Abstract
A new D3h symmetric triptycene derivative has been synthesized with the aim of obtaining molecules that are able to assemble into porous structures, and can be used in the development of new ligands. The synthesis involves a Diels-Alder reaction as the key step, followed by an oxidation and the formation of a maleimide ring. Triptycene trimaleimide furnished single crystals which have been analyzed by means of X-ray diffraction.
Keywords: tripodal ligand, cycloaddition, symmetry, triptycene
Introduction
The advantages offered by high-symmetry molecules are widely recognized in catalysis and can be readily applied in several other fields (e.g., material science, liquid crystal technology and nanoscience). In recent years we have been involved in the synthesis and application of C3, [1,2] C3v [3,4,5,6,7] and D3 [8] symmetric molecules. More recently, we decided to extend our attention to D3h molecules and in particular to the functionalization of triptycenes. Triptycenes are members of an interesting class of compounds which have a high level of symmetry associated with a very rigid framework. Owing to these properties, they have been used extensively as molecular scaffolds in supramolecular chemistry, as well as in materials science. [9,10,11]
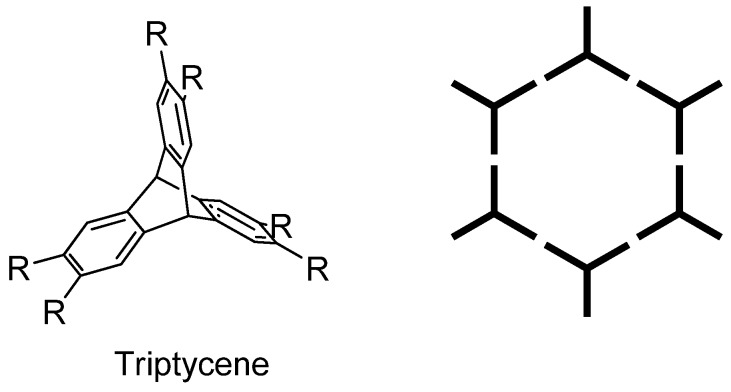
Indeed, by using the strategy of molecular tectonics, a hypothetical molecule possessing D3h symmetry and linking sites at the extremities might lead to a porous structure via the formation of a hexagonal network (Figure 1). [12] A tecton based on a triptycene quinoxaline ligand has been used for the formation of pores. [13] In this case, copper atoms bridge the quinoxaline units to give a honeycomb structure in the crystalline state.
Figure 1.
Triptycene (R=H) and binding motifs for pore formation.
Results and Discussion
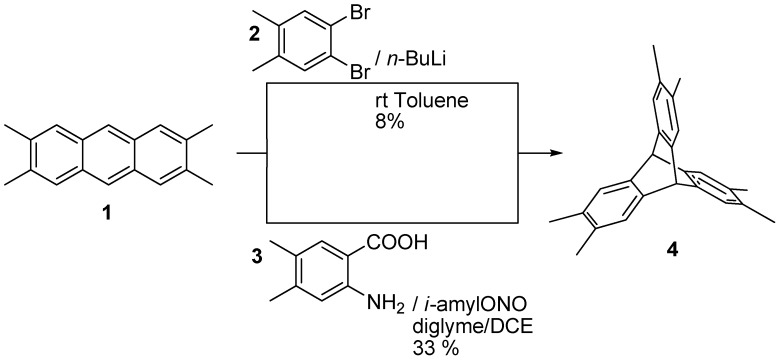
We chose to synthesize a related triptycene-based tecton with D3h symmetry in the hope that it would also assemble in the solid state into a honeycomb structure with large pores. Instead of using metal atoms to link the organic moieties, the plan was to utilize non-covalent intermolecular interactions, viz. hydrogen bonds, between suitably positioned donor and acceptor groups to directionally control the aggregation of the molecules. The binding functionality was the well known maleimide motif, which has been used extensively as a recognition motif for the formation of supramolecular aggregates. [14,15] The synthetic strategy was based on the preparation of the D3h-hexamethyl triptycene followed by oxidation to hexacarboxylic acid and cyclisation to the maleimide.The intermediate hexaacid is itself an interesting platform for future developments, such as dendrimer chemistry, or as a core for liquid crystals. Of the two common approaches for the synthesis of functionalized triptycenes – i.e. addition of functional groups to unfunctionalized triptycene and Diels-Alder cycloaddition of benzyne to anthracene, both of which contain the desired functionalities - we followed the second procedure by starting from tetramethylanthracene synthesized according to a literature procedure. [16] Two different precursors of the benzyne derivative were studied (Scheme 1). The first comes from the addition of n-butyllithium to 1,2-dibromo-4,5-dimethylbenzene, while the second is the 4,5-dimethyl equivalent of anthranilic acid. 1,2-dibromo-4,5-dimethylbenzene can be synthesized readily from xylene, while the anthranilic derivative requires a longer synthesis starting from 3,4-dimethylaniline. [17] The slow addition of n-butyllithium to a solution of 2 and 1 furnished the desired product in low yield. Attempts to optimize the yield did not give significant improvements. The generation of the benzyne via aprotic diazotization of the corresponding anthranilic derivative produced the expected product in fair yields. [18,19] The reaction was performed under standard conditions and the product was obtained after flash chromatography (silica gel hexane/dichloromethane 95:5) and crystallization from dichloromethane/toluene.
Scheme 1.
Synthesis of hexamethyl compound 4.
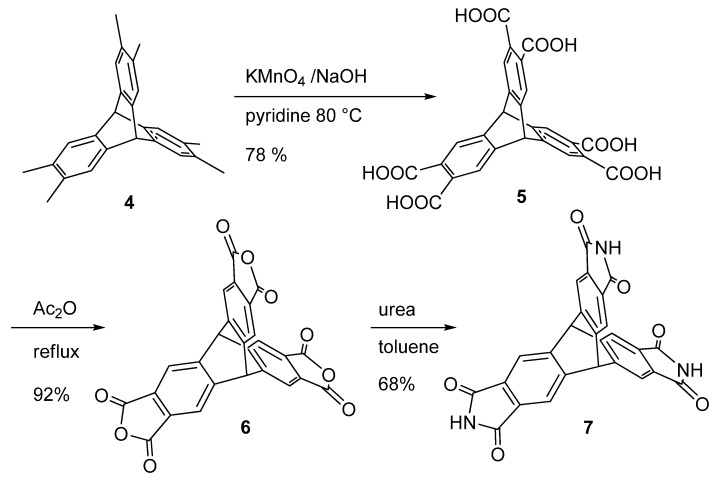
Several conditions for the oxidation of compound 4 were tried. Indeed, mild conditions using only potassium permanganate resulted in the formation of partially oxidized products, whereas strongly basic potassium permanganate results in the production of the desired product, but at the same time, over-oxidation products, such as 1,2,4,5-benzenetetracarboxylic acid, are generated. The best conditions were obtained by using the oxidizing agent in pyridine in the presence of a water solution of NaOH (Scheme 2). This afforded the desired hexacarboxylic acid in 78% yield. A similar procedure was reported in the literature during this work. [20,21].
Scheme 2.
Synthesis of trimaleimido derivative 7.
The synthesis of the trimaleimido derivative 7 followed a procedure reported in the literature for phthalic acids. [22] The acid 5 was converted to the corresponding anhydride by refluxing in acetic anhydride. The resulting compound was then treated with urea in boiling xylene to afford the tris-maleimide 7. The formation of the product was confirmed by NMR and ESI-MS analysis.
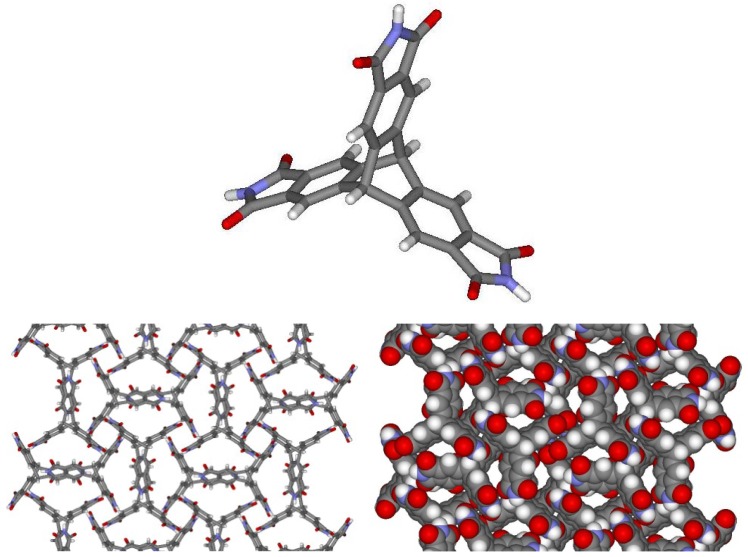
The compound proved difficult to purify, this being achieved finally by filtration followed by recrystallisation from an acetone-DMF mixture. The resulting crystals were analyzed by means of X-ray diffraction which revealed that compound 7 had crystallized as a 1:1 solvate with disordered DMF. The crystals were merohedrally twinned via a 2-fold rotation about (1 1 0). In the crystal, the molecules of 7 are not aligned in the expected honeycomb structure with open pores. Instead, a more compact arrangement has ensued (Figure 2). Although all of the maleimide groups are involved in intermolecular hydrogen bonds, the sought recognition pattern was not produced. Nonetheless, a sufficiently open packing of the molecules of 7 permits the inclusion of DMF molecules in the lattice. For each molecule of 7, one N-H group forms a hydrogen bond with the O-atom of the DMF molecule. The second N-H group forms a bifurcated hydrogen bond with the O-atom of the DMF molecule and with an O-atom of an adjacent trimaleimido molecule. The third N-H group forms a hydrogen bond with a different O-atom of another adjacent trimaleimido molecule. The combination of all of the interactions links the trimaleimido and DMF molecules into an extended three-dimensional framework. In addition, two independent C-H…O hydrogen bonds are observed between some maleimide carbonyl groups and H-atoms of aromatic rings. So far, attempts to obtain polymorphs with different packing arrangements by using alternative solvents have not been successful.
Figure 2.
Crystal structure and crystal packing of molecule 7.
Experimental
General
Solvents were purchased from Sigma-Aldrich and used without further purification. 1H- and 13C-NMR spectra were recorded using a Bruker Ultrashield spectrometer operating at 300 MHz (1H) and 75.47 MHz (13C). ESi-MS experiments were performed on a Agilent ESI-IonTrap instrument by direct flow injection using methanol as mobile phase. Compound 5 was synthesised according to [21].
Synthesis of triptycene-2,3,6,7,14,15-hexacarboxytriimide (7)
Hexacid 5 (518 mg, 1 mmol) was added to acetic anhydride (20 mL) and the solution refluxed (165 °C) for 24 h. The acetic anhydride was removed in vacuo to afford the crude phthalic anhydride 6 (445 mg, yield 96%), which was dissolved in xylenes (25 mL), urea (480 mg, 8 mmol) was added and the mixture stirred at reflux for 20 hours. The residual solvent was removed in vacuo and the resulting solid was washed with water to remove unreacted urea. Filtration, followed by recrystallisation from an acetone-DMF mixture (3:1) furnished 278 mg (yield 58%) of brown crystals; m.p. > 250 °C (dec.); ESI-MS: m/z 462 (M+H+); 1H-NMR (DMSO): δ = 13.1 (bs, 3 H), 7.80 ( s, 6 H), 6.14 (s, 3 H) ppm; 13C-NMR (DMSO): δ = 169,0, 147.1, 131.5, 125.1, 35.0 ppm; Anal. Calcd for C26H11N3O6: C, 67.68; H, 2.40; N, 9.11. Found: C, 66.85; H, 2.82; N, 9.11.
X-ray crystallography
Crystals suitable for X-ray structure determination were obtained by slow diffusion of acetone into a DMF solution of 7. Crystal data for 7: C26H11N3O6·C3H7NO, M = 534.48, space group: I (tetragonal), a = 20.4247(3) Å, c = 12.0229(3) Å, V = 5015.6(2) Å3, Z = 8, F(000) = 2208, μ(Mo Kα) = 0.104 mm-1Dx = 1.416 g cm-3, 2θ(max) = 60º, T = 160 K, Nonius KappaCCD diffractometer, 39549 measured reflections, 3832 independent reflections, 3144 reflections with I > 2σ(I), refinement on F2 with SHELXL97, 395 parameters, 74 restraints, R(F) [I > 2σ(I) reflections] = 0.0510, wR(F2) [all data] = 0.1138, goodness of fit = 1.040, Δρmax = 0.30 e Å-3. The crystal was merohedrally twinned by a 2-fold rotation about (1 1 0); major twin fraction 0.530(2). The DMF molecule is disordered over two orientations with the major orientation present in 63(1)% of the molecules. CCDC-647558 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Conclusions
In summary, we have investigated the possibility of building a honeycomb-structured porous crystal by using triptycene as the basic structural component. Along the way, other molecules with other potential applications have been synthesized.
Acknowledgements
This work was co-founded by MURST within the PRIN national framework.
Footnotes
Sample Availability: Not available.
References
- 1.Fabris F., Pellizzaro L., Zonta C., De Lucchi O. A novel C3-symmetric triol as chiral receptor for ammonium ions. Eur. J. Org. Chem. 2007:283–291. [Google Scholar]
- 2.Longhi G., Fabris F., Zonta C., Fornili S.L. Molecular dynamics simulation of small water-binding cavitands. Chem. Phys. Lett. 2006;423:312–316. doi: 10.1016/j.cplett.2006.03.093. [DOI] [Google Scholar]
- 3.Zonta C., Cossu S., De Lucchi O. Synthesis of benzotri(benzonorbornadienes) (BTBNDs): Rigid, cup-shaped molecules with high electron density within the cavity. Eur. J. Org. Chem. 2000:1965–1971. [Google Scholar]
- 4.Paulon A., Cossu S., De Lucchi O., Zonta C. Palladium-catalysed cyclotrimerisation reactions of polycyclic alkenes under the Stille and Grigg coupling conditions. Chem. Commun. 2000:1837–1838. [Google Scholar]
- 5.Cossu S., De Lucchi O., Paulon A., Peluso P., Zonta C. anti-Selective Heck-type cyclotrimerization of polycyclic bromoalkenes. Tetrhaedron Lett. 2001;42:3515. doi: 10.1016/S0040-4039(01)00495-6. [DOI] [Google Scholar]
- 6.Borsato G., De Lucchi O., Fabris F., Groppo L., Lucchini V., Zambon A. Efficient cyclotrimerization of bicyclic vic-bromostannylalkenes promoted by copper(I) thiophen-2-carboxylate. J. Org. Chem. 2002;67:7894. doi: 10.1021/jo020396s. [DOI] [PubMed] [Google Scholar]
- 7.Zonta C., Fabris F., De Lucchi O. The pyrrole approach toward the synthesis of fully functionalized cup-shaped molecules. Org. Lett. 2005;6:1003–1006. doi: 10.1021/ol047569f. [DOI] [PubMed] [Google Scholar]
- 8.Borsato G., Crisma M., De Lucchi O., Lucchini V. "Hexacarboxytrindanes": Benzene rings with homotopic faces as scaffolds for the construction of D-3 chiral architectures. Angew. Chem. Int. Ed. 2005;44:7435. doi: 10.1002/anie.200502262. [DOI] [PubMed] [Google Scholar]
- 9.Long T.M., Swager T.M. Using "internal free volume" to increase chromophore alignment. J. Am. Chem. Soc. 2002;124:3826–3827. doi: 10.1021/ja017499x. [DOI] [PubMed] [Google Scholar]
- 10.Swager T.M. Iptycenes in the design of high performance polymers. Acc. Chem. Res. 2008;41:1181–1189. doi: 10.1021/ar800107v. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C., Chen C.F. Synthesis and structure of 2,6,14-and 2,7,14-trisubstituted triptycene derivatives. J. Org. Chem. 2006;71:6626–6629. doi: 10.1021/jo061067t. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw D., Claridge J.B., Cussen E.J., Prior T.J., Rosseinsky J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 2005;38:273–282. doi: 10.1021/ar0401606. [DOI] [PubMed] [Google Scholar]
- 13.Chong J.H., MacLachlan M.J. Robust non-interpenetrating coordination frameworks from new shape-persistent building blocks. Inorg. Chem. 2006;45:1442–1444. doi: 10.1021/ic052123w. [DOI] [PubMed] [Google Scholar]
- 14.Theobald J.A., Oxtoby N.S., Phillips M.A., Champness N.R., Beton P.H. Controlling molecular deposition and layer structure with supramolecular surface assemblies. Nature. 2003;424:1029–1031. doi: 10.1038/nature01915. [DOI] [PubMed] [Google Scholar]
- 15.Heinz T., Rudkevich D.M., Rebek J. Pairwise selection of guests in a cylindrical molecular capsule of nanometre dimensions. Nature. 1998;394:764–766. doi: 10.1038/29501. [DOI] [Google Scholar]
- 16.Barnett E.B., Goodway N.F., Watson J.W. Anthracene derivatives. X. Chem. Ber. 1933;66:1876–1886. [Google Scholar]
- 17.Baker B.R., Schaub R.E., Joseph J.P., McEvoy F.J., Williams J.H. An antimalarial alkaloid from hydrangea. XV. Synthesis of 5-, 6-, 7-, and 8-derivatives with two identical substituents. J. Org. Chem. 1952;17:149–156. doi: 10.1021/jo01135a015. [DOI] [Google Scholar]
- 18.Friedman L., Logullo F.M. Arynes via aprotic diazotization of anthranilic acids. J. Org. Chem. 1969;34:3089–3092. doi: 10.1021/jo01262a065. [DOI] [Google Scholar]
- 19.Guenzi A., Johnson C.A., Cozzi F., Mislow K. Dynamic gearing and residual stereoisomerism in labeled bis(9-triptycyl)methane and related molecules. Synthesis and stereochemistry of bis(2,3-dimethyl-9-triptycyl)methane, bis(2,3-dimethyl-9-triptycyl)carbinol, and bis(1,4-dimethyl-9-triptycyl)methane. J. Am. Chem. Soc. 1983;6:1438–1448. [Google Scholar]
- 20.Rybáčková M., Bělohradský M., Holý P., Pohl R., Závada J. Versatile synthesis of triptycene di- and tetracarboxylic acids. Synthesis. 2006:2039–2042. [Google Scholar]
- 21.Rybáčková M., Bělohradský M., Holý P., Pohl R., Závada J. Synthesis of highly symmetrical triptycene tetra- and hexacarboxylates. Synthesis. 2007:1554–1558. [Google Scholar]
- 22.Mazzocchi P.H., Wilson P., Khacilk W.F., Klingler L., Minamikawa S. Photochemical additions of alkenes to phthalimides. Mechanistic investigations on the stereochemistry of alkene additions and the effect of aryl substituents on the regiochemistry of alkene additions. J. Org. Chem. 1983;48:2981–2989. doi: 10.1021/jo00166a009. [DOI] [Google Scholar]