Abstract
Background
The feasibility of liver transplantation (LT) in elderly recipients remains a topic of debate.
Methods
This cohort study evaluated the impact of recipient's age on LT outcome between January 2007 and May 2016 covered by the Korean National Health Insurance system (n = 9415). Multilevel regression models were used to determine the impact of recipient's age on in-hospital and long-term mortality after LT.
Results
All patients had a first LT, with 2473 transplanted with liver from deceased donors (DD) and 6942 from living donors. The mean age was 52.2 ± 9.0 years. Most LT were performed on patients in their 50s (n = 4290, 45.6%) and 0.9% (n = 84) of the LT was performed on patients older 70 years. The overall in-hospital mortality was 6.3%, and the 3-year mortality was 11.3%. The in-hospital mortality included, 13.5% associated with DDLT and 3.7% involved living donor LT. When compared with that for patients aged 51 to 55 years, the risk of death among recipients older than 70 years was about fourfold higher after adjusting for baseline liver disease (odds ratio, 4.1; 95% confidence interval, 2.21-7.58), and was nearly threefold higher after adjusting for baseline liver disease and perioperative complications (odds ratio, 2.92; 95% confidence interval, 1.37-6.24). Also, the cost of LT increased significantly with age.
Conclusions
The data show that age remains an important risk factor for LT, suggesting that LT should be considered with caution in elderly recipients.
The authors examined mortality risk for a large cohort of liver transplant recipients between 2007 and May 2016 covered by the Korean National Health Insurance system (n = 9415). Both costs and mortality were significantly increased in the cohort over 70 years old. Supplemental digital content is available in the text.
Liver transplantation (LT) is a lifesaving procedure for patients with end-stage liver disease. Refinements in surgical techniques and postoperative care have improved allograft and patient survival.1 Formerly marginal donors, such as hepatitis B virus (HBV) carriers and steatotic liver donors, have been successfully and increasingly used as donors.2 Nevertheless, the number of patients waiting for a donor liver greatly exceeds the number of available grafts, and many death without LT have been reported due to the shortage of donated organs.3
Donor liver allocation is usually guided by the model for end-stage liver disease (MELD) score.4 Although the MELD score represents the need for urgent LT,5 it was not designed to predict survival after LT. Many clinicians have investigated futile transplantations, and the risk factors for early mortality after LT include advanced age, renal insufficiency, acute liver failure, ABO incompatibility, retransplantation, and longer ischemic time.6-8
The prevalence of end-stage liver disease in elderly patients indicated for LT has increased as societies continue to age. Furthermore, medical advances (such as better immunosuppression and new surgical techniques) have increased the likelihood of successful LT in elderly patients.9 In the late 1980s, patients older than 50 years were recognized as eligible for LT, and the age of eligibility has since increased.6 There are some reports of low rejection rate in elderly patients, which may indicate a favorable effect on graft survival.10-14 However, some experts suggest that LT is contraindicated in the elderly because of comorbid conditions and the increased risk of postoperative complications that adversely affect their long-term survival.8,15 However, it has been difficult to draw conclusions from these studies due to limitations inherent in the retrospective design, and single-center nature. Accordingly, we used a nationwide database in Korea to evaluate the impact of recipients’ age on LT outcomes.
MATERIALS AND METHODS
Data Sources
The Health Insurance Review and Assessment Service (HIRA), a government-affiliated organization, reviews all national health insurance (NHI) claims and assesses their accuracy and quality. All Koreans are covered by the NHI, and HIRA claim data are available to researchers who submit their work profile for approval. The claims data include clinical visits, surgical and medical treatments, medical resources used, medication and diagnostic codes.16 The NHI codes use the Korean Classification of Diseases, 6th edition. Korean Classification of Diseases, 6th edition is the modified version of the International Classification of Disease, 10th revision, and is adapted for use in the Korean health system. This study was approved by HIRA.
Study Populations
This study includes patients who underwent LT between January 2007 and May 2016. In Korea, the allocation priority for LT based on the Child-Pugh scoring system during our study periods. We included adult patients (aged 18 years and older) who were assigned surgical procedural codes for LT (Q8040-Q8050) combined with anesthesia fee codes. We excluded patients who underwent re-LT (Q8140-Q8150), multiple organ transplantations (Q8061-Q8062, Q8080, Q8101-Q8103, Q8121-Q8123, R3280) and patients who underwent both DDLT (Q8040-Q8044) and living donor LT (LDLT) (Q8045-Q8050) in the same hospitalization period. Our Institutional Review Board approved the analyses and waived the requirement for informed consent since we only used deidentified data from HIRA. Ethical approval for this study was exempted by the Samsung Medical Center Institutional Review Board (SMC 2017-01-001).
Definitions
Liver transplantation was classified into 5 categories based on etiology of liver disease: malignant (C22, C220, C222, C227, C229), viral, alcoholic (K70, K700-704, K7010, K7011, K7030, K7031, K7040, K7041, K709), fulminant, and others. Viral hepatitis was defined as liver disease associated with hepatitis B (HBV; B15, B150, B159) or hepatitis C (B171, B182) virus infection. We included only patients treated with HBV-related medication to exclude a viral carrier. In Korea, the disease code for hepatitis C virus is only granted to patients identified by the Korea Centers for Disease Control and Prevention. However, liver disease associated with hepatitis A virus infection was classified as fulminant hepatitis. We defined fulminant hepatitis as a rapidly progressive liver injury at the time of symptom onset without preexisting liver disease. It includes hepatitis associated with hepatitis A virus infection (B15, B150, B159), toxic hepatitis (K71, K710-719, K7110, K7111, K7150, K7151), and acute liver failure (K72, K720, K7200, K7201). Alcoholic liver disease refers to any alcohol-related condition, with or without any disease. Other codes included metabolic liver disease (E80, E800, E800-807, E83, E830-835, E838, E839), Budd-Chiari syndrome (I82, I820), biliary cirrhosis (K743-745), biliary atresia (Q442, Q443), and primary sclerosing cholangitis (K83, K830).
Procedures for concomitant medical therapy included mechanical ventilation (M5857, M5858, M5860) for more than 3 days, intermittent hemodialysis ( O7020), continuous renal replacement therapy (O7051-7054) and extracorporeal membrane oxygenation (ECMO) (O1901-O1904). We considered dialysis in patients treated with renal replacement therapy (intermittent hemodialysis or continuous renal replacement therapy). We identified the use of vasopressor drugs, such as dobutamine, dopamine, epinephrine, and norepinephrine, for more than 2 days using the Korea drug and anatomical therapeutic chemical codes.17
Statistical Analysis
The primary outcome was the impact of the recipient’s age on in-hospital death. Secondary outcomes involved short- and long-term results. We considered the length of stay (LOS) in the intensive care unit (ICU) and medical costs as short-term outcomes. We investigated long-term outcomes, after discharge, such as 1-, 2-, and 3-year mortalities, and readmission rates. We used the operational definition of death after LT in the absence of obvious statement for death in HIRA data set. Liver transplantation recipients who had no insurance claims for an additional year were considered dead, and the date of death was defined accordingly. We classified LT participants into 2 groups based on the type of donor (DDLT and LDLT). In addition, patients were stratified according to age into 8 groups: younger than 40 years, 41 to 45 years, 46 to 50 years, 51 to 55 years, 56 to 60 years, 61 to 65 years, 66 to 70 years, and 71 years or older. We calculated the odds ratios (ORs) with 95% confidence intervals (CIs) for hospital mortality after LT in different age groups. The effect of individual characteristics on mortality was analyzed using multilevel models with individual LT patients (level 1) nested within the 56 hospitals (level 2). Multilevel analysis recognizes the hierarchical structures in the data and explains the variations in dependent variables at one level as a function of variables defined at various levels, along with interactions within and between levels. We used the PROC GLIMMIX procedures in the SAS software for the analysis. We used 2 models with increasing degrees of adjustment to account for potential confounding factors. Model 1 was adjusted for sex, presence of fulminant hepatic failure, alcohol-related hepatic failure, viral hepatitis, neoplasm, and donor type alone compared with the whole group. Model 2 was further adjusted for vasopressor drugs, renal replacement therapy, requirement for mechanical ventilation and ECMO. We considered a P value less than 0.05 as significant for all analyses. Statistical analyses were performed using SAS and Visual Analytics software. Additionally, we investigated for consistency in operational death reported in HIRA and the actual death as Cohen κ coefficient (Table S1, SDC, http://links.lww.com/TP/B565). We considered a Cohen κ value of more than 80% as good, 60% to 80% as substantial, 40% to 60% as moderate, 20% to 40% as fair, and less than 20% as poor quality.
RESULTS
Baseline Characteristics
During the study period, a total 9614 patients received LT. We excluded 90 of these patients who underwent re-LT, 73 patients who received both DDLT and LDLT surgical codes during the same hospitalization period and 36 multiorgan transplantations (Figure 1).
FIGURE 1.
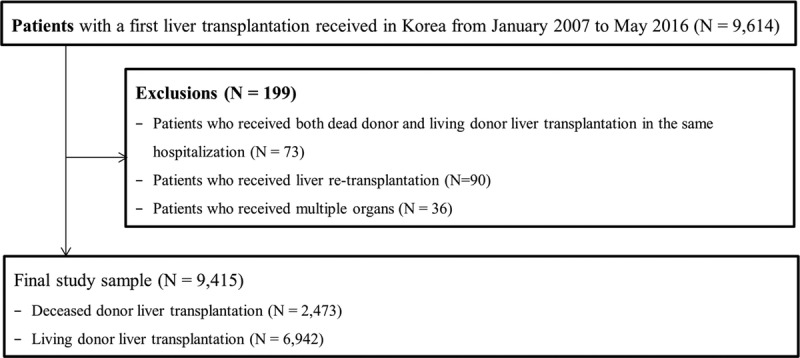
Flow chart outlining the study population.
The mean (SD) age of study patients was 52.2 (9.0) years (Table 1) and 45.6% of the LT procedures were performed in patients in their 50s whereas 0.9% (n = 84) of the LT procedures were conducted in patients aged above 70 years (Table S1, SDC, http://links.lww.com/TP/B565). Viral liver disease was the most common cause (45.4%) of LT. Among all the patients, 1233 (23.4%) received care with a mechanical ventilator, 1051 (19.4%) underwent renal replacement therapy, and 1081 (21.7%) needed vasopressor support. The volume of LT steadily increased from 2007 (n = 663, 17.7%) to 2015 (n = 1231, 31.5%) in Korea.
TABLE 1.
Demographic and clinical characteristics of liver transplantation patients
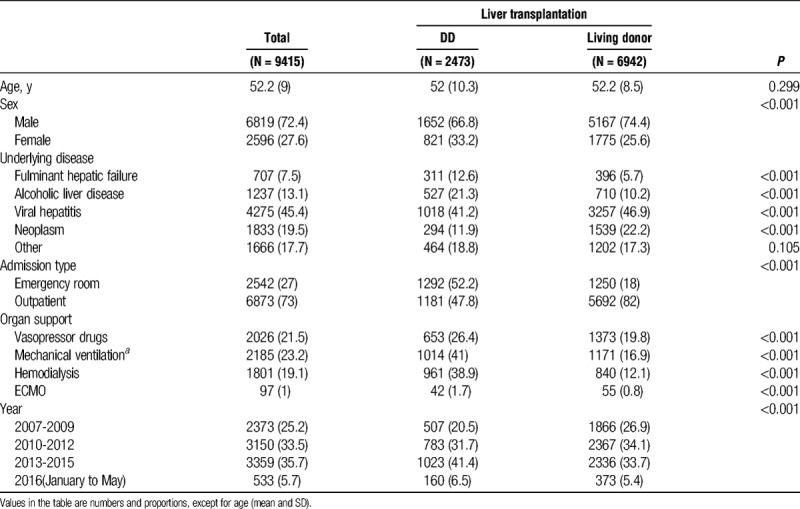
Compared with patients who underwent LDLT, DDLT recipients were more likely to be diagnosed with fulminant hepatic failure (12.6% vs 5.7%; P < 0.001) and alcoholic liver disease (21.3% vs 10.2%; P < 0.001), and display higher frequency of treatment with vasopressor drugs (26.4% vs 19.8%, P < 0.001), mechanical ventilation (41.0% vs 16.9%, P < 0.001), renal replacement therapy (38.9% vs 12.1%, P < 0.001), and ECMO (1.7% vs 0.8%, P < 0.001). While the volume of LDLT was steady, the volume of DDLT gradually increased from 2007 (17.7%) to 2015 (31.5%) as shown in Figure 2. The volume of LT was significantly increased especially in elderly patients aged 70 and above, from 2007 (0.1%) to 2015 (1.3%) as shown in Table S2, SDC (http://links.lww.com/TP/B565).
FIGURE 2.
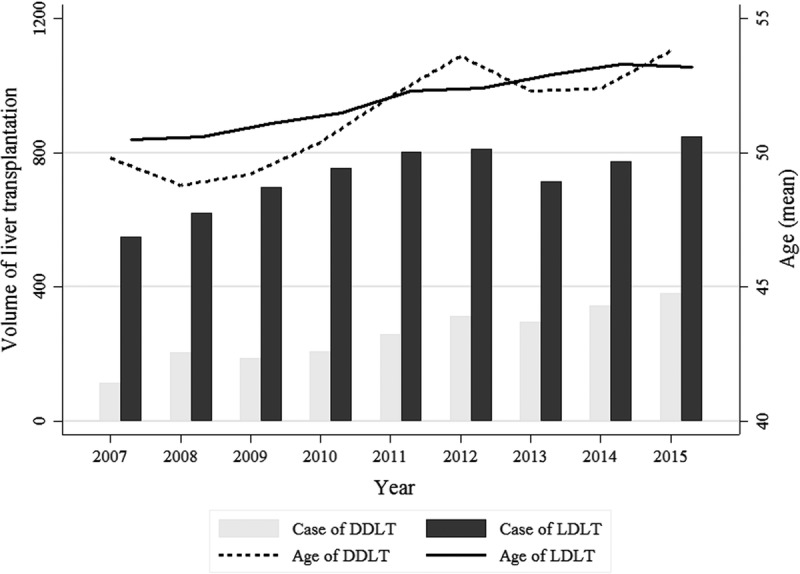
Volume and mean age of liver transplantation by year in Korea, 2007 to 2015.
Short-term Outcomes According to Age Groups
Of the 9415 patients who received LT, 591 (6.3%) died after LT before hospital discharge. In-hospital death increased with age. In patients aged 70 and above, the in-hospital mortality exceeded 20% (Table 2). In all LT recipients, the median hospital LOS was 33 days (interquartile range [IQR], 25-50) and the median ICU LOS was 10 days (IQR, 6-18). No statistically significant differences were found in the hospital LOS and total cost among the age groups (LOS, P = 0.43; total cost, P = 0.06)
TABLE 2.
Hospital outcomes after liver transplantation
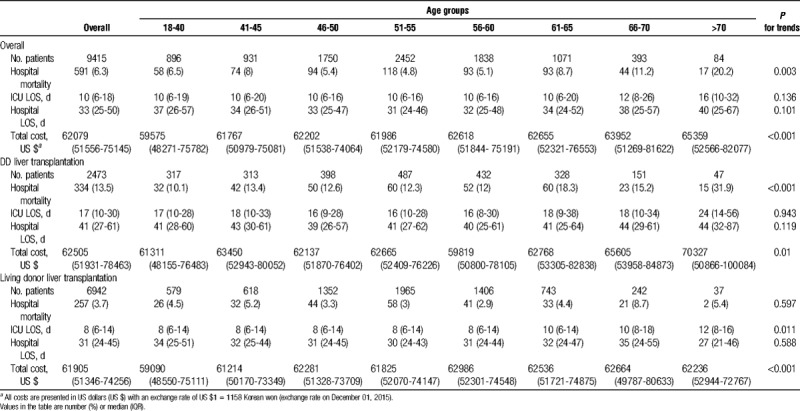
Compared with LDLT, the mortality associated with DDLT was higher in all age groups (13.5% vs 3.7%, P < 0.001). The hospital LOS for DDLT patients was longer (41 days vs 31 days, P < 0.001) and the total cost for DDLT patients was also higher (US $62 860 vs US $61 922; P = 0.002) across all age groups compared with LDLT patients. Among LDLT recipients, there was no significant increase in in-hospital mortality with age (P for trend = 0.597) and ICU LOS (P = 0.01). In LDLT recipients, the total cost increased significantly with age (P < 0.001).
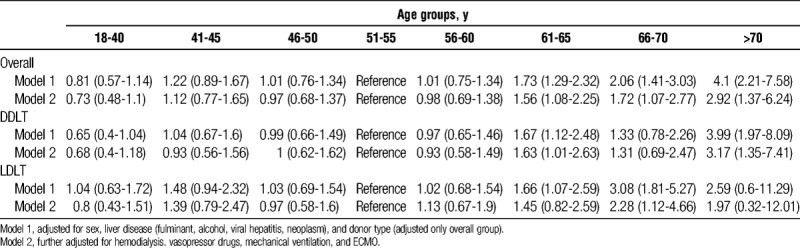
The risk of in-hospital death among patients undergoing LT continued to increase as the age of patients receiving LT increased above 60 years (Table 3). Compared with the risk of in-hospital mortality in patients aged 50 years, the risk of death in recipients above 70 years of age was about four-fold higher after adjusting for baseline liver disease (OR, 4.1; 95% CI, 2.21-7.58; P < 0.001). The effect of age on the risk of in-hospital mortality in patients underwent LT was also similar after adjusting for baseline liver disease and perioperative complications (OR, 2.92; 95% CI, 1.37-6.24; P = 0.006). The age-related effect on in-hospital mortality was greater in LDLT recipients. In DDLT patients, a steep rise in the risk of death occurred starting from 70 years of age, whereas it increased from 66 years of age in LDLT patients.
TABLE 3.
ORs (95% CI) for hospital mortality after liver transplantation by age group
Long-term Outcomes After Liver Transplantation
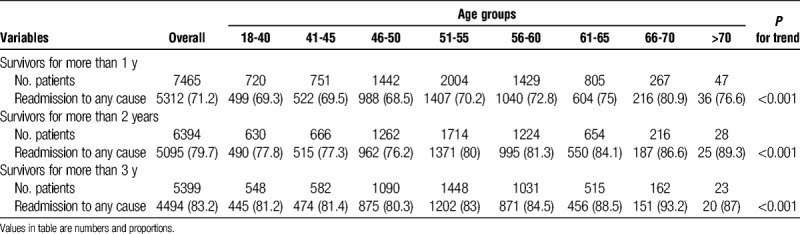
Of the 9415 patients who received LT, 7524 (79.9%) patients who received LT from January 2007 to May 2015 were analyzed for long-term mortality. Overall, 1-year mortality was 5.8% (n = 318), 2-year mortality was 9.2% (n = 501), and 3-year mortality was 11.3% (n = 615). The 1-year mortality rate was significantly higher in DDLT than in LDLT recipients (7.2% vs 5.5%, P = 0.027). However, there were no statistically significant differences in 2-year and 3-year mortalities between DDLT and LDLT. The overall rate of readmission was 71.2% (n = 5312) in survivors more than 1 year, 79.7% (n = 5095) in survivors more than 2 years, and 83.2% (n = 4494) in survivors more than 3 years. Readmission rate was related to increased age (Table 4).
TABLE 4.
Post outcomes for a year after discharge of liver transplantation patients by age groups
DISCUSSION
This nationwide analysis investigated the impact of age on outcomes in patients who underwent LT from 2007 to 2016 in Korea. The mean age of the recipients increased over time. In addition, the age of patients who received LT was an important component of the increased risk of in-hospital mortality after the first LT. In particular, the in-hospital mortality and risk of in-hospital death increased sharply in recipients of LT older than 70 years compared with the reference group (age, 51-55 years), especially in DDLT.
Significant advances in surgical techniques, anesthesia, critical care and infection control have led to improved graft survival after LT.18 As the proportion of elderly individuals in the general population increases, the demand for LT in the elderly also rises.13 The age of recipients has steadily increased since the 1980s, from 50 years to more than 60 years10,12 and currently is above 70 years.11,13,14 In our study, this increase in the mean age of LT recipients has occurred gradually from 50.4 years in 2007 to 54.2 years in 2016. In particular, transplantation among patients aged 65 and above increased from 2.3% (n = 15) in 2007 to 12.9% (n = 106) in 2015, which is comparable to the situation in the United States. According to United Network for Organ Sharing (UNOS),19 the number of patients aged 65 years or older in 2002 was 7.6%, which had nearly doubled to 14.6% in 2012. However, this trend has yet to be reflected in postoperative management.20 To date, the management of elderly recipients after LT is not significantly different from that of younger recipients. With respect to immune senescence,10 older LT recipients displayed a lower rate of rejection and a higher incidence of chronic renal failure and malignancies than younger recipients.21 However, management protocols after LT do not vary between elderly and younger recipients.22,23 Further studies are needed to reduce additional morbidity and mortality in elderly LT recipients.
Many studies have reported the success and feasibility of LT in the elderly10,13,24–26 and have included recipients 70 years and older.14,27,28 Data from the Mayo Clinic obtained from 1998 to 2004 show that the 5-year survival rate of LT recipients aged above 70 years (73%) was almost similar to that of recipients younger than 60 years (76%).13 This positive survival in the elderly has been shown in retrospective, single center studies, and patients older than 70 years have lower MELD scores at transplantation than their younger cohorts.29 Older patients also reported a better nutritional state than the younger control group.13
In other hands, Sharpton et al30 reported that being older than 70 years is a risk factor for graft loss and mortality, especially, with MELD scores higher than 28. The UNOS database revealed a lower survival rate in elderly (55%) than in younger LT recipients,31 and data from the European Liver Transplant Registry showed higher mortality in older recipients.32 Mortality rate in elderly population was similarly higher than in younger recipients in our data (P < 0.001, Table 2). The outcome of UNOS was similar to our in-hospital mortality. In our study, age was an independent risk factor for death after adjusting for underlying liver diseases, comorbidities and severity. Mortality after LT in recipients older than 70 years (20.2%) was 4.2-fold higher than in patients aged 51 to 55 years (4.8%). The risk of death increased sharply among patients aged greater than 70 years. In the case of patients undergoing DDLT, the risk of death was affected more by age than in LDLT patients. Liver transplantation recipients who received transplant from DDs often had a more severe pretransplantation condition compared with those who received the liver from a living donor.
Although medical conditions are better in the elderly than in younger recipients, the elderly are more vulnerable to infection or organ failure in postoperative care.33 In our study, older recipients were supported by mechanical ventilation and renal replacement therapy in the perioperative period more than younger patients. Advanced age was also associated with higher medical costs and higher readmission rates after discharge. Similar to other studies,34,35 medical costs in older recipients on life support were higher than in younger recipients. Even if LT in the elderly was feasible, it was probably inadequate due to organ shortage. This finding supports the current condition of LT, which is relatively contraindicated for patients at an advanced age in the absence of a specific and successfully implemented protocol. Further studies are required to improve the outcomes of elderly recipients considering their greater odds of renal dysfunction and immune-senescence.
Several limitations need to be considered to interpret our findings. First, the administrative data regarding claims were designed for reimbursement purposes and related to comorbidities, procedures, and outcomes, but were not important determinants of liver status (eg, MELD or CTP scores), including test results. Our ability to adjust for disease severity in recipients was limited. Second, claims data cannot be used to identify donor or transplant-related factors that influence mortality, such as ABO compatibility and cold ischemic time. Third, we identified treated items, but not the dates of procedures due to the use of administrative claims data, which increased the difficulty in ascertaining the exact date of transplantation within the hospital stay. Therefore, we were unable to demonstrate the hazard ratios of mortality. We might not be fully able to estimate the risk of in-hospital death after transplantation. However, our data could be used to estimate the risk of mortality during pretransplantation, intratransplantation, and posttransplantation. We adjusted for other factors with a multilevel regression model to accurately estimate the effect of age on LT mortality. Fourth, our data were analyzed in-hospital mortality without operational definition. Therefore, we determined long-term mortality based on insurance claim history. The crude long-term outcomes were validated using our institutional data and HIRA data set. The consistency in long-term mortality between 2 data sets was good in LDLT and fair-to-moderate in DDLT (Table S1, SDC, http://links.lww.com/TP/B565). Other cohort studies with longer follow-up periods are needed to corroborate our findings. Finally, our study was conducted in Korea, a country in which LDLT is popular, and our findings may not be applicable to Western countries following different systems.
In addition, we understand that the novelty of our validation study may be disputed. However, validation studies are indispensable to address reproducibility in various populations. Furthermore, compared with other single-center studies, our study is more likely to result in unbiased information on outcomes in LT recipients after adjusting for differences in experience among hospitals. The NHI database, which covers all Koreans, also excludes publication bias and contributes to robust data.
In conclusion, the age of transplant recipients is increasing with aging societies. Our data show that age is still an important risk factor for LT, and further studies investigating posttransplantation protocols and treatments are indicated for elderly patients to improve the efficiency of liver transplantation in older patients. LT, especially DDLT, in elderly patients requires abundant caution, until outcomes are further improved.
Supplementary Material
Footnotes
This study was supported by Samsung Medical Center grant (SMX1151381).
The authors declare no conflicts of interest.
The study was reviewed by the Institutional Review Board (IRB) of Samsung Medical Center (IRB SMC 2017-01-001) and was exempted because it involved only deidentified administrative data collected previously.
The authors cannot share their data because of administrative data of Korean government.
E.G. participated in its design and drafted the article. J.M.K., K.J., and G.Y.S. participated in its design and coordination. H.P. and D.K. performed the statistical analysis and helped to draft the article. J.C. directed the statistical analysis form the HIRA database. J.P. participated in its design and coordination and contributed as a corresponding author.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol. 2013;10:746–751. [DOI] [PubMed] [Google Scholar]
- 2.deLemos AS, Vagefi PA. Expanding the donor pool in liver transplantation: extended criteria donors. Clin Liver Dis. 2013;2:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–1776. [DOI] [PubMed] [Google Scholar]
- 5.Briceno J, Ciria R, de la Mata M. Donor-recipient matching: myths and realities. J Hepatol. 2013;58:811–820. [DOI] [PubMed] [Google Scholar]
- 6.Keswani RN, Ahmed A, Keeffe EB. Older age and liver transplantation: a review. Liver Transpl. 2004;10:957–967. [DOI] [PubMed] [Google Scholar]
- 7.Bilbao I, Armadans L, Lazaro JL, et al. Predictive factors for early mortality following liver transplantation. Clin Transplant. 2003;17:401–411. [DOI] [PubMed] [Google Scholar]
- 8.Desai MN, Mange CK, Crawford M, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa H, Todo S. Evolution of immunosuppression in liver transplantation: contribution of cyclosporine. Transplant Proc. 2005;36:274S–284S. [DOI] [PubMed] [Google Scholar]
- 10.Kuramitsu K, Egawa H, Keeffe EB, et al. Impact of age older than 60 years in living donor liver transplantation. Transplantation. 2007;84:166–172. [DOI] [PubMed] [Google Scholar]
- 11.Wilson GC, Quillin RC, Wima K, et al. Is liver transplantation safe and effective in elderly (> = 70 years) recipients? A case-controlled analysis. Hpb. 2014;16:1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikegami T, Bekki Y, Imai D, et al. Clinical outcomes of living donor liver transplantation for patients 65 years old or older with preserved performance status. Liver Transpl. 2014;20:408–415. [DOI] [PubMed] [Google Scholar]
- 13.Aduen JF, Sujay B, Dickson RC, et al. Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc. 2009;84:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oezcelik A, Dayangac M, Guler N, et al. Living donor liver transplantation in patients 70 years or older. Transplantation. 2015;99:1436–1440. [DOI] [PubMed] [Google Scholar]
- 15.Petrowsky H, Rana A, Kaldas FM, et al. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014;259:1186–1194. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Jeon K, Chung CR, et al. A nationwide analysis of intensive care unit admissions, 2009–2014—the Korean ICU National Data (KIND) study. J Crit Care. 2017;44:24–30. [DOI] [PubMed] [Google Scholar]
- 17.Korea Pharmaceutical Information Service. Korea Pharmaceutical Information vol 2016.
- 18.Fernandez TMA, Gardiner PJ. Critical care of the liver transplant recipient. Curr Anesthesiol Rep. 2015;5:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aloia TA, Knight R, Gaber AO, et al. Analysis of liver transplant outcomes for United Network for Organ Sharing recipients 60 years old or older identifies multiple model for end-stage liver disease–independent prognostic factors. Liver Transpl. 2010;16:950–959. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14(Suppl 1):69–96. [DOI] [PubMed] [Google Scholar]
- 21.Herrero JI, Lucena JF, Quiroga J, et al. Liver transplant recipients older than 60 years have lower survival and higher incidence of malignancy. Am J Transplant. 2003;3:1407–1412. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Carithers R. Liver transplantation in the elderly: an evidence based review on patient outcome, selection and management strategies. J Biomed Sci Eng. 2014;7:651–661. [Google Scholar]
- 23.EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizumi T, Shirabe K, Soejima Y, et al. Living donor liver transplantation in patients older than 60 years. Transplantation. 2010;90:433–437. [DOI] [PubMed] [Google Scholar]
- 25.Collins BH, Pirsch JD, Becker YT, et al. Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation. 2000;70:780–783. [DOI] [PubMed] [Google Scholar]
- 26.Garcia CE, Garcia RF, Mayer AD, et al. Liver transplantation in patients over sixty years of age. Transplantation. 2001;72:679–684. [DOI] [PubMed] [Google Scholar]
- 27.Kwon JH, Yoon YI, Song GW, et al. Living donor liver transplantation for patients older than age 70 years: a single-center experience. Am J Transplant. 2017;17:2890–2900. [DOI] [PubMed] [Google Scholar]
- 28.Safdar K, Neff GW, Montalbano M, et al. Liver transplant for the septuagenarians: importance of patient selection. Transplant Proc. 2004;36:1445–1448. [DOI] [PubMed] [Google Scholar]
- 29.Lipshutz GS, Hiatt J, Ghobrial RM, et al. Outcome of liver transplantation in septuagenarians: a single-center experience. Arch Surg. 2007;142:775–781; discussion 781–774. [DOI] [PubMed] [Google Scholar]
- 30.Sharpton SR, Feng S, Hameed B, et al. Combined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomes. Transplantation. 2014;98:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz JJ, Pappas L, Thiesset HF, et al. Liver transplantation in septuagenarians receiving model for end-stage liver disease exception points for hepatocellular carcinoma: the national experience. Liver Transpl. 2012;18:423–433. [DOI] [PubMed] [Google Scholar]
- 32.Burroughs AK, Sabin CA, Rolles K, et al. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225–232. [DOI] [PubMed] [Google Scholar]
- 33.Winnick AM, Karabicak I, Distant DA. Elderly Transplant Recipients. In: Rosenthal RA, Zenilman ME, Katlic MR, Principles and Practice of Geriatric Surgery. New York, NY: Springer New York; 2011:1335–1350. [Google Scholar]
- 34.Habka D, Mann D, Landes R, et al. Future economics of liver transplantation: a 20-year cost modeling forecast and the prospect of bioengineering autologous liver grafts. PLoS One. 2015;10:e0131764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvalaggio PR, Dzebisashvili N, MacLeod KE, et al. The interaction among donor characteristics, severity of liver disease and the cost of liver transplantation. Liver Transpl. 2011;17:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


