Abstract
A new geldanamycin analogue was isolated from Streptomyces hygroscopicus A070101. The structure was elucidated as 11-methoxy-17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin (1) on the basis of extensive 1D and 2D NMR as well as HRESI-MS spectroscopic data analysis. Compound 1 showed considerable cytotoxicity (SRB) against human cancer cell lines (breast cancer MCF-7, skin melanoma SK-MEL-2 and lung carcinoma COR-L23).
Keywords: Streptomyces hygroscopicus, 11-methoxy-17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin, geldanamycin
Introduction
Steroid hormone receptors are generally intracellular receptors and initiate signal transduction for steroid which lead to changes in gene expression over a time period ranging from hours to days [1]. During the translation, steroid receptors are assembled into a multi-protein complex containing hsp90 (one of the most abundant proteins expressed in cells) [2], p23, an immunophilin, and often some hsp70 [3]. Geldanamycin analogues were found to bind to hsp90 and disrupt its function, impedes dexamethasone-dependent trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus, led many of them are protooncogenic and play a prominent role in cancer [4]. For example, geldanamycin and its analog 17-AAG showed significant cytotoxicity against human cancer cell line SKBr3 (IC50 = 41 and 33 nM, respectively) [5]. During our continued work on bioactive bacteria and fungi, a Streptomyces hygroscopicus strain was isolated from the soil of Chang-Bai Mountain. A new geldanamycin analogue was isolated from a 20 L fermentation of this strain. Its structure was elucidated as 11-methoxy-17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin (1, Figure 1) on the basis of extensive 1D and 2D NMR as well as HRESI-MS spectroscopic data analysis. Compound 1 was tested with five-day in vitro SRB cytotoxicity against human tumors cell lines and showed considerable cytotoxicity against human cancer cell lines (breast cancer MCF-7, skin melanoma SK-MEL-2 and lung carcinoma COR-L23).
Figure 1.
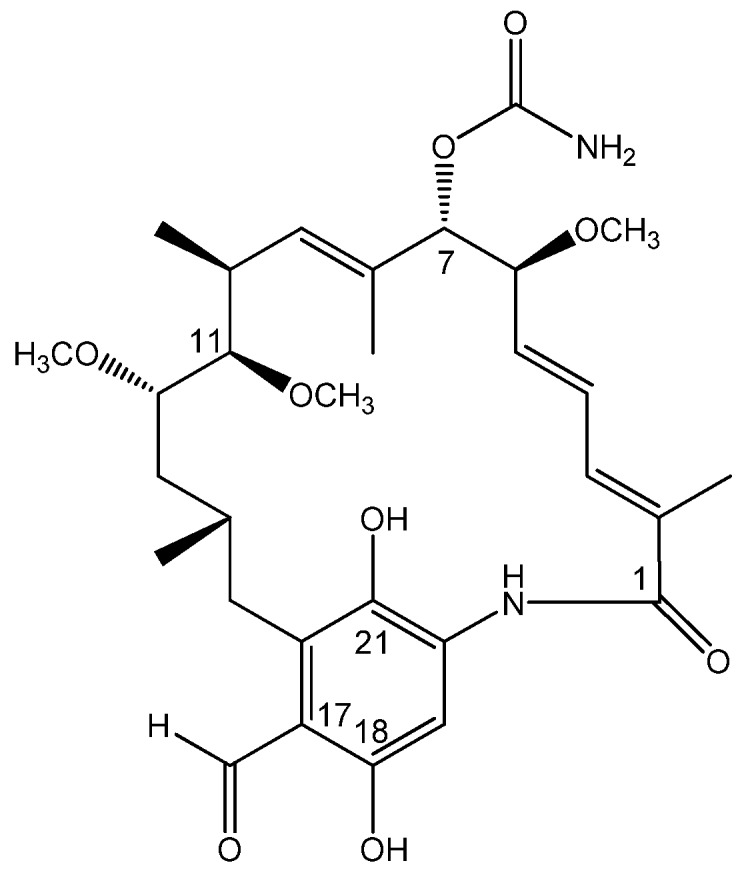
Structure of 11-methoxy-17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin (1).
Results and Discussion
Characterization of compound 1
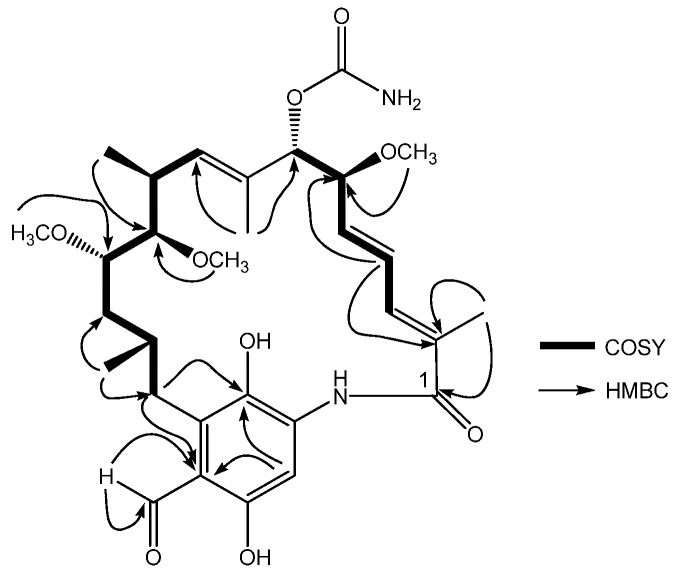
The molecular formula of compound 1 was determined as C30H42N2O9 on the basis of its HR-ESI-MS (m/z 575.2981 [M+H]+, calcd. 575.2969) and NMR data (Table 1). Analysis of the 13C-NMR spectrum and DEPT experiments, allowed the identification of seven methyl groups, two methylenes, eleven methines and ten quaternary carbons. Analysis of the 1H-1H COSY (Figure 2) and HMQC spectra suggested the presence of two 1H-1H spin systems: H3-H4- H5-H6-H7, H9-H10-H11-H12-H13-H14-H15. The chemical shifts of the protons and carbons (Table 1) were similar to those of the previously reported compound, 17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin, a geldanamycin analogue isolated from recombinant S. hygroscopicus strain [6]. The main differences between the two metabolites concerned a newly appeared methoxyl group [δC 57.2, δH 3.37 (s)] in compound 1. The location of the methoxyl group was established taking into account the correlation observed between 11-OCH3 (δ 3.37) and C-11 (δ 156.8) in the HMBC experiment of 1 (Figure 2). The coupling constant between the H5 and H6 (11.2 Hz), H9 and H10 (8.8 Hz), were similar to the literature values 10.4 Hz and 9.6 Hz [6], respectively, led to determine the relative stereochemistry same as reported compound KOSN 1645.
Table 1.
The NMR data of compound 1.a
| No. | Compound 1 | KOSN 1645 b | |||
|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | DEPT | 1H-NMR | 13C-NMR | |
| 1 | - | 169.1 | C | - | 167.3 |
| 2 | - | 135.2 | C | - | 136.7 |
| 3 | 7.01 (d, J = 12.0) | 124.3 | CH | 6.85 (d, J = 11.2) | 124.7 |
| 4 | 6.22 (t, J = 12.0) | 128.6 | CH | 6.35 (t, J = 11.2) | 127.1 |
| 5 | 5.24 (t, J = 11.2) | 133.3 | CH | 5.71 (t, J = 10.4) | 132.9 |
| 6 | 3.99 (d, J = 11.2) | 79.6 | CH | 4.31 (d, J = 10.4) | 80.9 |
| 7 | 4.83 (s) | 80.7 | CH | 4.96 (s) | 82.8 |
| 8 | - | 132.6 | C | - | 133.4 |
| 9 | 5.47 (d, J = 8.8) | 133.1 | CH | 5.94 (d, J = 9.6) | 133.4 |
| 10 | 2.51 (m) | 32.1 | C | 2.81 (qn, J = 7.6) | 32.3 |
| 11 | 3.73 (m) | 72.9 | CH | 3.63 (m) | 74.7 |
| 12 | 3.57 (m) | 79.3 | CH | 3.5 (m) | 80.3 |
| 13 | 1.86 (m) | 33.7 | CH2 | 1.88 (br) | 35.4 |
| 14 | 1.83 (m) | 29.3 | CH | 1.6-1.8 (ov) | 29.2 |
| 15 | 2.58 (m) | 33.1 | CH2 | 2.72 (br) | 33.4 |
| 16 | - | 126.9 | C | - | 127.3 |
| 17 | - | 114.2 | C | - | 113.1 |
| 18 | - | 159.3 | C | - | 159.4 |
| 19 | 7.71 (s) | 105.6 | CH | 7.93 (s) | 104.7 |
| 20 | - | 132.3 | C | - | 135.6 |
| 21 | - | 140.6 | C | - | 136.4 |
| 2-Me | 1.65 (s) | 11.8 | CH3 | 1.59 (s) | 12.4 |
| 8-Me | 1.68 (s) | 11.3 | CH3 | 1.79 (s) | 12.6 |
| 10-Me | 0.91 (d, J = 7.2) | 10.9 | CH3 | 0.93 (d, J = 7.2) | 12.1 |
| 14-Me | 0.97 (d, J = 8.8) | 23.1 | CH3 | 0.95 (d, J = 8.8) | 22.4 |
| 6-OMe | 3.29 (s) | 55.9 | CH3 | 3.36 (s) | 56.9 |
| 11-OMe | 3.37 (s) | 57.2 | CH3 | - | - |
| 12-OMe | 3.31 (s) | 57.7 | CH3 | 3.22 (d) | 57.1 |
| 17-CHO | 9.81 (s) | 191.0 | C | 10.09 (s) | 193.2 |
| 7-carbamate | 8.50 (s) | 155.5 | C | 8.73 (s) | 156.5 |
a Compound 1 was measured in DMSO-d6 and chemical shifts are expressed in ppm; b1H and 13C-NMR.
Figure 2.
COSY and HMBC of compound 1.
data (in CDCl3) of KOSN 1645 were extracted from [6].
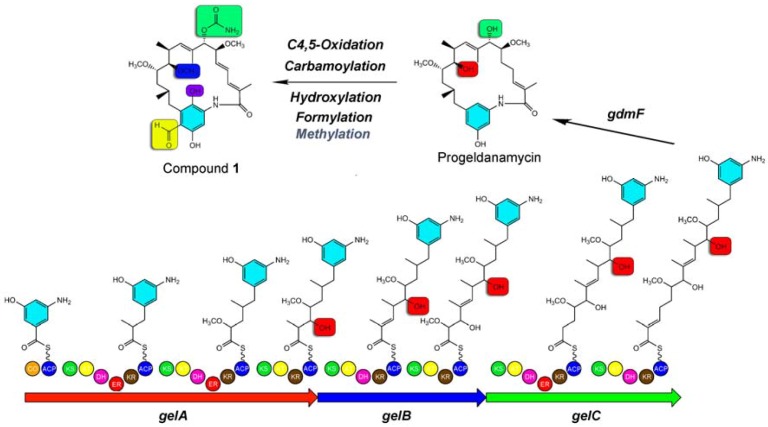
The biosyntheses of geldanamycin analogues were reported to be involved in the assembly of 3-amino-5-hydroxybenzoic acid (AHBA) as a starter unit, following elongation with the acyl-Coenzyme A substrates malonyl-CoA, methylmalonyl-CoA, and 2-methoxymalonyl-ACP, the polyketide intermediate undergoes intra-molecular lactamization by gdmF to form progeldanamycin [5,7] (Figure 3). The compound 1 isolated in this study and previously reported herbimycin A [6], proposed that an O-methylation step exist after the formation of polyketide backbone which may lead to identify a new O-methyltransferase. To prove this hypothesis, mutant lines could be established for screening of this 11-O-methyltransferase.
Figure 3.
Speculated biosynthetic pathway of compound 1.
Bioactivity results
Compound 1 was tested in anti-tumor bioassays and showed significant cytotoxicity (SRB) against several human cancer cell lines: breast cancer MCF-7 (IC50 = 142 nM), skin melanoma SK-MEL-2 (IC50 = 496 nM) and lung carcinoma COR-L23 (IC50 = 278 nM).
Conclusions
In summary, we have isolated a new geldanamycin analogue 11-methoxy-17-formyl-17-demethoxy-18-O-21-O-dihydrogeldanamycin (1) from a 20 L of Streptomyces hygroscopicus A070101 fermentation broth. Compound 1 showed considerable cytotoxicity against human cancer cell lines (breast cancer MCF-7, skin melanoma SK-MEL-2 and lung carcinoma COR-L23).
Experimental
General
The 1H- and 13C-NMR spectra were measured on a Bruker Avance DRX 500 NMR spectrometer in DMSO-d6, using TMS as an internal standard. Chemical shifts (δ) are expressed in parts per million (ppm), with the coupling constants (J) reported in Hertz (Hz). The HR-ESI mass spectrum was obtained from a MDS SCIEX API QSTAR-MS instrument. TLC was performed with silica gel plates (Macherey -Nagel, SilG / UV254, 0.20mm); Semi-preparative HPLC was carried out with Agilent 1100 on a Zorbax C18 column (250 x 10 mm, Phenomenex, Torrance, CA), UV absorption data (λ280) were analyzed with Agilent Chemstation Ver 8.01. All solvents used in this study were HPLC grade, purchased from the Chinese Chemical Group, Beijing, China.
Bacterial strain and growth conditions
Bacterial strain A070101 was obtained from Chang-Bai Mountain soil during a systematic screening of ginsenoside-glucosidase-produced bacteria [10] and was identified as Streptomyces hygroscopicus by professor Zhao-Yang He (Jilin Agricultural University). Geldanamycin production medium (GPM), consist of sucrose (50 g/liter), peptone (2 g/liter), tryptone (2 g/liter), yeast-extract (2 g/liter), Gerber's oatmeal (5 g/liter), and Brer Rabbit molasses (10 ml/liter) (pH = 7.0), was used to produce geldanamycin homologues [11]. The fermentation was carried in fifty 1 L flasks at 28 °C for 7 days.
Extraction and isolation of compound 1
The lyophilized culture broth was extracted with 80% EtOH at room temperature. The extract was concentrated under reduced pressure to give the pale brown residue (625 mg) that was fractionated by reverse phase (C-8) chromatography using H2O, aqueous MeOH (30%, 60%, 90%) and MeOH to give four fractions: the 90% MeOH fraction was further fractionated on a Sephadex LH- 20 column (CHCl3: MeOH=1:1) and then purify by semi-preparative HPLC to yield compound 1 (3.6 mg, 0.18 mg/L, whereas yield of geldanamycin was reported as ~10 mg/liter) [6].
In vitro cytotoxicity assays
Five-day in vitro SRB cytotoxicity tests against human tumors cell lines were carried out at the Cell Culture Laboratory, Pharmaceutical College, Jilin University, using modified protocols for MCF-7 (breast cancer), SK-MEL-2 (skin melanoma) and COR-L23 (lung carcinoma), the normal cells were used as control [12]. Generally, 5x103/mL cells were placed in a 24-well plate and treated with compound 1. The plate was incubated at 37 °C for 5 days. Then the medium was removed from the 24-well plate, and 10% ice-cold TCA (trichloroacetic acid, 1 mL) was added. The plate was kept at 4 °C for two hours after which was washed four times with cold water, then stained with SRB (Sulforhodamine B, Sigma St. Louis, MO, USA). After washing with 1% acetic acid, the bound dye was solubilized with Tris base A (Sigma) and 100 μL of each sample were transferred into a 96-well plate, and then read at 492 nm.
Acknowledgements
This work was supported by the grant from National Natural Science Foundation of China (30971885), Major Program of Transgenic Species Development of Ministry of Agriculture of China (2009ZX08009-062B) and Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period (2009BADB3B05).
Footnotes
Sample Availability: Samples are available from the authors (contact drxiang@hotmail.com).
References and Notes
- 1.Evans R. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csermely P., Schnaider T., Soti C., Prohászka Z., Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 3.Czar M., Galigniana M., Silverstein A., Pratt W. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- 4.Stebbins C., Russo A., Schneider C., Rosen N., Hartl F., Pavletich N. Crystal structure of an hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/S0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 5.Patel K., Piagentini M., Rascher A., Tian Z., Buchanan G., Regentin R., Hu Z., Hutchinson C., McDaniel R. Engineered biosynthesis of geldanamycin analogs for Hsp90 Inhibition. Chem. Biol. 2004;11:1625–1633. doi: 10.1016/j.chembiol.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z., Liu Y., Tian Z., Ma W., Starks C., Regentin R., Licari P., Myles D., Hutchinson C. Isolation and characterization of novel geldanamycin analogues. J. Antibiot. 2004;57:421–428. doi: 10.7164/antibiotics.57.421. [DOI] [PubMed] [Google Scholar]
- 7.Rascher A., Hu Z., Buchanan G., Reid R., Hutchinson C. Insights into the biosynthesis of the benzoquinone ansamycins geldanamycin and herbimycin, obtained by gene sequencing and disruption. Appl. Environ. Microbiol. 2005;71:4862–4871. doi: 10.1128/AEM.71.8.4862-4871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetcher L., Tian Z., McDaniel R., Rascher A., Revill W., Hutchinson C., Hu Z. Rapid engineering of the geldanamycin biosynthesis pathway by Red/ET recombination and gene complementation. Appl. Environ. Microbiol. 2005;71:1829–1835. doi: 10.1128/AEM.71.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Y., Lee D., Kim W., Jeong J., Kim C., Sohng J., Lee J., Paik S., Lee J. Inactivation of the carbamoyltransferase gene refines post-polyketide synthase modification steps in the biosynthesis of the antitumor agent geldanamycin. J. Am. Chem. Soc. 2004;126:11142–11143. doi: 10.1021/ja047769m. [DOI] [PubMed] [Google Scholar]
- 10.Ruan C., Zhang H., Zhang L., Liu Z., Sun G., Lei J., Qin Y., Zheng Y., Li X., Pan H. Biotransformation of ginsenoside Rf to Rh1 by recombinant β-Glucosidase. Molecules. 2009;14:2043–2048. doi: 10.3390/molecules14062043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deboer C., Meulman P., Wnuk R., Peterson C. Geldanamycin, a new antibiotic. J. Antibiotics. 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]
- 12.Baruch B., Lina W., Einat B., Jardena N., Jacob S., Haim G., Eyal F. Variable cytotoxicity of amifostine in malignant and non-malignant cell lines. Oncol. Rep. 2003;10:1609–1613. [PubMed] [Google Scholar]