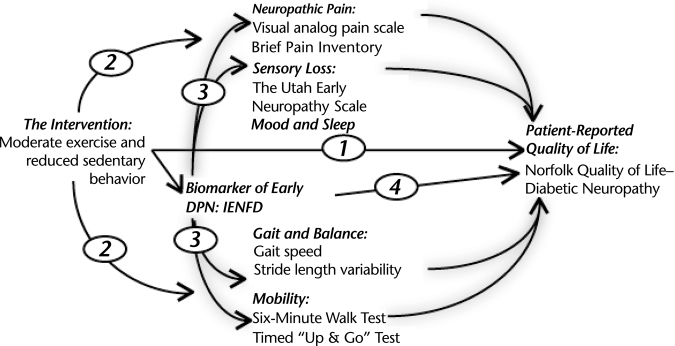
Mediation analyses will be performed using the conceptual model presented in the Figure. This analysis will explore the extent to which the intervention affects quality of life directly via a general health effect, independent of IENFD or mediated by IENFD.
Figure.
Conceptual model for the mediation analysis. This analysis will explore the extent to which the intervention affects quality of life directly via a general health effect (1) or via intermediary variables such as pain, sensory loss, fall risk, and mobility. The treatment effects can either occur independent of intraepidermal nerve fiber density (IENFD) effects (2) or be “mediated” by IENFD (3). Lastly, it is possible that a portion of the treatment benefit could be mediated purely by IENFD without influence of the other intermediate variables (4). DPN=diabetic peripheral neuropathy.
Data monitoring.
An independent safety monitor (Jennifer Majersik, MD, University of Utah) will review all significant adverse events (see below for details) or concerns about data inaccuracy on an ongoing basis with quarterly reports. The study will be modified or stopped if it is determined by the safety monitor in consultation with the University of Utah Institutional Review Board (IRB) and investigators that the outlined research plan is unsafe for participants.
Adverse events.
Adverse events that are unexpected, related to study procedures, and place participants at increased risk of harm will be reported to the University of Utah IRB as soon as possible, but in all cases within 10 working days. An adverse event is considered unexpected if it is not a known risk of the study procedures and is not consistent with the expected natural progression of any underlying disease or condition of the participant. The Common Terminology Criteria for Adverse Events (version 4.0, 2010)41 will be used to report the grade or severity of each adverse event. All grade 1 to 2 adverse events (mild or moderate) will be reported to the local site principal investigator; grade 3 to 5 adverse events will be reported to the primary principal investigator (J.R.S.) and the safety monitor at the University of Utah. Serious adverse events that are unexpected and possibly related to the study must be reported within 24 hours to the University of Utah IRB. Serious adverse events are defined as death, life-threatening events, hospital admission, permanent disability, or congenital anomaly.
Ethics and Dissemination
Regulatory oversight for this study is provided by the University of Utah IRB. A reliance agreement was established for this study between the 2 institutions for consistency in the informed consent document and compliance with federal regulations. The reliance agreement established the University of Utah IRB as the authority for reviewing the study, approving consent forms, and reviewing protocol amendments. The KUMC IRB completed a facilitated review, with the decision to accept and rely on the approval issued by the University of Utah IRB.
The informed consent process will be initiated when potential participants meet with the primary investigators or other IRB-approved study personnel. At the meeting, the protocol will be described in detail, and the informed consent form will be reviewed in detail. Potential participants will be offered the opportunity to ask questions or take additional time to review. Once the consent statement has been signed, the participant will be enrolled, and screening assessments may proceed. Each participant in the ADAPT study will be asked to contribute DNA and serum plasma to a biobank as an optional item on the consent form. Access to these samples and guidelines for future study will be governed under the individual IRB agreements at the participating institutions.
Information about potential and enrolled participants will be kept confidential through secure storage of signed consent forms and printed study forms, storage of electronic data in the REDCap database, and encrypting data electronically transferred between the 2 study sites.
Financial and other competing interests will be managed via existing conflict of interest reporting and management systems at each participating institution. Any reported conflict will be addressed and resolved following established procedures.
The full protocol will be made available to investigators or members of the public upon request. The final trial data set will be available to the investigators at each participating institution. Once the data have been analyzed and the primary results published, the full data set will be made available to other investigators with a specific research question upon formal request in compliance with the agreed-on NIH data-sharing plan. The results of the ADAPT study will be made publicly available on ClinicalTrials.gov and disseminated to participants via an end-of-study newsletter.
Discussion
It is well known that lifestyle modification has substantial metabolic benefit for people with type 2 diabetes. The ADAPT study will address questions about benefits of an intensive exercise, sedentary behavior counseling, and dietary counseling specifically for people with DPN. Our preliminary data indicate that DPN may be more responsive than other diabetic complications to metabolic improvements due to the regenerative capacity of small unmyelinated axons.8,9,33 These preliminary results and the design of the ADAPT trial build on a growing literature that addresses the benefits and feasibility of exercise and behavioral strategies to reduce sedentary behavior.
Exercise has consistently demonstrated benefits to metabolism and health outcomes in people with diabetes.42–44 Ironically, patients with DPN have historically been cautioned to limit their activity to non–weight-bearing exercise out of concern for the risk of foot ulcers, infection, or neurogenic arthropathy (Charcot joint).45 However, recent evidence suggests that a walking program does not increase the ulcer risk,5,46 and greater improvements in endurance and activity level were found following weight-bearing exercise compared with non–weight-bearing exercise in people with DPN.47 Emerging research has shown promising effects of exercise on functional neuropathy outcomes, including balance and fall risk,48–50 cutaneous reinnervation,8,9 and decreased pain and fatigue.22,51 These results have led to a paradigm shift in the use of physical activity to improve symptoms and decrease neuropathy complications. However, self-administered exercise programs may not be effective in this population,52 and even supervised exercise is associated with frequent adverse events requiring ongoing modification of the exercise program.22
Reduction of sedentary behavior is an alternative approach. Diabetic peripheral neuropathy in older adults is strongly associated with decreased activity level.53 Sedentary behavior is associated with multiple metabolic risk factors, including increased waist circumference, high lipid and cholesterol levels, decreased glycemic control, and increased blood pressure.10–12 During prolonged sitting time, postural muscles are inactive, leading to reduced glucose uptake and unbalanced regulation of lipoprotein lipase, which is a key enzyme in lipid metabolism.54,55 Studies of interventions that target sedentary behavior have primarily focused on healthy adults.24–26 However, 2 recent studies13,14 have investigated the effect of reducing sedentary time in people with diabetes. These small randomized trials demonstrated significant improvements in insulin sensitivity through the use of short-duration, light activity to reduce sustained sedentary time.
An intervention to reduce sedentary behavior is an attractive complement to exercise-based therapies because of minimal physiological stress or pain, reduced risk of adverse events, and potentially greater sustainability. A recent meta-analysis of interventions to reduce sedentary time reported promising results with multicomponent “lifestyle interventions” directed at physical activity or sedentary behavior with a dietary/nutrition component.24 Other recommendations from the literature include the use of objective measures to capture sedentary behavior, addressing sedentary behavior in home and daily environments and situations, and the application of behavioral modification theories and motivational interviewing with participants.24–26
The ADAPT protocol was designed by a collaborative, interprofessional team of physical therapist and neurologist researchers at 2 institutions. This study is of particular interest to physical therapists due these specific innovations:
Emphasis on reducing sedentary behavior. The inclusion of actigraphy-based counseling and motivational interviews is a promising approach to address adherence issues commonly encountered in exercise intervention studies (for example, see Praet et al56).
Co-primary outcomes that include a patient-reported quality-of-life measure (NQOL-DN) and a biomarker (IENFD). Most clinical trials of people with DPN use nerve conduction studies or clinical examination scales as primary outcome measures, but these measures are unresponsive to change in early DPN, and their clinical significance has not been established.30 A secondary benefit of this study will be the establishment of the minimal clinically important difference in IENFD by evaluating the relationship between change in IENFD and change in the NQOL-DN scores and change in the participant-reported measures of pain, sleep, and general health.
Based on preliminary results of our study team at both sites, we anticipate that participants in the intensive intervention group will experience significant increases in fitness, endurance, and activity level, resulting in weight loss and improvement in lipids and glycated hemoglobin. We expect that the rate of decline of IENFD at the distal thigh will be reduced by half over 18 months and that the NQOL-DN will improve significantly by 9 months and remain significantly improved for the duration of the study. A positive outcome of this study would support development of a multicenter effectiveness trial to explore the practical clinical utility of this type of intervention for DPN.
There are several weaknesses of our study design and potential study problems. Because the intensive intervention includes several distinct elements (exercise, sedentary behavior, dietary counseling), we will not know which of these components have the greatest influence on the outcomes. No information about dose effect of the intervention or sustainability of the intervention outside the 18-month period will be gained from the study. As with any long-term intervention study, we anticipate challenges with recruitment and attrition. We developed a strong recruitment plan, budgeted for funds to compensate participants after completing assessment and intervention sessions, and accounted for 20% attrition when calculating our sample size. Study recruitment and retention will be monitored closely throughout the study, and adjustments will be made as needed.
The ADAPT trial has the potential to demonstrate that an intensive lifestyle intervention approach may be a sustainable, clinically effective approach for people with DPN that meaningfully improves patient outcomes and could immediately affect patient care and future clinical trials.
Appendix.
Appendix.
Interview Guide
References
- 1. Pop-Busui R, Lu J, Lopes N, Jones TL; BARI 2D Investigators. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Periph Nerv Syst. 2009;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mueller MJ, Minor SD, Sahrmann SA et al. . Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compated with age-matched controls. Phys Ther. 1994;74:299–303. [DOI] [PubMed] [Google Scholar]
- 3. Thurman DJ, Stevens JA, Rao JK; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:473–479. [DOI] [PubMed] [Google Scholar]
- 4. Jernigan SD, Pohl PS, Mahnken JD, Kluding PM. Diagnostic accuracy of fall risk assessment tools in people with diabetic peripheral neuropathy. Phys Ther. 2012;92:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of Medical Care in Diabetes—2016: Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care. 2016;39(suppl 1):S23–S35. [DOI] [PubMed] [Google Scholar]
- 6. Callaghan B, Little A, Feldman EL, Hughes R. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groover AL, Ryals JM, Guilford BL et al. . Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154:2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kluding PM, Pasnoor M, Singh R et al. . The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith AG, Russell JW, Feldman EL et al. . Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. [DOI] [PubMed] [Google Scholar]
- 10. Cooper AR, Sebire S, Montgomery AA et al. . Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012;55:589–599. [DOI] [PubMed] [Google Scholar]
- 11. Helmerhorst HJ, Wijndaele K, Brage S et al. . Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58:1776–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper AJ, Brage S, Ekelund U et al. . Association between objectively assessed sedentary time and physical activity with metabolic risk factors among people with recently diagnosed type 2 diabetes. Diabetologia. 2014;57:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Dijk JW, Venema M, van Mechelen W et al. . Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care. 2013;36:3448–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunstan DW, Kingwell BA, Larsen R et al. . Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lauria G, Bakkers M, Schmitz C et al. . Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Sys. 2010;15:202–207. [DOI] [PubMed] [Google Scholar]
- 16. Vinik EJ, Hayes RP, Oglesby A et al. . The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7:497–508. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R et al. . Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dyck PJ, Albers JW, Andersen H et al. ; Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27:620–628. [DOI] [PubMed] [Google Scholar]
- 19. Singleton JR, Bixby B, Russell JW et al. . The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Sys. 2008;13:218–227. [DOI] [PubMed] [Google Scholar]
- 20. Billinger SA, Tseng BY, Kluding PM. Modified total body recumbent stepper exercise test to obtain peak oxygen consumption in people with chronic stroke. Phys Ther. 2008;88:1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Billinger SA, Loudon JK, Gajewski BJ. Validity of a total body recumbent stepper exercise test to assess cardiorespiratory fitness. J Strength Cond Res. 2008;22:1556–1562. [DOI] [PubMed] [Google Scholar]
- 22. Kluding PM, Pasnoor M, Singh R et al. . Safety of aerobic exercise in people with diabetic peripheral neuropathy: single-group clinical trial. Phys Ther. 2015;95:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Office of Disease Prevention and Health Promotion. Be Active Your Way: A Guide for Adults. Available at: https://health.gov/paguidelines/guidelines/activeguide.aspx. Accessed November 24, 2015.
- 24. Martin A, Fitzsimons C, Jepson R et al. . Interventions with potential to reduce sedentary time in adults: systematic review and meta-analysis. Br J Sports Med. 2015;49:1056–1063. [DOI] [PubMed] [Google Scholar]
- 25. Prince SA, Saunders TJ, Gresty K, Reid RD. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: a systematic review and meta-analysis of controlled trials. Obes Rev. 2014;15:905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owen N, Sugiyama T, Eakin EE et al. . Adults' sedentary behavior determinants and interventions. Am J Prev Med. 2011;41:189–196. [DOI] [PubMed] [Google Scholar]
- 27. Tesfaye S, Boulton AJ, Dyck PJ et al. . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy W, Wendelschafer-Crabb G, Johnson T. Quantification of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. [DOI] [PubMed] [Google Scholar]
- 29. Zochodne D. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve. 2007;36:144–166. [DOI] [PubMed] [Google Scholar]
- 30. Dyck PJ, Norell JE, Tritschler H et al. . Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30:2619–2625. [DOI] [PubMed] [Google Scholar]
- 31. Smith AG, Howard JR, Kroll R et al. . The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65–69. [DOI] [PubMed] [Google Scholar]
- 32. Singleton JR, Marcus RL, Jackson JE et al. . Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol. 2014;1:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singleton JR, Marcus RL, Lessard MK et al. . Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boyd A, Barlow P, Pittenger G. Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2010;3:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyd A, Casselini C, Vinik E. Quality of life and objective measures of diabetic neuropathy in a prospective placebo-controlled trial of ruboxistaurin and topiramate. J Diabetes Sci Technol. 2011;5:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verbeke G, Molenberghs A. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000. [Google Scholar]
- 37. Littell R, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–1819. [DOI] [PubMed] [Google Scholar]
- 38. Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol. 2001;54:343–349. [DOI] [PubMed] [Google Scholar]
- 39. Westfall P, Young S. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. New York, NY: John Wiley & Sons Inc; 1993. [Google Scholar]
- 40. Schafer JK. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 41. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Published May 28, 2009 (v4.03: June 14, 2010). Available at: http://evs.nci.nih.gov/ftpl/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 42. Davidson LE, Hudson R, Kilpatrick K et al. . Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Int Med. 2009;169:122–131. [DOI] [PubMed] [Google Scholar]
- 43. Larose J, Sigal RJ, Boule NG et al. . Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc. 2010;42:1439–1447. [DOI] [PubMed] [Google Scholar]
- 44. Sigal RJ, Kenny GP, Boule NG et al. . Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Int Med. 2007;147:357–369. [DOI] [PubMed] [Google Scholar]
- 45. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12–S54. [DOI] [PubMed] [Google Scholar]
- 46. LeMaster J, Mueller MJ, Rieber G et al. . Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1385–1398. [DOI] [PubMed] [Google Scholar]
- 47. Mueller MJ, Tuttle LJ, LeMaster JW et al. . Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Allet L, Armand S, De Bie R et al. . The gait and balance of patients with diabtes can be improved: a randomised controlled trial. Diabetologia. 2010;53:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song C, Petrofsky J, Lee S et al. . Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–811. [DOI] [PubMed] [Google Scholar]
- 50. Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209. [DOI] [PubMed] [Google Scholar]
- 51. Yoo M, D'Silva LJ, Martin K et al. . Pilot study of exercise therapy on painful diabetic peripheral neuropathy. Pain Med. 2015;16:1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kruse RL, LeMaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “Feet First” randomized controlled trial. Phys Ther. 2010;90:1568–1579. [DOI] [PubMed] [Google Scholar]
- 53. van Sloten TT, Savelberg HH, Duimel-Peeters IG et al. . Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res Clin Pract. 2011;91:32–39. [DOI] [PubMed] [Google Scholar]
- 54. Hamilton MT, Healy GN, Dunstan DW et al. . Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardiovasc Risk Rep. 2008;2:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551(pt 2):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Praet SF, van Rooij E, Wijtvliet A. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2008;51:736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zelman D, Gore M, Dukes E et al. . Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage. 2005;29:401–410. [DOI] [PubMed] [Google Scholar]
- 58. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 59. Podsiadlo D, Richardson S. The timed “up and go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 60. Franchignoni F, Godi M, Guglielmetti S et al. . Enhancing the usefulness of the Mini-BESTest for measuring dynamic balance: a Rasch validation study. Eur J Phys Rehabil Med. 2015;51:429–437. [PubMed] [Google Scholar]