Abstract
New 5-(2-thienyl)-1,2,4-triazoles and 5-(2-thienyl)-1,3,4-oxadiazoles namely, N-[3-mercapto-5-(2-thienyl)-1,2,4-triazol-4-yl]-N'-arylthioureas 4a–e, 2-arylamino-5-(2-thienyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles 5a–e, 3-arylaminomethyl-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 7a–e, 3-(N-substituted anilinomethyl)-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 8a, b and 3-(4-substituted-1-piperazinylmethyl)-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 9a–f, were prepared. The synthesized compounds were tested for in vitro activities against certain strains of Gram-positive and Gram-negative bacteria and the yeast-like pathogenic fungus Candida albicans. Compound 9a displayed marked broad spectrum antibacterial activity, while compounds 4d, 5e, 7b, 7c, 7d, 9b, 9c and 9d were highly active against the tested Gram-positive bacteria. None of the synthesized compounds were proved to be significantly active against Candida albicans.
Keywords: 2-thienyl derivatives; 1,2,4-triazoles; 1,3,4-oxadiazoles; microwave irradiation; antimicrobial activity
1. Introduction
In the past decades, the problem of multidrug resistant microorganisms has reached on alarming level around the world, and the synthesis of new anti-infective compounds has become an urgent need for the treatment of microbial infections. The 1,2,4-triazole nucleus has been incorporated into a wide variety of therapeutically important agents, mainly displaying antimicrobial [1,2,3,4,5] and anti-inflammatory activities [6,7,8,9]. In addition, several 1,3,4-oxadiazole derivatives were reported to exhibit good antimicrobial [10,11,12,13] and anti-inflammatory activities [13,14,15]. Moreover, the thiophene nucleus was proven to constitute the active part of several biologically active compounds [16,17,18,19,20]. In view of these findings, and in continuation to our interest in the synthesis and biological activities 1,2,4-triazoles and 1,3,4-oxadiazoles [7,12], the present investigation describes the synthesis as potential antimicrobial agents of new series of 1,2,4-triazoles and 1,3,4-oxadiazoles bearing 2-thienyl moieties.
2. Results and Discussion
2.1. Chemistry
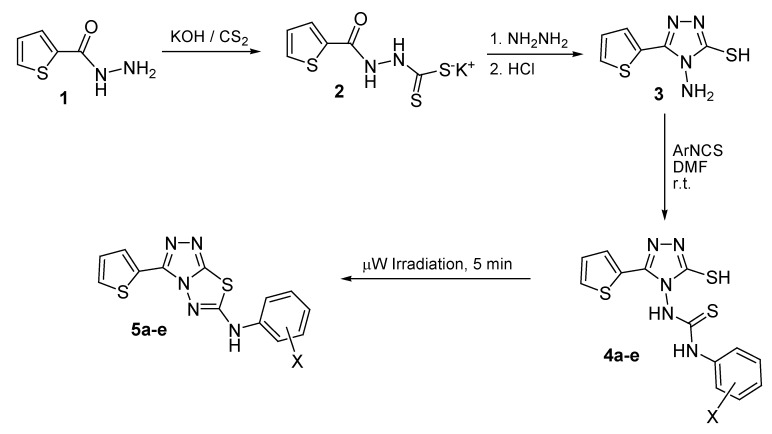
4-Amino-5-mercapto-3-(2-thienyl)-1,2,4-triazole (3), required as starting material, was prepared via treatment of an ethanolic potassium hydroxide solution of thiophene-2-carbohydrazide (1) with carbon disulphide to yield the intermediate dithiocarbazate 2, which was subsequently reacted with hydrazine to yield compound 3 [21]. It was reported that the nature of the reaction products of N-amino-1,2,4-triazoles with arylisothiocyanates was dependent on the reaction solvent and temperature [22,23]. Molina and Tárraga [22] reported the formation of the N,N'-disubstituted thiourea derivatives upon carrying out the reaction in N,N-dimethylformamide (DMF) at room temperature for 24 h and the formation of the 2,5-disubstituted-1,2,4-triazolo[3,4–b][1,3,4]thiadiazoles upon prolonged heating in DMF. On the other hand, carrying out the reaction under microwave irradiation in the absence of solvent yielded the corresponding 2,5-disubstituted-1,2,4-triazolo[3,4–b][1,3,4]thiadiazoles in good yield [23]. Thus, compound 3 was reacted with phenyl- or substituted phenylisothiocyanates in DMF at room temperature for 24 h to yield the corresponding N,N'-disubstituted thiourea derivatives 4a–e in excellent yields (85–90%). Compounds 4a–e were successfully dehydrosulphurized to the corresponding 2-arylamino-5-(2-thienyl)-1,2,4-triazolo[3,4–b][1,3,4]thiadiazole derivatives 5a–e in 92%–95% yields via exposure to microwave irradiation for 5 min (Scheme 1, Table 1).
Scheme 1.
Synthetic Pathway for Compounds 4a–e and 5a–e.
Table 1.
Crystallization solvents, melting points, yield percentages, molecular formulae, and molecular weights of compounds 4a–e, 5a–e, 7a–e, 8a, b and 9a–f.
| Comp. No. | X/R | Cryst. Solvent | M.p. (°C) | Yield (%) | Mol. Formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 4a | H | EtOH/H2O | 205-7 (Dec.) | 85 | C13H11N5S3 (333.45) |
| 4b | 3-F | EtOH/H2O | 169-71 (Dec.) | 89 | C13H10FN5S3 (351.45) |
| 4c | 4-F | EtOH/H2O | 174-6 (Dec.) | 85 | C13H10FN5S3 (351.45) |
| 4d | 4-Cl | EtOH/H2O | 169-71 (Dec.) | 88 | C13H10ClN5S3 (367.9) |
| 4e | 4-Br | EtOH | 210-2 (Dec.) | 90 | C13H10BrN5S3 (412.35) |
| 5a | H | EtOH | 292-4 | 94 | C13H9N5S2 (299.37) |
| 5b | 3-F | EtOH | 266-8 | 90 | C13H8FN5S2 (317.36) |
| 5c | 4-F | EtOH | 275-277 | 90 | C13H8FN5S2 (317.36) |
| 5d | 4-Cl | EtOH | >300 | 95 | C13H8ClN5S2 (333.82) |
| 5e | 4-Br | EtOH/CHCl3 | >300 | 95 | C13H8BrN5S2 (378.27) |
| 7a | 2-F | EtOH | 107-9 | 72 | C13H10FN3OS2 (307.37) |
| 7b | 4-F | EtOH | 123-5 | 81 | C13H10FN3OS2 (307.37) |
| 7c | 4-Cl | EtOH | 175-7 | 85 | C13H10ClN3OS2 (323.82) |
| 7d | 2-CF3 | EtOH | 133-135 | 68 | C14H10F3N3OS2 (357.37) |
| 7e | 3-CF3 | EtOH | 108-10 | 75 | C14H10F3N3OS2 (357.37) |
| 8a | CH3 | EtOH | 112-4 | 92 | C14H13N3OS2 (303.4) |
| 8b | C6H5CH2 | EtOH | 120-2 | 90 | C20H17N3OS2 (379.5) |
| 9a | CH3 | EtOH/H2O | 96-8 | 56 | C12H16N4OS2 (296.41) |
| 9b | C6H5 | EtOH | 131-3 | 70 | C17H18N4OS2 (358.48) |
| 9c | 4-FC6H4 | EtOH | 120-2 | 77 | C17H17N4OFS2 (376.46) |
| 9d | 2-CF3C6H4 | EtOH | 141-3 | 72 | C18H17F3N4OS2 (426.48) |
| 9e | C6H5CH2 | EtOH | 101-3 | 65 | C18H20N4OS2 (372.51) |
| 9f | 2-CF3C6H2CH2 | EtOH | 125-7 | 72 | C19H19N4OF3S2 (440.50) |
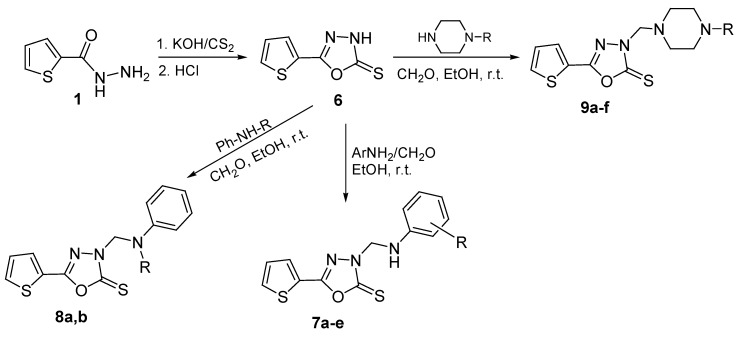
5-(2-Thienyl)-1,3,4-oxadiazoline-2-thione (6) was prepared via the reaction of the hydrazide 1 with carbon disulphide and potassium hydroxide, in ethanol, followed by acidification, following the previously reported methods [24,25,26]. Treatment of 6 with formaldehyde solution and primary aromatic amines, N-substituted anilines or N-substituted piperazines in ethanol at room temperature afforded good yields of the corresponding N-Mannich derivatives 7a–e, 8a, b and 9a–f, respectively (Scheme 2, Table 1). The structures of the synthesized compounds were confirmed by elemental analyses, IR, 1H-NMR, 13C-NMR, and mass spectral data.
2.2. Antimicrobial Testing
The newly synthesized compounds 4a–e, 5a–e, 7a–e, 8a, b and 9a–f were tested for their in vitro antimicrobial activity against a panel of standard strains of the Gram-positive bacteria (Staphylococcus aureus IFO 3060 and Bacillus subtilis IFO 3007), the Gram-negative bacteria (Escherichia coli IFO 3301 and Pseudomonas aeuroginosa IFO 3448), and the yeast-like pathogenic fungus Candida albicans IFO 0583. The primary screening was carried out using the agar disc-diffusion method [27] using Müller-Hinton agar medium. Sterile filter paper discs (8 mm diameter) were moistened with the test compound solution in dimethylsulphoxide of specific concentration 200 μg/disc were carefully placed on the agar cultures plates that had been previously inoculated separately with the microorganisms. The plates were incubated at 37 °C, and the diameter of the growth inhibition zones were measured after 24 h in case of bacteria and 48 h in case of Candida albicans. The results of the preliminary antimicrobial testing of compounds 4a–e, 5a–e, 7a–e, 8a, b and 9a–f (200 μg/disc), the antibacterial antibiotic Ampicillin trihydrate (100 μg/disc) and the antifungal drug clotrimazole (100 μg/disc) are shown in Table 2. The results revealed that the majority of the synthesized compounds showed varying degrees of inhibition against the tested microorganisms. In general, the best activity was displayed by compounds 4d, 4e, 5e, 7b, 7c, 7d, 9a, 9b, 9c and 9d, and the Gram-positive bacteria Bacillus subtilis is considered the most sensitive among the tested microorganisms. Compound 9a showed high broad-spectrum inhibitory activity against the tested microorganisms, while compounds 4d, 5e, 7b, 7c, 7d, 9b, 9c and 9d were selectively active against the Gram-positive bacteria (inhibition zone >15 mm). Moderate (inhibition zone 12–15 mm) or weak (inhibition zone 10–12 mm) antibacterial activity was observed for compounds 4a, 4b, 4c, 5b, 5c, 5d, 7a and 7e. Meanwhile, compounds 5a, 8a, 8b, 9e and 9f were inactive against the tested microorganisms. All the synthesized compounds were practically inactive against Candida albicans, compounds 9a, 9c and 9d produced weak activity relative to the antifungal drug clotrimazole. The minimal inhibitory concentration (MIC) for the most active compounds 4b, 4c, 4d, 4e, 5e, 7a, 7b, 7c, 7d, 9a, 9b. 9c and 9d against the same microorganism used in the primary screening was carried out using the microdilution susceptibility method in Müller-Hinton Broth and Sabouraud Liquid Medium [28]. The compounds, Ampicillin trihydrate and clotrimazole were dissolved in dimethylsulphoxide at concentration of 128 μg/mL. The twofold dilutions of the solution were prepared (128, 64, 32, …, 0.5 μg/mL). The microorganism suspensions at 106 CFU/mL (colony forming unit/mL) concentrations were inoculated to the corresponding wells. The plates were incubated at 36 °C for 24 and 48 h for the bacteria and Candida albicans, respectively. The MIC values were determined as the lowest concentration that completely inhibited visible growth of the microorganism as detected by unaided eye. The MIC of the most active compounds, the antibacterial antibiotic ampicillin trihydrate and the antifungal drug clotrimazole which are shown in Table 3, were in accordance with the results obtained in the primary screening.
Table 2.
Antimicrobial activity of compounds 4a–e, 5a–e, 7a–e, 8a, b and 9a–f (200 μg/8 mm disc), the broad spectrum antibacterial drug ampicillin (100 μg/8 mm disc) and the antifungal drug clotrimazole (100 μg/8 mm disc) against Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS), Escherichia coli IFO 3301 (EC), Pseudomonas aeuroginosa IFO 3448 (PA), and Candida albicans IFO 0583 (CA).
| Comp. No. | Diameter of Growth Inhibition Zone (mm)* | ||||
|---|---|---|---|---|---|
| SA | BS | EC | PA | CA | |
| 4a | 12 | 14 | – | – | – |
| 4b | 12 | 16 | – | – | – |
| 4c | 14 | 16 | – | – | – |
| 4d | 17 | 19 | – | – | – |
| 4e | 16 | 16 | 12 | – | – |
| 5a | – | – | – | – | – |
| 5b | 10 | 12 | – | – | – |
| 5c | 12 | 12 | – | – | – |
| 5d | 13 | 13 | – | – | – |
| 5e | 16 | 17 | 14 | – | – |
| 7a | 14 | 18 | 12 | 12 | – |
| 7b | 16 | 18 | 14 | 14 | – |
| 7c | 18 | 18 | 14 | 12 | – |
| 7d | 17 | 18 | 12 | 12 | – |
| 7e | 14 | 15 | 12 | – | – |
| 8a | – | – | – | – | – |
| 8b | – | – | – | – | – |
| 9a | 18 | 18 | 16 | 17 | 12 |
| 9b | 16 | 18 | 12 | 10 | – |
| 9c | 17 | 18 | 15 | 12 | 12 |
| 9d | 18 | 16 | 16 | 14 | 12 |
| 9e | – | – | – | – | – |
| 9f | – | – | – | – | – |
| Ampicillin | 19 | 18 | 16 | 15 | NT |
| Clotrimazole | NT | NT | NT | NT | 21 |
* (–): Inactive, no inhibition zone. (NT): Not tested.
Table 3.
The minimal inhibitory concentrations (MIC, μg/mL) of compounds 4b, 4c, 4d, 4e, 5e, 7a, 7b, 7c, 7d, 9a, 9b. 9c and 9d, the broad spectrum antibacterial drug Ampicillin and the antifungal drug Clotrimazole against Staphylococcus aureus IFO 3060 (SA), Bacillus subtilis IFO 3007 (BS), Escherichia coli IFO 3301 (EC), Pseudomonas aeuroginosa IFO 3448 (PA), and Candida albicans IFO 0583 (CA).
| Comp. No. | Minimal Inhibitory Concentration (MIC, μg/mL)* | ||||
|---|---|---|---|---|---|
| SA | BS | EC | PA | CA | |
| 4b | ND | 4 | ND | ND | ND |
| 4c | ND | 4 | ND | ND | ND |
| 4d | 4 | 1 | ND | ND | ND |
| 4e | 8 | 4 | ND | ND | ND |
| 5e | ND | 4 | ND | ND | ND |
| 7a | ND | 2 | ND | ND | ND |
| 7b | 4 | 2 | ND | ND | ND |
| 7c | 4 | 2 | ND | ND | ND |
| 7d | 2 | 1 | ND | ND | ND |
| 9a | 2 | 2 | 4 | 2 | ND |
| 9b | 4 | 2 | ND | ND | ND |
| 9c | 2 | 2 | ND | ND | ND |
| 9d | 2 | 4 | 4 | ND | ND |
| Ampicillin | 1 | 0.5 | 2 | 2 | ND |
| Clotrimazole | ND | ND | ND | ND | 2 |
* ND: Not determined.
The structure-antibacterial activity relationship of the synthesized compounds revealed that the acyclic N,N'-disubstituted thiourea derivatives 4a–e and their cyclic 2,5-disubstituted-1,2,4-triazolo[3,4–b][1,3,4]thiadiazoles analogues 5a–e are almost of equal activity against the tested Gram-positive bacteria and almost inactive against the Gram-negative bacteria. The antibacterial activity of the oxadiazole N-Mannich derivatives 7a–e, 8a, b and 9a–f indicated that antibacterial activity is mainly dependent on the aminomethyl substituents. The arylaminomethyl-1,3,4-oxadiazoline-2-thiones 7a–e were almost active against both the Gram-positive and Gram-negative bacteria, while N-methyl or benzyl derivatives 8a, b were completely inactive. Regarding the piperazine derivatives 9a–f, the methyl, phenyl, 4-fluorophenyl and 2-trifluoromethylphenyl derivatives 9a, 9b, 9c and 9d were highly active against the Gram-positive bacteria and to lesser extent to the Gram-negative bacteria. On the other hand, replacement of the methyl or aryl substituents in compounds 9a, 9b, 9c and 9d with a 4-benzyl- or 2-trifluoromethylbenzyl moieties 9e and 9f dramatically reduced the antimicrobial activity. None of the synthesized compounds were found to be active against Candida albicans, and only the piperazine derivatives 9a, 9c and 9d exhibited marginal activities.
3. Experimental
3.1. General
All melting points (°C, uncorrected) were determined using a Gallenkamp melting point apparatus. Microwave irradiation was performed using an Akai MW-GB092MP (800 W) unmodified domestic microwave oven operated at 2450 MHz. Infra red spectra were recorded in KBr disc using Jasco FT/IR 460 Plus spectrometer, and expressed in wave number υ (cm−1). NMR spectra were obtained on a Bruker AC 500 Ultra Shield NMR spectrometer at 500 MHz for 1H and 125 MHz for 13C, the chemical shifts are expressed in δ (ppm) downfield from tetramethylsilane (TMS). Electron impact mass spectra were recorded on a Shimadzu GC–MS-QP 5000 instrument. Elemental analyses (C, H, N, S) were in full agreement with the proposed structures within ± 0.4% of the theoretical values. The bacterial strains and Candida albicans fungus were obtained from the Institute of fermentation of Osaka (IFO), Osaka, Japan. The reference drugs ampicillin trihydrate (CAS 7177-48-2) and clotrimazole (CAS 23593-75-1) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
3.2. N-[3-Mercapto-5-(2-thienyl)-1,2,4-triazol-4-yl]-N'-arylthioureas 4a–e
The appropriate arylisothio-cyanate (2 mmol) was added to a solution of 4-amino-3-mercapto-5-(2-thienyl)-1,2,4-triazole (3, 0.4 g, 2 mmol) in dry DMF (8 mL), and the solution was stirred at room temperature for 24 h. Water (20 mL) was then added and the mixture was stirred for 20 min. The separated precipitate was filtered, washed with water and crystallized from ethanol.
4a: IR, υ (cm−1): 3,305 (NH), 3,021 (Ar-CH), 1,604, 1,452 (C=N), 1,374 (C=S); 1H-NMR (DMSO-d6): δ 6.78–7.03 (m, 3H, Ar-H), 7.15–7.32 (m, 5H, Ar-H & thiophene-H), 10.01 (s, 1H, NH), 10.62 (s, 1H, NH), 13.88 (s, 1H, SH); 13C-NMR: δ 123.52, 125.44, 127.01, 127.32, 127.89, 129.55, 136.42, 142.75 (Ar-C & thiophene-C), 143.53 (triazole C-5), 159.50 (triazole C-3), 169.54 (C=S); MS, m/z (rel. int.): 333 (M+, 6), 301 (9), 182 (54), 92 (88), 77, (72), 58 (100).
4b: IR, υ (cm−1): 3308 (NH), 3022 (Ar-CH), 1610, 1452 (C=N), 1322 (C=S). 1H NMR (DMSO-d6): δ 6.89–7.98 (m, 7H, Ar-H & Thiophene-H), 10.16 (s, 1H, NH), 10.95 (s, 1H, NH), 13.95 (s, 1H, SH). 13C NMR: δ 110.78, 114.76, 122.0, 125.50, 126.75, 127.95, 130.62, 136.20, 141.43, 160.25 (Ar-C & Thiophene-C), 146.53 (triazole C-5), 164.44 (triazole C-3), 172.82 (C=S). MS, m/z (Rel. Int.): 351 (M+, 1), 319 (3), 182 (61), 154 (9), 111 (77), 57 (100).
4c: IR, υ (cm−1): 3331 (NH), 3022 (Ar-CH), 1610, 1460 (C=N), 1371 (C=S). 1H NMR (DMSO-d6): δ 6.81–7.75 (m, 7H, Ar-H & Thiophene-H), 10.15 (s, 1H, NH), 10.97 (s, 1H, NH), 14.05 (s, 1H, SH). 13C NMR: δ 113.65, 123.05, 126.98, 127.90, 128.88, 132.24, 142.35, 159.88 (Ar-C & Thiophene-C), 147.90 (triazole C-5), 168.01 (triazole C-3), 171.98 (C=S). MS, m/z (Rel. Int.): 351 (M+, 4), 319 (11), 182 (69), 111 (71), 57 (100).
4d: IR, υ (cm−1): 3310 (NH), 3025 (Ar-CH), 1598, 1450 (C=N), 1377 (C=S). 1H NMR (DMSO-d6): δ 7.21–7.42 (m, 5H, Ar-H & Thiophene-H), 7.63 (d, 2H, Ar-H, J = 8.2 Hz), 10.01 (s, 1H, NH), 10.88 (s, 1H, NH), 13.88 (s, 1H, SH). 13C NMR: δ 124.11, 127.60, 127.96, 129.05, 129.54, 136.10, 143.25 (Ar-C & Thiophene-C), 148.88 (triazole C-5), 167.21 (triazole C-3), 168.33 (C=S). MS, m/z (Rel. Int.): 369 (M+ +2, 2), 367 (M+, 5), 335 (6), 333 (13), 182 (44), 126 (22), 111 (64), 57 (100).
4e: IR, υ (cm−1): 3343 (NH), 3010 (Ar-CH), 1600, 1442 (C=N), 1388 (C=S). 1H NMR (DMSO-d6): δ 7.22–7.98 (m, 7H, Ar-H & Thiophene-H), 10.80 (s, 1H, NH), 11.0 (s, 1H, NH), 14.06 (s, 1H, SH). 13C NMR: δ 121.07, 126.10, 128.43, 129.59, 130.12, 131.72, 135.29, 143.65 (Ar-C & Thiophene-C), 146.33 (triazole C-5), 167.29 (triazole C-3), 168.31 (C=S). MS, m/z (Rel. Int.): 413 (M+ +2, 5), 411 (M+, 4), 377 (8), 379 (10), 182 (23), 171 (25), 169 (25), 57 (100).
3.3. 2-Arylamino-5-(2-thienyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles 5a–e
The appropriate N,N'-disubstituted thiourea 4a–c (2 mmol) was placed in 50 mL open round bottom flask, and the mixture was irradiated in the microwave oven for 5 min. at 454 W (58%). On cooling, chloroform (10 mL) was added and the reaction mixture was stirred for 5 min., then filtered and the filtrate was evaporated in vacuo. The crude product was crystallized to yield the desired compounds 5a–e.
5a: IR, υ (cm−1): 3221 (NH), 3001 (Ar-CH), 1620, 1459 (C=N). 1H NMR (CDCl3): δ 6.44 (s, 1H, NH), 6.95–7.05 (m, 3H, Ar-H), 7.22–7.34 (m, 5H, Ar-H and Thiophene-H). 13C NMR: δ 117.50, 120.05, 125.44, 127.62, 129.82, 139.92, 142.45, 144.56 (Ar-C & Thiophene-C), 148.70 (C-8), 151.10 (C-5), 172.15 (C-2). MS, m/z (Rel. Int.): 300 (M+ +1, 2), 299 (M+, 24), 223 (26), 91 (65), 77 (100), 57 (13).
5b: IR, υ (cm−1): 3245 (NH), 3010 (Ar-CH), 1654, 1462 (C=N). 1H NMR (CDCl3): δ 6.93 (s, 1H, NH), 6.92 (s, 1H, Ar-H), 7.02–7.62 (m, 6H, Ar-H and Thiophene-H). 13C NMR: δ 105.66, 108.78, 113.23, 124.50, 127.90, 128.05, 130.85, 142.22, 144.60, 161.09 (Ar-C & Thiophene-C), 151.44 (C-8), 154.40 (C-5), 177.80 (C-2). MS, m/z (Rel. Int.): 317 (M+, 26), 207 (11), 110 (34), 96 (12), 57 (100).
5c: IR, υ (cm−1): 3241 (NH), 3012 (Ar-CH), 1623, 1466 (C=N). 1H NMR (CDCl3): δ 6.67–6.97 (m, 3H, Ar-H & NH), 7.15–7.62 (m, 5H, Ar-H and Thiophene-H). 13C NMR: δ 114.50, 119.02, 124.94, 127.24, 128.05, 136.65, 142.75, 150.76 (Ar-C & Thiophene-C), 151.98 (C-8), 156.34 (C-5), 178.05 (C-2). MS, m/z (Rel. Int.): 317 (M+, 33), 207 (23), 110 (42), 96 (9), 57 (100).
5d: IR, υ (cm−1): 3259 (NH), 3044 (Ar-CH), 1626, 1461 (C=N). 1H NMR (CDCl3): δ 6.44 (s, 1H, NH), 7.08 (d, 2H, Ar-H, J = 8.0 Hz), 7.45–7.63 (m, 5H, Ar-H & Thiophene-H). 13C NMR: δ 115.44, 122.90, 124.0, 127.10, 127.65, 130.22, 136.55, 141.80 (Ar-C & Thiophene-C), 149.51 (C-8), 155.25 (C-5), 174.85 (C-2). MS, m/z (Rel. Int.): 335 (M+ +2, 7), 333 (M+, 24), 207 (35), 126 (19), 113 (7), 111 (20), 57 (100).
5e: IR, υ (cm−1): 3251 (NH), 3014 (Ar-CH), 1661, 1465 (C=N). 1H NMR (CDCl3): δ 6.52 (s, 1H, NH), 7.16 (d, 2H, Ar-H, J = 8.2 Hz), 7.33–7.68 (m, 5H, Ar-H & Thiophene-H). 13C NMR: δ 112.23, 118.24, 124.90, 127.02, 127.65, 132.45, 142.05, 144.50 (Ar-C & Thiophene-C), 146.60 (C-8), 153.90 (C-5), 172.82 (C-2). MS, m/z (Rel. Int.): 379 (M+ +2, 51), 377 (M+, 62), 207 (52), 172 (42), 170 (41), 158 (8), 156 (10), 57 (100).
3.4. 3-Arylaminomethyl-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 7a–e, 3-(N-substituted anilinomethyl)- 5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 8a,b and 3-(4-substituted-1-piperazinylmethyl)-5-(2-thienyl)-1,3,4-oxadiazoline-2-thiones 9a–f
Τhe appropriate primary aromatic amine, N-substituted aniline or 1-substituted piperazine (2 mmol) and 37% formaldehyde solution (0.5 mL) were added to a solution of 5-(2-thienyl)-1,3,4-oxadiazoline-2-thione (6, 0.37 g, 2 mmol) in ethanol (8 mL), and the mixture was stirred at room temperature for 2 h and allowed to stand overnight. The separated precipitate was filtered, washed with cold ethanol, dried, and crystallized.
7a: IR, υ (cm−1): 3336 (NH), 3034 (Ar-CH), 2898 (CH2), 1604, 1466 (C=N) 1258 (C-O-C). 1H NMR (CDCl3): δ 5.42 (s, 1H, NH), 5.64 (s, 2H, CH2), 6.80–7.50 (m, 5H, Ar-H & Thiophene-H), 7.53 (d, 1H, Thiophene-H, J = 5.0 Hz), 7.71 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 58.92 (CH2), 114.90, 116.21, 118.82, 123.43, 125.55, 127.23, 128.31, 130.80, 140.10, 154.95 (Ar-C & Thiophene-C), 156.90 (C=N), 177.30 (C=S). MS m/z (Rel. Int.): 307 (M+, 1), 207 (14), 183 (90), 105 (22), 57 (100).
7b: IR, υ (cm−1): 3342 (NH), 3052 (Ar-CH), 2912 (CH2), 1612, 1458 (C=N) 1266 (C-O-C). 1H NMR (CDCl3): δ 5.47 (d, 1H, NH, J = 8.5 HZ), 5.69 (s, 2H, CH2), 6.86–7.89 (m, 5H, Ar-H & Thiophene-H), 7.56 (d, 1H, Thiophene-H, J = 4.5 Hz), 7.69 (d, 1H, Thiophene-H, J = 4.5 Hz). 13C NMR: δ 58.92 (CH2), 115.49, 117.12, 123.43, 128.31, 130.80, 140.10, 155.24 (Ar-C & Thiophene-C), 158.74 (C=N), 176.25 (C=S). MS m/z (Rel. Int.): 307 (M+, 2), 207 (65), 183 (88), 105 (78), 57 (100).
7c: IR, υ (cm−1): 3349 (NH), 3040 (Ar-CH), 2891 (CH2), 1619, 1460 (C=N) 1265 (C-O-C). 1H NMR (CDCl3): δ 5.40 (s, 1H, NH), 5.65 (s, 2H, CH2), 6.65 (d, 2H, Ar-H, J = 8.5 Hz), 7.22 (d, 2H, Ar-H, J = 8.5 Hz), 7.43 (t, 1H, Thiophene-H, J = 5.0 Hz), 7.56 (d, 1H, Thiophene-H, J = 5.0 Hz), 7.69 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 58.92 (CH2), 115.49, 117.12, 123.43, 128.31, 129.95, 131.02, 134.23, 136.42 (Ar-C & Thiophene-C), 156.44 (C=N), 176.98 (C=S). MS m/z (Rel. Int.): 323 (M+, 1), 217 (25), 183 (85), 125 (88), 127 (23), 57 (100).
7d: IR, υ (cm−1): 3360 (NH), 3043 (Ar-CH), 2880 (CH2), 1615, 1451 (C=N) 1254 (C-O-C). 1H NMR (CDCl3): δ 4.45 (s, 1H, NH), 5.58 (s, 2H, CH2), 6.95–7.07 (m, 2H, Ar-H), 7.20–7.23 (m, 1H, Ar-H), 7.45–7.60 (m, 3H, Ar-H & Thiophene-H), 7.72 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 57.88 (CH2), 113.61, 121.38, 121.90, 122.16, 123.37, 125.63, 126.88, 128.39, 130.99, 131.23, 146.85 (Ar-C, CF3 & Thiophene-C), 156.02 (C=N), 176.04 (C=S). MS m/z (Rel. Int.): 357 (M+, 0.5), 183 (56), 174 (8), 145 (75), 57 (100).
7e: IR, υ (cm−1): 3322 (NH), 3065 (Ar-CH), 2887 (CH2), 1622, 1460 (C=N) 1249 (C-O-C). 1H NMR (CDCl3): δ 4.44 (s, 1H, NH), 5.62 (s, 2H, CH2), 6.87–7.0 (m, 2H, Ar-H), 7.17–7.52 (m, 4H, Ar-H & Thiophene-H), 7.68 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 57.54 (CH2), 108.55, 112.36, 120.24, 121.64, 122.17, 124.25, 126.82, 126.98, 128.0, 131.05, 132.25, 143.98 (Ar-C, CF3 & Thiophene-C), 157.08 (C=N), 177.20 (C=S). MS m/z (Rel. Int.): 357 (M+, 0.5), 183 (21), 174 (6), 145 (64), 57 (100).
8a: IR, υ (cm−1): 3322 (NH), 3021 (Ar-CH), 2910, 2852 (CH2, CH3), 1618, 1450 (C=N), 1371 (C=S), 1251 (C-O-C). 1H NMR (CDCl3): δ 3.28 (s, 3H, CH3), 5.64 (d, 2H, NCH2N), 6.83–7.36 (m, 6H, Ar-H & Thiophene-H), 7.55 (d, 1H, Thiophene-H, J = 5.0 Hz), 7.70 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 39.54 (CH3), 66.80 (CH2), 113.72m 119.12, 123.65, 128.30, 129.36, 130.36, 131.07, 146.69 (Ar-C & Thiophene-C), 155.89 (C=N), 176.32 (C=S). MS m/z (Rel. Int.): 303 (M+, 1), 282 (22), 193 (65), 97 (91), 57 (100).
8b: IR, υ (cm−1): 3325 (NH), 3012, 3027 (Ar-CH), 2913, 2905 (CH2), 1604, 1451 (C=N), 1370 (C=S), 1259 (C-O-C). 1H NMR (CDCl3): δ 4.99 (s, 2H, CH2Ph), 5.77 (s, 2H, NCH2N), 6.87–6.90 (m, 1H, Ar-H), 7.05–7.07 (m, 2H, Ar-H), 7.11–7.18 (m, 1H, Ar-H), 7.25–7.41 (m, 7H, Ar-H & Thiophene-H), 7.56 (d, 1H, Thiophene-H, J = 5.0 Hz), 7.69 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 55.05 (CH2Ph), 56.41 (NCH2N), 114.26, 119.43, 123.69, 126.67, 127.20, 128.28, 128.74, 129.39, 130.77, 131.0, 137.87, 146.44 (Ar-C & Thiophene-C), 155.93 (C=N), 176.31 (C=S). MS m/z (Rel. Int.): 397 (M+, 1), 341 (12), 282 (27), 159 (75), 92 (98), 57 (100).
9a: IR, υ (cm−1): 3330 (NH), 3004 (Ar-CH), 2918, 2810 (CH2, CH3), 1588, 1441 (C=N), 1381 (C=S), 1261 (C-O-C). 1H NMR (CDCl3): δ 2.33 (s, 3H, CH3), 2.56–2.62 (m, 4H, Piperazine-H), 2.96–3.02 (m, 4H, Piperazine-H), 5.19 (s, 2H, CH2), 7.36–7.44 (m, 2H, Thiophene-H), 7.71 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: δ 43.62 (CH3), 52.20, 54.42 (Piperazine-C), 67.71 (CH2), 126.42, 127.98, 128.36, 130.85 (Thiophene-C), 157.28 (CH=N), 176.44 (C=S). MS m/z (Rel. Int.): 296 (M+, 0.5), 183 (35), 113 (22), 98 (28, 57 (100).
9b: IR, υ (cm−1): 3333 (NH), 3023 (Ar-CH), 2921, 2816 (CH2), 1592, 1445 (C=N), 1380 (C=S), 1266 (C-O-C). 1H NMR (CDCl3): δ 3.0–3.3.08 (m, 4H, Piperazine-H), 3.18–3.26 (m, 4H, Piperazine-H), 5.14 (s, 2H, CH2), 6.87–6.96 (m, 3H, Ar-H), 7.18 (t, 1H, Thiophene-H, J = 4.3 Hz), 7.22–7.32 (m. 2H, Ar-H), 7.59 (d, 1H, Thiophene-H, J = 4.5 Hz), 7.75 (d, 1H, Thiophene-H, J = 4.5 Hz). 13C NMR: δ 49.37, 50.30 (Piperazine-C), 70.40 (CH2), 116.45, 120.06, 123.67, 128.33, 129.14, 130.77, 130.98, 151.26 (Ar-C & Thiophene-C), 155.47 (C=N), 177.76 (C=S). MS m/z (Rel. Int.): 358 (M+, 1), 341 (4), 183 (14), 77 (88), 57 (100).
9c: IR, υ (cm−1): 3336 (NH), 3023 (Ar-CH), 2909, 2875, 2798 (CH2), 1601, 1456 (C=N), 1378 (C=S), 1260 (C-O-C). 1H NMR (CDCl3): δ 3.02–3.10 (m, 4H, Piperazine-H), 3.18 (s, 4H, Piperazine-H), 5.24 (s, 2H, CH2), 6.96 (d, 2H, Ar-H, J = 7.5 Hz), 7.35–7.41 (m, 3H, Ar-H & Thiophene-H), 7.52 (d, 1H, Thiophene-H, J = 5.0 Hz), 7.70 (d, 1H, Thiophene-H, J = 5.0 Hz). 13C NMR: 50.12, 51.60 (Piperazine-C), 69.95 (CH2), 115.23, 117.23, 125.28, 127.43, 127.46, 129.37, 145.01, 149.606 (Ar-C), 157.0 (CH=N), 177.49 (C=S). MS m/z (Rel. Int.): 376 (M+, 2), 183 (34), 95 (87), 57 (100).
9d: IR, υ (cm−1): 3338 (NH), 3022 (Ar-CH), 2911, 2849 (CH2), 1589, 1438 (C=N), 1378 (C=S), 1267 (C-O-C). 1H NMR (CDCl3): δ 3.01–3.10 (m, 4H, Piperazine-H), 3.23–3.31 (m, 4H, Piperazine-H), 5.14 (s, 2H, CH2), 7.05–7.12 (m, 3H, Ar-H), 7.18–7.20 (m, 1H, Ar-H), 7.34 (t, 1H, Thiophene-H, J = 7.0 Hz), 7.60 (d, 1H, Thiophene-H, J = 7.0 Hz), 7.75 (d, 1H, Thiophene-H, J = 7.0 Hz). 13C NMR: δ 48.84, 50.11 (Piperazine-C), 70.29 (CH2), 112.52, 116.16, 119.05, 123.19, 123.26, 125.35, 128.34, 129.58, 130.80, 131.0, 151.35 (Ar-C, CF3 & Thiophene-C), 155.52 (C=N), 177.77 (C=S). MS m/z (Rel. Int.): 426 (M+, 1), 340 (43), 282 (32), 183 (6), 145 (22), 83 (11), 57 (100).
9e: IR, υ (cm−1): 3344 (NH), 3023 (Ar-CH), 2921, 2841 (CH2), 1601, 1454 (C=N), 1372 (C=S), 1258 (C-O-C). 1H NMR (CDCl3): δ 2.56 (s, 4H, Piperazine-H), 2.95 (s, 4H, Piperazine-H), 3.58 (s, 2H, CH2Ar), 5.12 (s, 2H, NCH2N), 7.13–7.44 (m, 6H, Ar-H & Thiophene-H), 7.52 (d, 1H, Thiophene-H, J = 7.0 Hz), 7.77 (d, 1H, Thiophene-H, J = 7.0 Hz). 13C NMR: δ 50.18, 52.90 (Piperazine-C), 63.02 (CH2Ar), 70.41 (NCH2N), 123.68, 127.16, 128.26, 128.33, 129.21, 130.72, 130.96, 137.84 (Ar-C, Thiophene-C), 155.36 (C=N), 177.69 (C=S). MS m/z (Rel. Int.): 372 (M+, 1), 281 (40), 183 (9), 92 (66), 77 (65), 57 (100).
9f: IR, υ (cm−1): 3341 (NH), 3014 (Ar-CH), 2922, 2781 (CH2), 1604, 1457 (C=N), 1372 (C=S), 1251 (C-O-C). 1H NMR (CDCl3): δ 2.54 (s, 4H, Piperazine-H), 2.88–2.96 (m, 4H, Piperazine-H), 3.67 (s, 2H, CH2Ar), 5.08 (s, 2H, NCH2N), 7.19–7.20 (m, 1H, Ar-H), 7.31–7.34 (m, 1H, Ar-H), 7.48 (t, 1H, Thiophene-H, J = 7.0 Hz), 7.60–7.63 (m, 2H, Ar-H & Thiophene-H), 7.75–7.77 (m, 2H, Ar-H & Thiophene-H). 13C NMR: δ 50.36, 53.04 (Piperazine-C), 58.07 (CH2Ar), 70.56 (NCH2N), 123.80, 125.66, 125.71, 126.74, 128.30, 128.48, 128.72, 130.29, 130.68, 130.86, 137.61 (Ar-C, CF3 & Thiophene-C), 155.40 (C=N), 177.78 (C=S). MS m/z (Rel. Int.): 440 (M+, 0.5), 357 (41), 281 (26), 183 (15), 159 (65), 83 (26), 57 (100).
4. Conclusions
In this study, new series of 1,2,4-triazoles and 1,3,4-oxadiazoles carrying 2-thienyl moieties as potential antimicrobial agents. The new derivatives were characterized by elemental analyses, IR, 1H NMR, 13C NMR, and mass spectral data. The new derivatives were tested for in vitro antimicrobial activities against a panel of Gram-positive bacteria (Staphylococcus aureus IFO 3060 and Bacillus subtilis IFO 3007), the Gram-negative bacteria (Escherichia coli IFO 3301 and Pseudomonas aeuroginosa IFO 3448) and the yeast-like pathogenic fungus Candida albicans IFO 0583. Compound 9a displayed marked broad spectrum antibacterial activity, while compounds 4d, 5e, 7b, 7c, 7d, 9b, 9c and 9d were highly active against the tested Gram-positive bacteria. None of the synthesized compounds were proved to be significantly active against Candida albicans. Though, the mechanism of the antibacterial activity needs further investigations, which are in progress.
Scheme 2.
Synthetic Pathway for Compounds 7a–e, 8a, b and 9a–f.
Acknowledgements
The financial support of the Research Center of the College of Pharmacy, King Saud University (Project # C.P.R.C. 237) and Drug Exploration & Development Chair (DEDC) is greatly appreciated. The author is greatly indebted to Elsayed E. Habib, department of Microbiology, University of Mansoura, Egypt, for performing the antimicrobial testing.
Footnotes
Sample Availability: Samples of the compounds are available from the author.
References
- 1.Demirbas A., Sahin D., Demirbas N., Karaoglu S.A. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur. J. Med. Chem. 2009;44:2896–2903. doi: 10.1016/j.ejmech.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Bayrak H., Demirbas A., Karaoglu A.S., Demirbas N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009;44:1057–1066. doi: 10.1016/j.ejmech.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Eswaran S., Adhikari A.V., Shetty N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009;44:4637–4647. doi: 10.1016/j.ejmech.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Bayrak H., Demirbas A., Demirbas N., Karaoglu S.A. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009;44:4362–4366. doi: 10.1016/j.ejmech.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Isloor A.M., Kalluraya B., Shetty P. Regioselective reaction: Synthesis, characterization and pharmacological studies of some new Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2009;44:3784–3787. doi: 10.1016/j.ejmech.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Navidpour L., Shafaroodi H., Abdi K., Amini M., Ghahremani M.H., Dehpour A.R., Shafiee A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg. Med. Chem. 2006;14:2507–2517. doi: 10.1016/j.bmc.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Al-Deeb O.A., Al-Omar M.A., El-Brollosy N.R., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic Acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]propionic acids and related derivatives. Arzneim.-Forsch./Drug Res. 2006;56:40–47. doi: 10.1055/s-0031-1296699. [DOI] [PubMed] [Google Scholar]
- 8.Tozkoparan B., Küpeli E., Yeşilada E., Ertan M. Preparation of 5-aryl-3-alkylthio-l,2,4-triazoles and corresponding sulfones with antiinflammatory–analgesic activity. Bioorg. Med. Chem. 2007;15:1808–1814. doi: 10.1016/j.bmc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Kumar H., Javed S.A., Khan S.A., Amir M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem. 2008;43:2688–2698. doi: 10.1016/j.ejmech.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 10.Navarrete-Vázquez G., Molina-Salinas G.M., Duarte-Fajardo Z.V., Vargas-Villarreal J., Estrada-Soto S., González-Salazar F. Synthesis and antimycobacterial activity of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines. Bioorg. Med. Chem. 2007;15:5502–5508. doi: 10.1016/j.bmc.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Padmavathi V., Reddy G.S., Padmaja A., Kondaiah P., Shazia A. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2009;44:2106–2112. doi: 10.1016/j.ejmech.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.El-Emam A.A., Al-Deeb O.A., Al-Omar M.A., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Kadi A.A., El-Brollosy N.R., Al-Deeb O.A., Habib E.E., Ibrahim T.M., El-Emam A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007;42:235–242. doi: 10.1016/j.ejmech.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Jayashankar B., Lokanath Rai K.M., Baskaran N., Sathish H.S. Synthesis and pharmacological evaluation of 1,3,4-oxadiazole bearing bis(heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009;44:3898–3902. doi: 10.1016/j.ejmech.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari S.V., Bothara K.G., Raut M.K., Patil A.A., Sarkate A.P., Mokale V.J. Design, Synthesis and Evaluation of Antiinflammatory, Analgesic and Ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg. Med. Chem. 2008;16:1822–1831. doi: 10.1016/j.bmc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Masunari A., Tavares L.C. A new class of nifuroxazide analogues: Synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2007;15:4229–4236. doi: 10.1016/j.bmc.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 17.Foroumadi A., Oboudiat M., Emami S., Karimollah A., Saghaee L., Moshafi M.H., Abbas Shafiee A. Synthesis and antibacterial activity of N-[2-[5-(methylthio)thiophen-2-yl]-2-oxoethyl] and N-[2-[5-(methylthio)thiophen-2-yl]-2-(oxyimino)ethyl]piperazinylquinolone derivatives. Bioorg. Med. Chem. 2006;14:3421–3427. doi: 10.1016/j.bmc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli R., Pier Giovanni Baraldi P.V., Carrion M.D., Cara C.L., Cruz-Lopez O., Preti D., Tolomeo M., Grimaudo S., Di Cristina A., Zonta N., Balzarini J., Brancale A., Sarkar T., Hamel E. Design, synthesis, and biological evaluation of thiophene analogues of chalcones. Bioorg. Med. Chem. 2008;16:5367–5376. doi: 10.1016/j.bmc.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Shiradkar M.R., Padhalingappa M.B., Bhetalabhotala S., Akula K.C., Tupe D.A., Pinninti R.R., Thummanagoti S. A novel approach to cyclin-dependent kinase 5/p25 inhibitors: A potential treatment for Alzheimer’s disease. Bioorg. Med. Chem. 2007;15:6397–6406. doi: 10.1016/j.bmc.2007.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Chabert J.F., Marquez B., Neville L., Joucla L., Broussous S., Bouhours P., David E., Pellet-Rostaing S., Marquet B., Moreau N., Lemaire M. Synthesis and evaluation of new arylbenzo[b]thiophene and diarylthiophene derivatives as inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Bioorg. Med. Chem. 2007;15:4482–4497. doi: 10.1016/j.bmc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Reid J.R., Heindel N.D. Improved syntheses of 5-substituted-4-amino-3-mercapto-(4H)-1,3,4-triazoles. J. Heterocycl. Chem. 1976;13:925–926. doi: 10.1002/jhet.5570130450. [DOI] [Google Scholar]
- 22.Molina P., Tàrraga A. A novel preparation of s-triazolo[3,4-b]-1,3,4-thiadiazole derivatives. Synthesis (Stuttgart) 1983:411–413. doi: 10.1055/s-1983-30360. [DOI] [Google Scholar]
- 23.Al-Abdullah E.S., Shehata I.A., Al-Deeb O.A., El-Emam A.A. Microwave-assisted dehydrosulphurization: An efficient, solvent-free synthesis of 5-(1-adamantyl)-2-arylamino-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Heterocycles. 2007;71:379–388. [Google Scholar]
- 24.Oussaid B., Moeini L., Garrigues B., Villemin D. Synthesis of thiophenyl oxadiazoles and oxazoles. Phosphorus Sulfur Silicon Relat. Elem. 1993;85:23–30. doi: 10.1080/10426509308038178. [DOI] [Google Scholar]
- 25.Turilli O., Gandino M. 2-Mercapto-5-heterocyclic-substituted-1,3,4-oxadiazoles. Ann. Chim. (Rome) 1963;53:1687–1696. [Google Scholar]
- 26.Yamada N., Kataoka Y., Nagami T., Hong S., Kawai S., Kuwano E. 5-Aryl-1,3,4-oxadiazole-5-thiols as new series of trans-cinnamate 4-hydroxylase inhibitors. J. Pesticide Sci. (Tokyo) 2004;29:205–208. doi: 10.1584/jpestics.29.205. [DOI] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards (NCCLS) Approved standard document M7A; Villanova, PA, USA: 1985. [Google Scholar]
- 28.Murray P.R., Baron E.J., Pfaller M.A., Tenover F.C., Yolken R.H. In: Manual of Clinical Microbiology. Wood G.L., Washington J.A., editors. American Society for Microbiology; Washington, DC, USA: 1995. [Google Scholar]