Abstract
Background
Secondary lymphedema (SL) is a possible side effect of breast cancer treatment. Current data describe a positive influence of exercise on upper lymphedema. This systematic review evaluates studies examining a potential preventive effect of exercise on SL incidence.
Methods
A systematic literature search of PubMed, LIVIVO, and the Cochrane Library was performed.
Results
8 randomized controlled trials were included in the review. 3 studies investigated the effect of strength training, and 5 the effect of combined exercise therapy. 5 studies included participants without lymphedema at study entry, and 3 studies included both patients with and without lymphedema. The partly significant results showed that progressive strength training as well as combined dynamic exercise therapies consisting of physiotherapy, physical therapy, and/or kinesiotherapy are safe and can prevent SL. Onset as well as diagnosis of lymphedema were significantly decreased in 5 studies in the exercise group.
Conclusion
All 8 studies included indicate a potential preventive effect of exercise on SL; however, further research is needed.
Keywords: Breast cancer, Lymphedema, Exercise, Prevention, Prophylaxis
Introduction
Over 1.8 million women worldwide are diagnosed with breast cancer every year [1]. Due to improved medical treatment options and early screening and detection, breast cancer survival rates have increased significantly in the last years, and more than 80% of patients can be cured [1]. Given the improved survival rates, dealing with treatment-associated side effects such as secondary lymphedema (SL) is a new challenge in cancer care [2]. SL is likely to occur after the surgical removal of lymph nodes or in conjunction with radiotherapy [3]. SL is generally described as ‘arm swelling and dysfunction’ [4] and is defined as an increase in arm circumference by more than 2 cm [5] or as an accumulation of excessive protein-rich liquid in a part of the body where lymphatic vessels have been damaged [6]. About 20–30% of all breast cancer patients develop SL [7].
The American College of Sports Medicine (ACSM) roundtable on exercise guidelines for cancer survivors describes that exercise during and after cancer treatment is safe and can help patients improve their physical capacity and quality of life [8]. The ACSM guidelines indicate specific exercise programs oriented towards impairments associated with disease and medical treatment [8]. Strength exercise does not have any negative effects on an existing SL [9]; instead, it has beneficial effects such as improvement of strength [2] and lower exacerbation rates [10].
However, these recommendations do not include any information about the prevention of SL in breast cancer patients. According to our knowledge, no systematic review on solely the topic of prevention has been published so far. Therefore, we conducted a systematic review to analyze possible preventive effects of exercise on the incidence of SL in breast cancer patients.
Methods
In order to analyze the possible preventive effect of exercise on SL in breast cancer patients, a comprehensive literature search was performed. 3 reviewers independently searched the available literature in the PubMed, LIVIVO, and Cochrane Library databases in order to identify randomized controlled exercise intervention studies with breast cancer patients focusing on the prevention of SL. The literature search was completed in June 2016. The keywords ‘breast cancer’, ‘lymphedema’, ‘prevention’, ‘exercise’, ‘physical activity’, ‘physical fitness’, ‘physical exercise’, ‘sport endurance’, ‘resistance training’, ‘strength training’, ‘weight training’, ‘physiotherapy’, ‘physical therapy’, ‘kinesiotherapy’, ‘movement, ‘aerobic’, and ‘sport’ were used. Only randomized and controlled studies with more than 20 subjects were included. Parameters such as inclusion and exclusion criteria were defined and are listed in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| • Randomized controlled trials |
| • Female sex |
| • Studies including only subjects without secondary lymphedema at study entry |
| • Mixed studies including subjects with and without secondary lymphedema at study entry |
| • Surgical removal of at least 1 lymph node |
| • Exercise including manual lymphatic drainage |
| • Studies were included from 2006 to 2016 (June) |
| • Studies including at least 20 subjects |
| • Studies in English and German language |
| Exclusion criteria |
| • Male sex |
| • Studies including only subjects with secondary lymphedema at study entry |
| • No surgical removal of any lymph nodes |
| • Reviews |
| • Case reports |
| • Expert opinions |
| • Animal studies |
| • Studies combining exercise and nutrition |
Results
A total of 8 randomized controlled intervention studies investigating the preventive effects of exercise on the development of SL in breast cancer patients were identified (tables 2, 3, fig. 1). The studies by Schmitz et al. [11], Sagen et al. [12], Torres Lacomba et al. [5], De Rezende et al. [13], and Zimmermann et al. [14] enrolled only subjects without lymphedema at study entry. The studies by Ahmed et al. [15], Devoogdt et al. [16], and Zhang et al. [17] included both subjects with and without lymphedema at study entry. In total, 1,810 participants were investigated, and 1,780 of these were included in the post-analysis. 5 studies provided details on age; participants were on average 54 years old. 4 studies reported that their subjects received radiotherapy, chemotherapy, or hormone therapy during the intervention period.
Table 2.
Studies considering the preventive effect of exercise on secondary lymphedema
| Authors, year [ref.] | Subjects | Design and measuring point | Intervention | Outcome |
|---|---|---|---|---|
| Schmitz et al., 2010 [11] | - n = 154 (134) – age 36–75 years – included: 1–5 years post unilateral non-metastatic BC with radiation and/or chemotherapy; currently cancer-free, no medical conditions that limit participation; surgery: ALND: ≥2 lymph nodes (range: 2–26; IG: Ø 8; CG: Ø 9); ≥5: 94 women |
- randomized controlled equivalence trial – at baseline and 12 months |
IG: 1 year progressive weight lifting – first 13 weeks supervised instruction, followed by 9 months unsupervised exercise: 2/week for 90 min; 3 sets of each exercise, 10 repetitions/set – 10 min cardiovascular warm-up; range of motion stretching of major muscle groups; – 5–15 min resistance training to stabilize spinal and deep abdominal muscles: 9 strength training exercises with resistance machines and free weights (chest, back, shoulders, quadriceps, hamstrings, gluteal, biceps, triceps); end of session: stretching (held 30 s). – upper body exercises: seated row, supine dumbbell press, lateral or front raises, bicep curls, triceps pushdowns; dumbbells/variable resistance machines: start: no weight or 1 pound, next week: 0.5–1 pound increase, 1 set of 0.5 pound wrist weights and 2 pairs of dumbbell in 1 pound increments (up to 10 pounds) – lower body exercises: leg press and leg curl, back and leg extension; progressive, lift maximum weight that can be lifted in each exercise 8–10 times/set, up to 3 sets/exercise over first 3–4 weeks – increasing weight (without symptoms) CG: no exercise |
- incident lymphedema onset (≥5% increase in inter-limb volume difference): IG 11%; CG 17% (p = 0.003) – ≥ 5 lymph nodes removed: incident lymphedema onset: IG 7%; CG 22% (p = 0.001) – clinical defined lymphedema onset: IG: 1 woman (1.5%); CG: 3 women (4.4%); (p = 0.12) – IG became stronger and had lower percentage of body fat than CG |
| Sagen et al., 2009 [12] | - n = 204 – Ø age 55 ± 10 years (32–75 years) – included: early-stage BC surgery: mastectomy or breast-conserving with ALND; with or without radiotherapy, chemotherapy, hormone treatment |
- prospective randomized controlled trial – at baseline, 3, 6, and 24 months after surgery |
IG (n = 104): 6 months no activity restrictions (NAR) in daily living and moderate resistance exercise program: – progressive resistance training, 2–3/week with trainer for 45 min (first 2 weeks: 0.5 kg weights, 15 repetitions/set; individual weight increase) CG (n = 100): 6 months activity restrictions (AR) program with usual care program: – avoid heavy strenuous activities; no lifting or carrying >3 kg – low-dose physical therapy program (6 techniques of passive manual stretching emphasizing flexibility and light massage to the affected shoulder, arm, scar; 1/week for 45 min) |
- no significant difference between IG and CG in arm volume, difference in volume between affected arm and control arm, or lymphedema at 3, 6, or 24 months – lymphedema development from baseline to 2 years increased significantly in both groups (p < 0.001) – lymphedema: IG 5%; CG 7% at 3 months to 13% at 2 years for both groups – arm volume and arm lymphedema increased with time in both groups (p = 0.05) – home physical exercise rate significantly higher at 3 and 6 months in IG (p < 0.001) but did not differ at 2 years |
| Torres Lacomba et al., 2010 [5] | - n = 120 (116) – included: unilateral surgery with ALND, and adjuvant chemotherapy |
- randomized single-blinded clinical trial – 4 weeks (shortly after completion of intervention), 3, 6, 12 months after surgery |
IG (n = 60) (59): physiotherapy program (3 weeks; 3/week): – MLD technique used for treatment of postoperative edema (modification of strokes described by Leduc) – progressive massage of scar tissue (progressing from Jacquet and Leroy pincer to Wetterwald pincer) – stretching exercises for levator scapulae, upper trapezius, pectoralis major, medial and lateral rotators muscles of the shoulder – progressive active and action-assisted shoulder exercises – educational therapy (printed information about lymphatic system; concepts of normal load vs. overload; secondary lymphedema: source and preventive interventions; possible precipitating factors) CG (n = 60) (57): educational therapy only |
- statistically significant difference in diagnosis of lymphedema (>2 cm increase in arm circumference): CG 14 (25%); IG 4 (7%) (p = 0.010) - 1-year follow-up volume ratio between affected arms increased by 5.1% in CG and 1.6% in IG (p = 0.0065) - lymphedema diagnosed 4 times faster in CG (p = 0.010) - better survival rate in IG than in CG |
| De Rezende et al., 2006 [13] | - n = 60 – Ø age 54 years – included: first surgery for invasive BC, modified radical mastectomy or quadrant-ectomy with ALND; (neo-)adjuvant chemotherapy; 3 exercises started 1 day, others 48 h after surgery |
- prospective randomized controlled clinical trial |
IG: kinesiotherapy: – flexion, extension, abduction, adduction, internal and external rotation; 19 exercises: 10 repetitions (60-s interval between exercises) CG: biomechanical physiologic movements of the shoulder: – flexion, extension, abduction, adduction, internal and external rotation without defined number of repetitions or sequence; duration: 40 min, 3/week for 42 days |
- significantly better shoulder mobility in IG – no significant difference in lymphedema or arm circumference between IG and CG – statistically significant increase in circumference 7.5 cm above humeroradial joint in CG (p = 0.0332) |
| Zimmermann et al., 2012 [14] | - n = 67 – age 34–81 years (Ø IG: 60.3 years, Ø CG: 58.6 years) – breast-conserving therapy (n = 40) or modified mastectomy (n = 27) – SLND (n = 32) (Ø 2) – ALND (n = 35) (Ø 17) – adjuvant therapies, radiation, chemotherapy, or endocrine therapy |
- randomized controlled study – before surgery, at 2, 7, 14 days, and at 3, 6 months |
for all subjects from day 2: standard physiotherapy program (exercises of limb and chest physical therapy) IG (n = 33): MLD: 5/week during first 2 weeks; 2/week from day 14 to 6 months after surgery CG (n = 34): self-applied drainage |
- CG: mean values of arm volume measurements on operated side increased continually from 2nd day of surgery – IG: mean values increased on day 2 after surgery and started to resolve by day 7 – 6 months after surgery: CG: significant increase in arm volume on operated side (p = 0.0033); IG: no statistically significant increase in volume of upper limb on operated side – intergroup differences in mean values of volume of lymphedema: noticeable from day 7 after surgery and still evident at study end – 3 months after surgery: CG: 6% volume increase, up to 10% at 6 months; IG: ULL on operated side did not occur |
IG = Intervention group; CG = control group; BC = breast cancer; ALND = axillary lymph nodes dissection; SLND = sentinel lymph node dissection; MLD = manual lymph drainage; ULL = upper limb lymphedema; Ø = average.
Table 3.
Studies considering the preventive and rehabilitative effect of exercise on secondary lymphedema
| Authors, year [ref.] | Subjects | Design and measuring point | Intervention | Outcome |
|---|---|---|---|---|
| Ahmed et al., 2006 [15] | - n = 45 – Ø age 52 years – included: 4–36 months post-treatment; surgery: ALND; 13 women prevalent lymphedema at baseline |
- randomized controlled trial – at baseline and 6 months |
IG (n = 23): 6 months WTBS: warm-up; weight training; cool down – 2/week for 60 min with ACSM fitness coach in groups of 4 for 3 months, months 3–6 in pairs without trainer – 9 exercises (resistance machines and free weights) – upper body: start with no weight or half-pound wrist weights – lower body: 8–10/set; increment to 3 sets of each exercise over first 2–3 weeks – stretching exercises: increase ROM CG (n = 22): usual care |
- no variation in incidence of clinical diagnosis of lymphedema or onset of lymphedema symptoms in IG (p = 0.40) or CG (p = 0.22) – IG: no change in arm circumferences ≥2 cm after 6-month intervention – from baseline to 6 months: 3 women in CG, no woman in IG had increment in lymphedema symptoms (p = 0.22) – at 6 months: 2 women in IG, 1 woman in CG self-reported onset of lymphedema from baseline (p = 0.40) |
| Devoogdt et al., 2011 [16] | - n = 160 (154); IG: 4; CG: 2 with prevalent lymphedema – included: unilateral surgery with ALND; (neo-)adjuvant chemotherapy and/or radiation; guidelines and exercise therapy as soon as possible after surgery, MLD 5 weeks after surgery |
- randomized single blinded controlled trial – at 1, 3, 6, 12 months after axillary surgery |
both groups: 6-month exercise program: – guidelines about prevention of lymphedema – exercise therapy (mobilization of shoulder, stretching breast muscles, scar tissue massage, exercise schemes), 30-min sessions, start 2/week, 1/week, once/2 weeks IG (n = 79) (75): MLD: – duration: 20 weeks; 40 sessions for 30 min with increasing and decreasing frequency (1/week to 3/week to 1/week) CG (n = 81) (79): same program without MLD |
- incidence rate for arm lymphedema in IG and CG: 12 months (p = 0.45) and 3/6 months after surgery: comparable – at 3, 6, and 12 months after surgery: incidence rate and time to developing arm lymphedema: comparable – time to developing arm lymphedema (≥200 ml) during first year after surgery: comparable (p = 0.44) – follow-ups: both groups similar increase in arm volume compared with to before surgery – MLD in addition to guidelines and exercise therapy has no medium to large effect in reducing the incidence of arm lymphedema |
| Zhang et al., 2016 [17] | - n = 1,000 – modified radical mastectomy |
- randomized controlled study – ULL assessed 24 h before surgery and at 1 week, 1, 3, 6, 12 months after surgery – self-MLD beginning 10–30 days after surgery |
both groups: 24 h before surgery: education training repeated after patient recovered from anesthesia, daily thereafter for 3 days, and on day of discharge (20–30 min/session) IG (n = 500): physical exercise and self-MLD: – starting with passive exercise within first 7 days after surgery – 7–30 days: exercises progressed to localized active exercise on affected upper limb – 3 sessions/day, 15 min/session – all patients continued remedial exercise for 6 months after surgery – self-MLD on surgical incision for 3 sessions/day for 30 min CG (n=500): physical exercise: same as in CG but no self-MLD |
- IG: significant improvements in scar contracture, shoulder abduction, upper limb circumference – self-MLD and physical exercise beneficial in preventing post-mastectomy scar formation, ULL, shoulder joint dysfunction – 1 month after surgery: no patients in IG versus 5 in CG had developed mild lymphedema – in CG: number of patients with lymphedema was 23, 25, 39 at 3, 6, 12 months post-surgery – in IG: lymphedema observed in 6, 9, 8 patients – MLD combined with physical exercise significantly reduces ULL after surgery, relative to physical exercise alone (p < 0.05 for 1–12 months) |
IG = Intervention group; CG = control group; BC = breast cancer; ALND = axillary lymph node dissection; SLND = sentinel lymph node dissection; MLD = manual lymph drainage; ULL = upper limb lymphedema; WTBS = weight training for breast cancer survivors; ACSM = American College of Sports Medicine; ROM = range of motion; Ø = average.
Fig. 1.
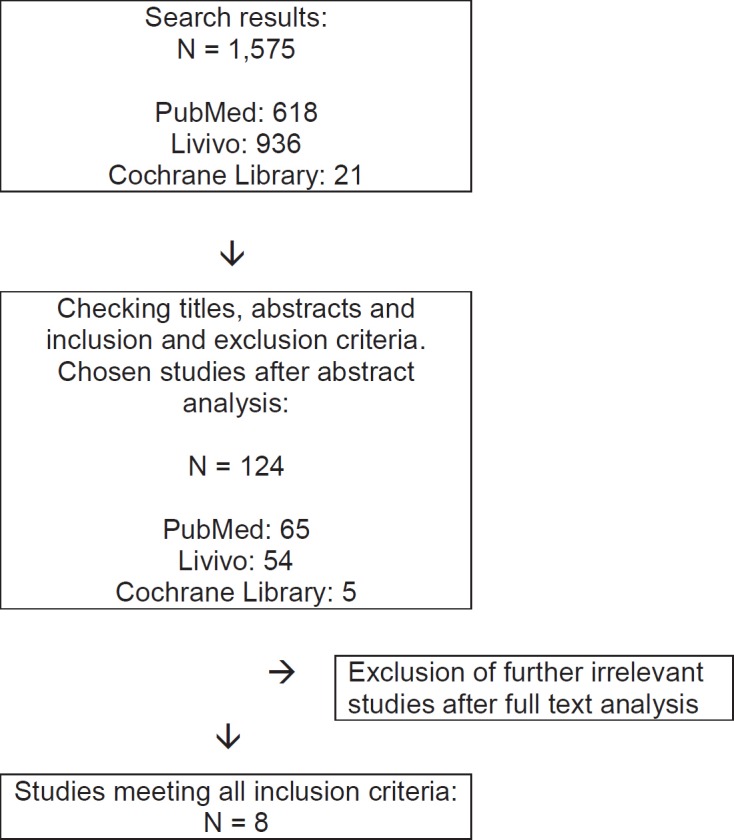
Selection of studies for systematic review.
The exercise intervention comprised either resistance training (n = 3) [11, 12, 15] or a combined exercise therapy (n = 5) consisting of physiotherapy, physical therapy, manual lymphatic drainage (MLD), stretching, massage, and/or kinesiotherapy [5, 13, 14, 16, 17]. Resistance training started between 4 months and 5 years after the end of primary treatment. 2 studies started combined exercise therapy 24–48 h after surgery. Intervention programs lasted from a minimum of 3 weeks to a maximum of 12 months. MLD in addition to exercise was administered in 4 studies [5, 14, 16, 17].
Preventive effects of exercise in relation to SL in breast cancer were observed. Significant effects could be identified in 5 studies [5, 11, 13, 14, 17]. 3 studies recognized significant effects of exercise in combination with MLD [5, 14, 17]. Schmitz et al. [11] reported only a minor incidence of SL in the intervention group undergoing a 1-year (twice weekly) progressive weightlifting intervention compared to the control group. This difference was even bigger in women who had at least 5 lymph nodes removed. The study conducted by Torres Lacomba et al. [5] observed a significantly lower incidence of lymphedema in the intervention group receiving physiotherapy (MLD, massage, stretching, progressive exercise, and educational therapy) compared to the control group receiving only educational material. Moreover, in the control group, onset of lymphedema was diagnosed 4 times earlier compared to the exercise group [5]. In the study by De Rezende et al. [13], the intervention group showed statistically significant better recovery of flexion, abduction, and external movements in the shoulder compared to the control group. Zimmermann et al. [14] performed a 6-month MLD intervention and showed 6 months after surgery a significant increase in arm volume on the operated side in the control group. Zhang et al. [17] combined physical exercise with self-administered MLD in their trial and showed that the combined intervention significantly reduced upper limb lymphedema after surgery compared to physical exercise alone.
Discussion
The aim of this study was to analyze the potential preventive effects of exercise-based therapies on SL incidence after breast cancer. To our knowledge, a systematic review on solely the topic of prevention was not conducted before. 8 randomized controlled studies [5, 11, 12, 13, 14, 15, 16, 17] could be identified. They showed that exercise in the form of progressive resistance training as well as combined exercise therapies consisting of physiotherapy, physical therapy, MLD, stretching, massage, and/or kinesiotherapy are safe and might have a preventive effect on SL incidence.
In the past, physicians believed that cancer patients must avoid exercise [8], and literature indicating that exercise can cause or exacerbate lymphedema still exists [15]. However, the present review revealed that 5 out of 8 trials reported significant preventive effects of resistance training and exercise on SL incidence. Park et al. [18] investigated the incidence and risk factors of SL in breast cancer patients. They demonstrated that women who exercised regularly, performed preventive self-care, and received information about the possible appearance of a lymphedema before local treatment had a lower risk of developing lymphedema. Proposed mechanisms included that exercise promotes the contractility of the skeletal muscles and subsequently provides primary pump mechanisms for lymph and venous drainage [19, 20].
Besides radiation and the number of surgically removed lymph nodes [16], overweight also contributes crucially to the development of SL [21, 22, 23]. In the study by Sagen et al. [12], a significant risk increase was observed in patients with a body mass index of >25 kg/m2 (p = 0.005). Shaw et al. [24] also referred to the relationship between overweight/obesity and the development of lymphedema [25]. According to Bicego et al. [19], further risk factors include obstruction, trauma, and inflammation [26]. Physical inactivity results in a decrease in lymph circulation. Physical exercise maintaining or improving the ‘range of motion’ of the shoulder therefore seems to be an effective and preventive measure. Additional benefits include improved muscle strength/fitness and maintenance of body weight.
A limitation of this systematic review is that probably not all studies covering the preventive effect of exercise on SL in breast cancer were identified in the literature. Also, we must take into consideration that other risk factors contribute to the development of SL as previously described. In this review, we included studies performed between 2006 and 2016 during which period surgical treatment and especially axillary staging shifted from axillary dissection level I-III to sentinel lymph node dissection. The risk of SL decreased during that time, making it difficult to compare these studies.
Due to the fact that the included studies evaluated different exercise intervention regimens, we are currently unable to provide clear and evidence-based exercise recommendations. The results published by Cavanaugh [27] underline the urgency of individualizing exercise guidelines. Further, Ahmed et al. [15] recommend that breast cancer patients should perform strength training of the upper body because this does not promote the risk or symptoms of lymphedema. Besides, Sagen et al. [12] recommend that patients with axillary lymph node dissection continue to exercise without restriction in daily living. In addition, considering early exercise intervention in women with breast cancer is important and necessary [27]. The studies by Ahmed et al. [15] and Schmitz et al. [11] show that progressive strength training can generate a preventive effect. Combined exercise therapy can result in similar effects [5, 14, 17]. Exercise additionally supports muscular pump function and should be performed at a moderate level of intensity and with a small number of repetitions. The application of MLD seems to have prophylactic effects only in combination with exercise, and current data does not show any evidence for MLD as a single primary prophylactic method [28]. To guarantee the safe and effective performance of the exercises, the support of a certified exercise therapist during the first months of the strength training is also advised [29].
Conclusion
A total of 8 randomized controlled trials yielded promising data supporting the preventive effect of exercise on SL in breast cancer patients. Results showed that exercise is safe and that it is important to maintain exercise in daily living. Exercise in the form of progressive resistance training as well as combined exercise therapies are safe and might have a preventive effect on SL incidence. Future investigations will have to differentiate between patients with and without axillary surgery, and exact exercise recommendations for therapists and the rehabilitation system should be based on further studies.
Disclosure Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Global Burden of Disease Cancer Collaboration The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–227. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson NL. Breast cancer-related lymphedema and resistance exercise: a systematic review. J Strength Cond Res. 2016;30:2656–2665. doi: 10.1519/JSC.0000000000001355. [DOI] [PubMed] [Google Scholar]
- 3.Bennett Britton TM, Purusgitham AD. Understanding breast cancer-related lymphoedema. Surgeon. 2009;7:120–124. doi: 10.1016/s1479-666x(09)80027-9. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning breast cancer survivors. Exerc Sport Sci Rev. 2010;38:17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi Á, Prieto Merino D, Mayoral del Moral O, Cerezo Téllez E, Minayo Mogollón E. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998;83:2798–2802. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2798::aid-cncr28>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Francis WP, Abghari P, Du W, Rymal C, Suna M, Kosir MA. Improving surgical outcomes: standardizing the reporting of Incidence severity of acute lymphedema after sentinel lymph node biopsy and axillary lymph node dissection. Am J Surg. 2006;192:636–639. doi: 10.1016/j.amjsurg.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL, American College of Sports Medicine American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 9.Keilani M, Hasenoehrl T, Neubauer M, Crevenna R. Resistance exercise and secondary lymphedema in breast cancer survivors - a systematic review. Support Care Cancer. 2016;24:1907–1916. doi: 10.1007/s00520-015-3068-z. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith T, Chittams J. Weight lifting for women at risk for breast cancer-related lymphedema. JAMA. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 12.Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009;48:1102–1110. doi: 10.3109/02841860903061683. [DOI] [PubMed] [Google Scholar]
- 13.De Rezende LF, Franco RL, de Rezende MF, Beletti PO, Morais SS, Gurgel MS. Two exercise schemes in postoperative breast cancer: comparison of effects on shoulder movement and lymphatic disturbance. Tumori. 2006;92:55–61. doi: 10.1177/030089160609200109. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann A, Wozniewski M, Szklarska A, Lipoxicz A, Szuba A. Efficacy of manual lymphatic drainage in preventing secondary lymphedema after breast cancer surgery. Lymphology. 2012;45:103–112. [PubMed] [Google Scholar]
- 15.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24:2765–2772. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- 16.Devoogdt N, Christiaens MR, Geraerts I, Truijen S, Smeets A, Leunen K, Neven P, Van Kampen M. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: randomised controlled trial. BMJ. 2011;343:d5326. doi: 10.1136/bmj.d5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Fan A, Yan J, He Y, Zhang H, Zhang H, Zhong Q, Liu F, Luo Q, Zhang L, Tang H, Xin M. Combining manual lymph drainage with physical exercise after modified radical mastectomy effectively prevents upper limb lymphedema. Lymphat Res Biol. 2016;4:104–108. doi: 10.1089/lrb.2015.0036. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs. 2008;17:1450–1459. doi: 10.1111/j.1365-2702.2007.02187.x. [DOI] [PubMed] [Google Scholar]
- 19.Bicego D, Brown C, Ruddick M, Storey D, Wong C, Harris SR. Exercise for women with or at risk for breast cancer-related lymphedema. Phys Ther. 2006;86:1398–1405. doi: 10.2522/ptj.20050328. [DOI] [PubMed] [Google Scholar]
- 20.Witte CL, Witte MH. Contrasting patterns of lymphatic and blood circulatory disorders. Lymphology. 1987;20:171–178. [PubMed] [Google Scholar]
- 21.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. doi: 10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 22.Hayes SB, Freedman GM, Li T, Anderson PR, Ross E. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys. 2008;72:1449–1455. doi: 10.1016/j.ijrobp.2008.02.080. [DOI] [PubMed] [Google Scholar]
- 23.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16:775–782. doi: 10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw C, Mortimer P, Judd PA. A randomized controlled trail of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110:1868–1874. doi: 10.1002/cncr.22994. [DOI] [PubMed] [Google Scholar]
- 25.Ochalek K. Prevention of lymphoedema. Wspolczesna Onkol. 2011;15:354–356. [Google Scholar]
- 26.Hull MM. Lymphedema in women treated for breast cancer. Semin Oncol Nurs. 2000;16:226–237. doi: 10.1053/sonc.2000.8117. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh KM. Effects of early exercise on the development of lymphedema in patients with breast cancer treated with axillary lymph node dissection. J Oncol Pract. 2011;7:89–93. doi: 10.1200/JOP.2010.000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuiver MM, ten Tusscher MR, Agasi-Idenburg CS, Lucas C, Aaronson NK, Bossuyt PM. Conservative interventions for preventing clinically detectable upper-limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy. Cochrane Database Syst Rev. 2015:CD009765. doi: 10.1002/14651858.CD009765.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]


