Abstract
BACKGROUND: At present, guidelines are lacking on platelet transfusion in patients with a traumatic intracranial bleed and history of antiplatelet therapy. The aspirin and P2Y12 response unit (ARU and PRU, respectively) assays detect the effect of aspirin and P2Y12 inhibitors in the cardiac population.
OBJECTIVE: To describe the reversal of platelet inhibition after platelet transfusion using the ARU and PRU assays in patients with traumatic brain injury.
METHODS: Between 2010 and 2015, we conducted a prospective comparative cohort study of patients presenting with a positive head computed tomography and a history of antiplatelet therapy. ARU and PRU assays were performed on admission and 6 hours after transfusion, with a primary end point of detection of disinhibition after platelet transfusion.
RESULTS: One hundred seven patients were available for analysis. Seven percent of patients taking aspirin and 27% of patients taking clopidogrel were not therapeutic on admission per the ARU and PRU, respectively. After platelet transfusion, 51% of patients on any aspirin and 67% of patients on any clopidogrel failed to be reversed. ARU increased by 71 ± 76 per unit of apheresis platelets for patients taking any aspirin, and PRU increased by 48 ± 46 per unit of apheresis platelets for patients taking any clopidogrel.
CONCLUSION: A significant percentage of patients taking aspirin or clopidogrel were not therapeutic and thus would be unlikely to benefit from a platelet transfusion. In patients with measured platelet inhibition, a single platelet transfusion was not sufficient to reverse platelet inhibition in almost half.
Keywords: Antiplatelet therapy, Aspirin, Aspirin response unit, Clopidogrel, Platelet transfusion, P2Y12 response unit, Traumatic brain injury
ABBREVIATIONS
- ARU
aspirin response unit
- GCS
Glasgow Coma Scale
- PRU
P2Y12 response unit
- TBI
traumatic brain injury
- TRALI
transfusion-related acute lung injury
Antithrombotic and antiplatelet therapies are commonly used by patients >40 years of age who sustain a traumatic brain injury (TBI). Although guidelines for the prevention of cardiovascular/cerebrovascular morbidity and mortality frequently support prescribing aspirin, P2Y12 inhibitors, warfarin, and other antithrombotic agents, the premorbid use of these medications may increase the risk and severity of traumatic intracranial hemorrhage and worsen long-term outcomes.1-5
Currently, standard clinical laboratory measures of platelet count and coagulation status do not adequately measure functional platelet status. There are no existing guidelines for platelet transfusion in the setting of TBI. Consequently, there are wide variations in practice regarding administering platelet transfusions to patients with a reported history of antiplatelet therapy who present with traumatic intracranial hemorrhage. Routine, reflexive transfusion of platelets may be unnecessary and potentially harmful because of complications such as transfusion-related acute lung injury (TRALI), hypersensitivity reactions including anaphylaxis, congestive heart failure exacerbations resulting from volume overload, and myocardial infarction in patients with coronary stents.6-12
Assays used to measure platelet dysfunction such as closure time, platelet aggregometry, thromboelastography platelet mapping, PFA-100, and others have proven problematic or unreliable for a variety of reasons.13 Recently, point-of-care aspirin response unit (ARU) and P2Y12 response unit (PRU) assays for evaluating aspirin- and thienopyridine-induced platelet inhibition have become widely available and have been extensively validated in the cardiac literature to detect the degree of platelet inhibition by these agents. The ARU and PRU assays enable point-of-care platelet function testing for patients taking aspirin, P2Y12 inhibitors (clopidogrel, ticlopidine, prasugrel), and glycoprotein IIb/IIIa inhibitors (abciximab or eptifibatide),14-18 with results available within 30 minutes, and have demonstrated nearly 100% sensitivity and 96% specificity for the detection of antiplatelet function.19 In this study, we describe the use of the ARU and PRU assays for monitoring the reversal of antiplatelet activity by platelet transfusion in the adult TBI population on antiplatelet agents with intracranial hemorrhage.
METHODS
Study Population
The study was performed under a Quality Assurance project through the Department of Neurosurgery. Deidentified data were extracted, including patient age, sex, mechanism of injury, Glasgow Coma Scale score, Marshall score, and history of aspirin or clopidogrel use. Patients with a history of antiplatelet use presenting to our institution's emergency department from November 2010 to January 2015 within 24 hours of a traumatic injury and having a noncontrast computed tomography (CT) scan of the head positive for intracranial hemorrhage had baseline ARU and PRU assays performed on arrival before platelet transfusion and a repeat assay performed 6 hours after platelet transfusion. This cohort of patients has previously been described by our group (Parry et al, unpublished data, September 2015). Patients were excluded from analysis if they were <16 or >95 years of age, had missing admission head CT scans, had a known bleeding diathesis, were taking anticoagulation therapy (warfarin, dabigatran, etc), or had unknown antithrombotic use. Patients were also excluded if antiplatelet reversal (platelet transfusion, desmopressin administration, etc) was performed at an outside hospital.
Sample Collection and Measurement of Platelet Function
Platelet dysfunction was evaluated with the VerifyNow assay (Accumetrics, San Diego, California). During collection of whole-blood samples for standard laboratory tests during presentation to the emergency department, 2 additional tubes of 2 mL whole blood were collected in standard citrated blood draw collection tubes for the assays and analyzed with standard, previously described procedures.15,20 Blood samples were again collected for the assays 6 hours after transfusion. An ARU count <550 was considered nontherapeutic, whereas a level >550 was considered nontherapeutic and indicated aspirin resistance in patients taking aspirin.19,21 A PRU count <240 was considered therapeutic, and a level >240 was considered nontherapeutic.22,23
Platelet Transfusion
Pooled donor platelets were transfused at the discretion of the attending neurosurgeon in the emergency department for patients presenting with a reported history of antiplatelet therapy and noncontrast CT of the head with intracranial hemorrhage. The number of apheresis units of platelets transfused between the admission and follow-up ARU and PRU assays was recorded.
Statistical Analysis
Continuous demographic characteristics were assessed for normality with the Kolmogorov-Smirnov test; normally distributed data were analyzed by the t test; and the remainder were compared by use of the Wilcoxon rank-sum test. Categorical data were analyzed by the Pearson χ2 or Fisher exact test. Differences between groups in multilevel ordinal measurements (ie, Marshall score, GCS) were tested with the Wilcoxon rank-sum (Mann-Whitney U) test. Change in ARU and PRU assay scores between before and after platelet transfusion was assessed with the Wilcoxon signed-rank test. Means are reported as mean ± SD; significant associations between outcomes and predictors are reported as odds ratios with 95% confidence intervals. Acceptable type I error was set a priori at α = 0.05 for all statistical tests. All data were analyzed with STATA 13.1 (StataCorp, College Station, Texas).
RESULTS
Baseline Characteristics
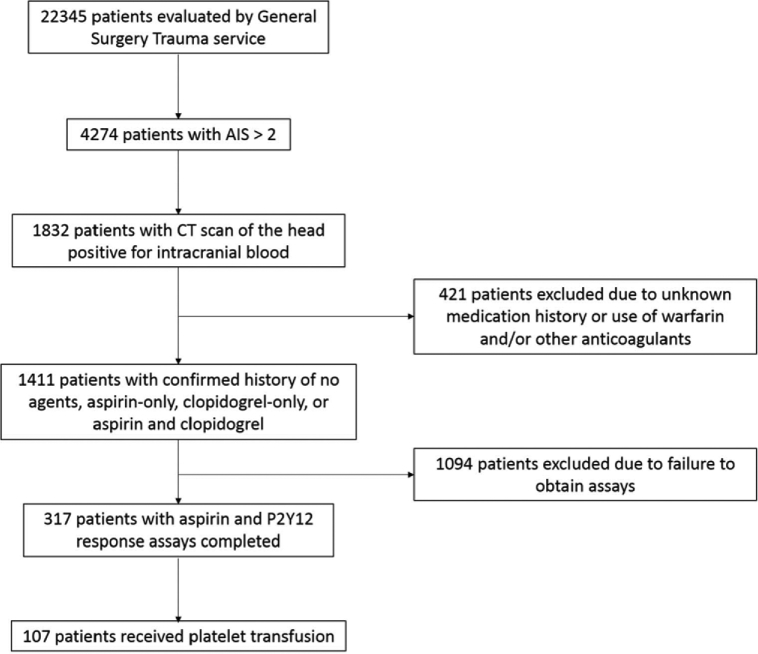
One hundred seven patients were included in the analysis (Table 1). These patients are a subgroup of patients from the study by Parry et al from our group who received platelet transfusion (Figure). The overall mean patient age was 75.5 ± 12.2 years. Men made up 65% of the sample; women, 35%. The mechanism of injury for the majority of patients was a fall at ground level or from height (87%). TBI severity on admission was mild in 84% (GCS score, 13-15), moderate in 8% (GCS score, 9-12), and severe in 8% (GCS score ≤ 8). The median GCS score on admission was 15 (interquartile range, 13-15), and the median Marshall score was 2 (interquartile range, 2-4). The mean platelet count on presentation was 222 ± 68. Sixty-two percent of all patients were taking aspirin only; 8% were taking clopidogrel only; and 30% were taking both aspirin and clopidogrel.
TABLE 1.
Baseline Characteristicsa
| Overall | |
|---|---|
| Patients who received platelet transfusion, n (%) | 107 (100) |
| Age, mean ± SD, y | 75.5 ± 12.2 |
| Female sex, % | 35 |
| Mechanism of injury, % | |
| Fall (ground level or from height) | 87 |
| Motor vehicle accident | 12 |
| Other | 0.01 |
| Injury severity | |
| Initial GCS score, median (interquartile range) | 15 (13-15) |
| Severe TBI (GCS score <8), % | 9 |
| Marshall score, median (interquartile range) | 2 (2-4) |
| Antiplatelet therapy, % | |
| Aspirin only | 62 |
| Clopidogrel only | 8 |
| Aspirin and clopidogrel | 30 |
aGCS, Glasgow Coma Scale; TBI, traumatic brain injury.
FIGURE.
Patient selection flow diagram. AIS, Abbreviated Injury Score; CT, computed tomography.
ARU Assay Results and Platelet Transfusion
Results for the ARU assay before and after transfusion with stratification by type of antiplatelet therapy are shown in Table 2. Ninety-three percent of patients with a history of any aspirin use had a therapeutic ARU. Half of patients with a history of using only clopidogrel had therapeutic ARU, while 89% of patients with any clopidogrel use (which includes those on dual therapy) were therapeutic. The mean ARU before transfusion for patients with any history of aspirin use was 449 ± 55. After transfusion, 47% of patients with any aspirin use were still therapeutic, which indicated that approximately 49% of patients with dysfunctional platelets taking aspirin were reversed. The mean ARU for patients on any aspirin increased by 71 ± 76 per unit of apheresis platelets transfused to 531 ± 71. Both the mean ARU and number of patients with therapeutic ARU were significantly different after platelet transfusion in patients with any aspirin use (P < .001 for both).
TABLE 2.
Aspirin Response Unit Assay Before and After Transfusiona
| n | % of Total | % Therapeutic T0, ARU | ARU T0, Mean (SD) | % Therapeutic T6, ARU | ARU T6, Mean (SD) | ARU Change, Mean (SD) | Apheresis Units of Platelets Transfused, Mean (SD) | ARU Change per Unit of Platelet Transfused, Mean (SD) | P, Fisher Exact Test for Therapeutic ARU | P, Wilcoxon Signed-Rank Test for ARU Change | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin only | 66 | 62 | 92 | 451 (52) | 46 | 534 (66) | 82 (83) | 1.3 (0.8) | 75 (79) | <.001b | <.001b |
| Clopidogrel only | 9 | 8 | 50 | 527 (111) | 60 | 536 (84) | 22 (138) | 1.9 (1.4) | 31 (75) | .20 | >.99 |
| Both agents | 32 | 30 | 94 | 444 (62) | 50 | 524 (81) | 77 (81) | 1.4 (0.6) | 63 (67) | <.001b | <.001b |
| Any aspirin | 98 | 92 | 93 | 449 (55) | 47 | 531 (71) | 80 (82) | 1.3 (0.7) | 71 (76) | <.001b | <.001b |
| Any clopidogrel | 41 | 38 | 89 | 454 (72) | 51 | 526 (80) | 71 (88) | 1.5 (0.8) | 59 (68) | <.001b | <.001b |
| Any antiplatelet agent | 107 | 100 | 91 | 452 (60) | 48 | 531 (71) | 78 (85) | 1.4 (0.8) | 69 (76) | <.001b | <.001b |
aARU, aspirin response unit; T0, at admission; T6; at 6 hours.
bIndicates significant result.
PRU Assay Results and Platelet Transfusion
Results for the PRU assay before and after transfusion with stratification by type of antiplatelet therapy are shown in Table 3. Seventy-three percent of patients with a history of any clopidogrel use had a therapeutic PRU. In contrast, 38% of patients with a history of only aspirin had therapeutic PRU assays. The mean PRU before transfusion for patients with any history of clopidogrel use was 189 ± 77. After transfusion, 49% of patients with any clopidogrel use were still therapeutic, which indicated that approximately 33% of patients with dysfunctional platelets taking clopidogrel were reversed. The mean PRU for patients on any clopidogrel increased by 48 ± 46 after platelet transfusion to 249 ± 88. Both the mean PRU and number of patients with therapeutic PRU were significantly different after platelet transfusion in patients with any clopidogrel use (P < .001 and P = .04, respectively).
TABLE 3.
P2Y12 Response Unit Assay Before and After Transfusiona
| n | % of Total | % Therapeutic T0, PRU | PRU T0, Mean (SD) | % Therapeutic T6, PRU | PRU T6, Mean (SD) | PRU Change, Mean (SD) | Apheresis Units of Platelets Transfused | PRU Change per Unit of Platelet Transfused, Mean (SD) | P, Fisher Exact Test for Therapeutic PRU | P, Wilcoxon Signed-Rank Test for PRU Change | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspirin only | 66 | 62 | 38 | 252 (60) | 0 | 305 (63) | 33 (64) | 1.3 (0.8) | 26 (60) | .05 | .21 |
| Clopidogrel only | 9 | 8 | 56 | 211 (82) | 33 | 259 (79) | 48 (50) | 1.9 (1.4) | 41 (47) | .64 | .03b |
| Both agents | 32 | 30 | 78 | 183 (76) | 53 | 246 (91) | 67 (52) | 1.4 (0.6) | 50 (46) | .06b | >.001b |
| Any aspirin | 98 | 92 | 65 | 206 (78) | 40 | 260 (88) | 60 (56) | 1.3 (0.7) | 45 (49) | .03b | >.001b |
| Any clopidogrel agent | 41 | 38 | 73 | 189 (77) | 49 | 249 (88) | 62 (52) | 1.5 (0.8) | 48 (46) | .04b | >.001b |
aPRU, P2Y12 response unit response unit; T0, at admission; T6; at 6 hours.
DISCUSSION
In the present study, aspirin and PRU assays detected platelet inhibition and the effect of platelet transfusion in patients with traumatic intracranial hemorrhage and a history of antiplatelet therapy. A significant percentage of patients taking aspirin or clopidogrel in our larger cohort of 317 patients with assays done on admission (Figure) were not therapeutic and thus would be unlikely to benefit from a platelet transfusion. In patients with measured platelet inhibition, the amount of apheresis platelets transfused at the attending neurosurgeon's discretion was not sufficient to reverse platelet inhibition in almost half. The ARU and PRU assays may have clinical utility for determining whether to transfuse platelets in patients with TBI.
Currently, evidence in the literature on the impact of premorbid antiplatelet therapy on long-term outcome after TBI is conflicting. It is likely that antiplatelet therapy is one of a number of factors, including genetic polymorphisms of platelet and cytochrome P450 enzymes, chronic illness, brain trauma-specific changes in the inflammatory milieu, and consumption of platelet granule contents, that can affect platelet function and overall hemostatic capacity at the time of TBI.24-27 Studying the impact of antiplatelet therapy on long-term outcome after TBI has been difficult in part because of the limited measures available of blood hemostasis that can be used in a trauma setting. Platelet count is a component of the standard complete blood count and is regularly used as a criterion for guiding platelet transfusion. However, measurement of platelet dysfunction has been difficult because previous tests have been limited by large sample volume requirements, long delays for results, prohibitively expensive equipment, or requirement for specialized personnel.13 As a result in part of the uncertainty of both the hemostatic capacity and whether outcome is influenced by reversal of platelet inhibition, there is wide variation in practice with respect to platelet transfusion for patients with TBI on premorbid antiplatelet therapy.
In this cohort of patients, a clinical history of antiplatelet therapy did not always portend platelet inhibition as measured by ARU and PRU assays at presentation. Seven percent and 27% of patients on any aspirin and any clopidogrel, respectively, were found to be nontherapeutic on admission. As noted previously, multiple factors may contribute to this incongruence. Previous systematic reviews examining normal platelet function despite a reported history of aspirin or clopidogrel use have found that 1 in 4 patients taking aspirin will be resistant and 1 in 5 patients taking clopidogrel will be resistant.28,29 Others are noncompliant with prescriptions. Identifying patients with functional platelets at the initial evaluation is important because platelet transfusion carries risk, including hypersensitivity side effects such as fever, rigors, or urticaria to much more serious complications such as TRALI, infection transmission, and immunomodulation.8,30-32 Studies have shown that the incidence of TRALI is higher in critically ill patients relative to others, and TRALI is more likely to occur after platelet transfusion compared with red blood cell transfusion.6,9-11
After transfusion of 1 unit of platelets, patients with a history of any aspirin use demonstrated a mean increase in ARU of 71 ± 76 per apheresis unit of platelets transfused, whereas patients with any clopidogrel use had an increase of 48 ± 46 in PRU per apheresis unit of platelets transfused. Fifty-one percent and 33% of patients taking any aspirin or any clopidogrel, respectively, had reversal of platelet dysfunction after platelet transfusion. Prior investigations of the ARU and PRU assays to monitor restoration of platelet function after transfusion have returned conflicting results. Bachelani et al33 reported reversal of aspirin-induced platelet dysfunction in approximately 65% of patients using the ARU assay in a TBI cohort with a mean increase in ARU of 70 ± 50 for every 6-pack of platelets. Naidech et al34 found, in a cohort of patients with spontaneous intracerebral hemorrhage, that platelet transfusion increases the ARU from a median of 448 to 586. Taylor et al35 studied a cohort of patients requiring lifesaving surgery for hemorrhagic shock or neurosurgery and reported an increase in ARU from a median of 420 to 630 after a 0.12-IU/kg platelet transfusion, with 94% of patients on aspirin reversed. However, they also reported that platelet transfusion for a history of clopidogrel use did not lead to reversal of platelet inhibition. Joseph et al36 found that transfusion of 1 apheresis unit of platelets in patients with traumatic intracerebral hemorrhage with premorbid 325 mg daily aspirin use led to reversal only 18% of the time. The discrepancy between the results of Joseph et al and our results and others may be due to a different distribution of patients taking high- vs low-dose aspirin therapy. One pack of apheresis platelets may not be enough to reverse strong platelet dysfunction; Vilahur et al37 found that reversal of platelet dysfunction in patients on aspirin and clopidogrel requires between 10 and 12.5 units of platelets.
One strategy for reversal of platelet dysfunction that we did not evaluate was desmopressin. Desmopressin is not used in our institution for the reversal of platelet dysfunction in patients with TBI. We excluded patients who received any reversal agents at outside hospitals, so we are sure that no patients in our cohort received desmopressin. Thus, we are unable to evaluate the utility of desmopressin as a reversal agent in the TBI population. An investigation by Kim et al38 showed that coadministration of platelets and desmopressin did not correlate with a lower incidence of early radiographic progression of intracranial hemorrhage in patients with TBI compared with patients who received neither agent. Desmopressin has been shown to be a safe and effective alternative for restoring platelet function in other contexts such as patients on antiplatelet therapy undergoing carotid endarterectomy or coronary artery bypass graft.39,40 Desmopressin may be a therapeutic tool that warrants further investigation in the TBI population.
The importance of monitoring platelet function in patients with TBI lies in monitoring platelet function not for its own sake but to determine whether it can be an effective tool for improving patient outcomes. Bachelani et al33 reported that neither clinical history of aspirin use nor initial ARU results predicted risk of intracranial hemorrhage progression, craniotomy, mortality, or poor outcome overall. Likewise, patients who were nonresponders to platelet transfusion as measured by ARU were not at a higher risk of mortality compared with responders, suggesting that reversal of platelet inhibition may not improve outcome. Joseph et al36 similarly report that there was no difference in progression of intracranial hemorrhage or need for neurosurgical intervention in patients with inhibited vs noninhibited platelets. However, it is difficult to draw conclusions from their results, given that only 4 of their 22 initially therapeutic patients were reversed with platelet transfusion. The relationship between reversal of antiplatelet therapy and outcome after TBI has yet to be firmly established. Our findings demonstrate that patients may need additional platelet transfusion after their initial transfusion to fully reverse their platelet dysfunction. However, because the decision to transfuse platelets was at the discretion of the attending neurosurgeon, our results may be biased. In addition, the ARU and PRU assays may have potential to guide the amount of platelets transfused initially based on assay values.
CONCLUSION
In this study, we demonstrate that reversal of platelet dysfunction in patients with TBI with a history of aspirin or clopidogrel use can be monitored with the ARU and PRU assays. Prospective studies are needed to determine whether reversal of platelet inhibition as measured by the ARU and PRU assays predicts likelihood of bleed progression and affects long-term outcome.
Disclosures
Support for this research was provided by the Walter L. Copeland Fund of The Pittsburgh Foundation and the University of Pittsburgh Clinical Scientist Training Program and Clinical and Translational Science Institute (UL1 TL1TR000005). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
We would like to thank the research nurses at the University of Pittsburgh Brain Trauma Research Center and the neurosurgery residents at the University of Pittsburgh for enrolling patients and collecting data for this manuscript.
REFERENCES
- 1. Lavoie A, Ratte S, Clas D et al. . Preinjury warfarin use among elderly patients with closed head injuries in a trauma center. J Trauma. 2004;56(4):802-807. [DOI] [PubMed] [Google Scholar]
- 2. Franko J, Kish KJ, O'Connell BG, Subramanian S, Yuschak JV. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006;61(1):107-110. [DOI] [PubMed] [Google Scholar]
- 3. Pearson TA, Blair SN, Daniels SR et al. . AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106(3):388-391. [DOI] [PubMed] [Google Scholar]
- 4. Amsterdam EA, Holmes DR Jr, Mukherjee D et al. . 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2014;64(24):e139-e228. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76. [DOI] [PubMed] [Google Scholar]
- 6. Eder AF, Herron R, Strupp A et al. . Transfusion-related acute lung injury surveillance (2003-2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47(4):599-607. [DOI] [PubMed] [Google Scholar]
- 7. Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10(6): 419-423. [DOI] [PubMed] [Google Scholar]
- 8. Blumberg N, Spinelli SL, Francis CW, Taubman MB, Phipps RP. The platelet as an immune cell: CD40 ligand and transfusion immunomodulation. Immunol Res. 2009;45(2-3):251-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapman CE, Stainsby D, Jones H et al. . Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. [DOI] [PubMed] [Google Scholar]
- 10. Vlaar AP, Binnekade JM, Prins D et al. . Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med. 2010;38(3):771-778. [DOI] [PubMed] [Google Scholar]
- 11. Gajic O, Rana R, Winters JL et al. . Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176(9):886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornet AD, Klein LJ, Groeneveld AB. Coronary stent occlusion after platelet transfusion: a case series. J Invasive Cardiol. 2007;19(10):E297-E299. [PubMed] [Google Scholar]
- 13. Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103(3 suppl):20A-26A. [DOI] [PubMed] [Google Scholar]
- 14. Lev EI, Patel RT, Maresh KJ et al. . Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006;47(1):27-33. [DOI] [PubMed] [Google Scholar]
- 15. Lee PY, Chen W-H, Ng W et al. . Low-dose aspirin increases aspirin resistance in patients with coronary artery disease. Am J Med. 2005;118(7):723-727. [DOI] [PubMed] [Google Scholar]
- 16. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41(6):961-965. [DOI] [PubMed] [Google Scholar]
- 17. Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a Verify Now-P2Y12 (R) cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol. 2006;28:315-322. [DOI] [PubMed] [Google Scholar]
- 18. Malinin A, Pokov A, Spergling M et al. . Monitoring platelet inhibition after clopidogrel with the Verify Now-P2Y12 rapid analyzer: the Verify Thrombosis Risk Assessment (VERITAS) study. Thromb Res. 2007;119(3):277-284. [DOI] [PubMed] [Google Scholar]
- 19. Blais N, Pharand C, Lordkipanidzé M, Sia YK, Merhi Y, Diodati JG. Response to aspirin in healthy individuals: cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B2. Thromb Haemost. 2009;102(2):404-411. [DOI] [PubMed] [Google Scholar]
- 20. Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119(19):2625-2632. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen HL, Kristensen SD, Thygesen SS et al. . Aspirin response evaluated by the VerifyNow aspirin system and light transmission aggregometry. Thromb Res. 2008; 123(2):267-273. [DOI] [PubMed] [Google Scholar]
- 22. Marcucci R, Gori AM, Paniccia R et al. . Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay a 12-month follow-up. Circulation. 2009;119(2):237-242. [DOI] [PubMed] [Google Scholar]
- 23. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention: results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52(14):1128-1133. [DOI] [PubMed] [Google Scholar]
- 24. Mega JL, Close SL, Wiviott SD et al. . Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354-362. [DOI] [PubMed] [Google Scholar]
- 25. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367(9510):606–617. [DOI] [PubMed] [Google Scholar]
- 26. Abaci A, Yilmaz Y, Caliskan M et al. . Effect of increasing doses of aspirin on platelet function as measured by PFA-100 in patients with diabetes. Thromb Res. 2005;116(6):465-470. [DOI] [PubMed] [Google Scholar]
- 27. Pareti F, Capitanio A, Mannucci L, Ponticelli C, Mannucci P. Acquired dysfunction due to the circulation of “exhausted” platelets. Am J Med. 1980;69(2):235-240. [DOI] [PubMed] [Google Scholar]
- 28. Hovens MMC, Snoep JD, Eikenboom JCJ, van der Bom JG, Mertens BJA, Huisman MV. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J. 2007;153(2):175-181. [DOI] [PubMed] [Google Scholar]
- 29. Snoep JD, Hovens M, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154(2):221-231. [DOI] [PubMed] [Google Scholar]
- 30. Ziai WC, Mirski MA. The slippery slope of platelet transfusion for intracerebral hemorrhage. Neurocrit Care. 2012;17(1):154-155. [DOI] [PubMed] [Google Scholar]
- 31. Heddle NM, Webert KE. Investigation of acute transfusion reactions. In: Practical Transfusion Medicine. 3rd ed Oxford, UK: Wiley-Blackwell; 2009:61-71. [Google Scholar]
- 32. Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood. 2011;117(5):1463-1471. [DOI] [PubMed] [Google Scholar]
- 33. Bachelani AM, Bautz JT, Sperry JL et al. . Assessment of platelet transfusion for reversal of aspirin after traumatic brain injury. Surgery. 2011;150(4):836-843. [DOI] [PubMed] [Google Scholar]
- 34. Naidech AM, Jovanovic B, Liebling S et al. . Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40(7):2398-2401. [DOI] [PubMed] [Google Scholar]
- 35. Taylor G, Osinski D, Thevenin A, Devys J-M. Is platelet transfusion efficient to restore platelet reactivity in patients who are responders to aspirin and/or clopidogrel before emergency surgery? J Trauma Acute Care Surg. 2013;74(5): 1367-1369. [DOI] [PubMed] [Google Scholar]
- 36. Joseph B, Pandit V, Sadoun M et al. . A prospective evaluation of platelet function in patients on antiplatelet therapy with traumatic intracranial hemorrhage. J Trauma Acute Care Surg. 2013;75(6):990-994. [DOI] [PubMed] [Google Scholar]
- 37. Vilahur G, Choi BG, Zafar MU et al. . Normalization of platelet reactivity in clopidogrel-treated subjects. J Thromb Haemost. 2007;5(1):82-90. [DOI] [PubMed] [Google Scholar]
- 38. Kim DY, O'Leary M, Nguyen A et al. . The effect of platelet and desmopressin administration on early radiographic progression of traumatic intracranial hemorrhage. J Neurotrauma. 2015;32(22):1815-1821. [DOI] [PubMed] [Google Scholar]
- 39. Gratz I, Koehler J, Olsen D et al. . The effect of desmopressin acetate on postoperative hemorrhage in patients receiving aspirin therapy before coronary artery bypass operations. J Thorac Cardiovasc Surg. 1992;104(5):1417-1422. [PubMed] [Google Scholar]
- 40. Ranucci M, Nano G, Pazzaglia A, Bianchi P, Casana R, Tealdi DG. Platelet mapping and desmopressin reversal of platelet inhibition during emergency carotid endarterectomy. J Cardiothorac Vasc Anesth. 2007;21(6):851-854. [DOI] [PubMed] [Google Scholar]