Abstract
Osteoarthritis is a degenerative disease that often causes patients to experience joint pain and deformity. It has been demonstrated that tumor necrosis factor (TNF)-α is associated with the progression of osteoarthritis; however, to the best of our knowledge, the mechanisms by which TNF-α simulates the progression of osteoarthritis and the signaling pathway(s) it influences remain unknown. Therefore, the aim of the present study was to investigate the therapeutic effects of TNF-α inhibitor in an iodoacetate-induced rat model of osteoarthritis and identify its potential mechanisms of action. Western blotting, ELISA and histological analyses were performed to assess the effects of the TNF-α inhibitor on osteoarthritis. The effects of TNF-α and phosphoinositide 3-kinase (PI3K) inhibition on synovial fibroblasts isolated from rats with osteoarthritis were tested in vitro. Furthermore, the expression of various inflammatory cytokines and the PI3K/protein kinase B (AKT) signaling pathway were assessed in vitro. The results indicated that the inflammatory factors TNF-α, interleukin (IL)-1β, IL-17a and IL-8 were upregulated in synovial fibroblasts taken from rats with osteoarthritis compared with normal rats. By contrast, TNF-α inhibition downregulated IL-1β, IL-17a and IL-8 expression in synovial fibroblasts in vitro. The PI3K/AKT pathway was also upregulated in synovial fibroblasts harvested from rats with osteoarthritis compared with that in normal rats. It was demonstrated that treatment with the TNF-α inhibitor downregulated the serum and protein levels of IL-1β, IL-17a and IL-8 in rats with osteoarthritis. Furthermore, treatment with the TNF-α inhibitor also decreased matrix metalloproteinase (MMP)-3, MMP-9, vascular endothelial growth factor and ADAMTS4 expression in synovial fibroblasts isolated from rats with osteoarthritis. Treatment with the TNF-α inhibitor also inhibited the PI3K/AKT pathway in synovial fibroblasts isolated from rats with osteoarthritis. Treatment with the PI3K inhibitor ameliorated TNF-α-induced increases in IL-1β, IL-17a and IL-8 expression in synovial fibroblasts isolated from rats with osteoarthritis. Furthermore, treatment with the TNF-α inhibitor decreased inflammation, as well as joint and cartilage destruction in vivo. Taken together, the results of the present study indicate that TNF-α inhibition may downregulate the expression of inflammatory factors in synovial fibroblasts, suggesting that TNF-α inhibition may be a novel method for treating osteoarthritis by downregulating the PI3K/AKT signaling pathway.
Keywords: osteoarthritis, tumor necrosis factor α, inflammation, phosphoinositide 3-kinase/protein kinase B
Introduction
Osteoarthritis is one of the most common joint diseases and is primarily caused by inflammation and synovial cell dysfunction (1). A number of factors, including patient age, body mass index, level of physical function and level of physical activity are associated with hip or knee osteoarthritis (2). The pathological causes of joint osteoarthritis development are complex (3).
The incidence of osteoarthritis (~8% of the population) has increased since 2012 and this disease poses a serious threat to human health and quality of life due to the pain and disability caused (4–6). Inflammation serves a crucial role in osteoarthritis and is associated with joint and cartilage destruction (7). Previous studies have demonstrated that anti-inflammatory therapies targeting the inflammatory factors accumulating in the synovial fluid of patients with osteoarthritis induce therapeutic effects (8–10).
The association between synovial inflammation and structural damage during the progression of osteoarthritis has been evaluated and it has been suggested that inflammatory cytokines may predict the prognosis of patients with osteoarthritis (11). The tumor necrosis factor (TNF) family is one such cytokine that may serve an important role in the inflammatory responses that occur during osteoarthritis (12). TNF-α-associated joint inflammation has been analyzed in patients with rheumatoid arthritis and osteoarthritis and TNF-α was identified as a potential target for rheumatoid arthritis therapy (13). Currently, anti-TNF-α-targeted therapy is being applied to treat patients with osteoarthritis and is achieving satisfactory outcomes by decreasing inflammation (14,15). A number of different agents that target TNF-α, including etanercept, trastuzumab, adalimumab and infliximab, have been developed to treat patients with osteoarthritis (16–18). However, to the best of our knowledge, the mechanisms mediated by TNF-α in the synovial fibroblasts of patients with osteoarthritis remain unknown.
The present study investigated the potential mechanism(s) of TNF-α in synovial fibroblasts taken from a monosodium iodoacetate-induced rat model of osteoarthritis in vitro. Levels of inflammatory cytokines in the synovial fibroblasts were also analyzed in vivo. The results of the present study indicate that TNF-α is able to regulate inflammation in a rat model of osteoarthritis by downregulating the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway in synovial fibroblasts.
Materials and methods
Ethics statement
The present study was performed in strict accordance with the Guide for the Ethics Committee of The Fourth Hospital affiliated to Harbin Medical University. All surgeries and euthanasia were performed under intravenous injection of sodium pentobarbital anesthesia (35 mg/kg, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). All mice were sacrificed using isoflurane (0.5 ml) euthanasia as described previously (19).
Animal model
A total of 36 female Sprague-Dawley rats (eight weeks old; body weight, 280–300 g) were purchased from Shanghai SLAC laboratory Animal Co., Ltd. (Shanghai, China). All rats were housed under controlled temperatures (23°C, 50% humidity) under a 12 h light/dark cycle with access to food and water ad libitum. All rats were identified by ear punching. A rat model of osteoarthritis was produced using intra-articular injections of monosodium iodoacetate (0.2 mg per rat; Sigma-Aldrich; Merck KGaA; n=24) administered at a volume of 30 µl once per day, for 10 days, as previously described (20); however, healthy control rats did not undergo this procedure (n=12). On day 10, rats were randomly assigned to one of three groups: A control group (n=12), a vehicle group (n = 12) and a TNF-α inhibitor group (n = 12). All rats received subcutaneous injections of vehicle (30 mg/kg/day; control group) or TNF-α inhibitor (30 mg/kg/day; cat. no. 1049741-03-8, Merck KGaA). Treatments were continued seven times and rats received the vehicle or TNF-α inhibitor once every 2 days for a total of 14 days. All rats were euthanized on day 14 prior to histological analysis. Rats were weighed following 14 days of treatment.
Cells and reagents
Synovial fibroblasts were isolated from the same rats as those described in the preceding paragraph and cultured in Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA) at 37°C in a 5% CO2 humidified atmosphere. Synovial fibroblasts were treated with the TNF-α inhibitor (2 mg/ml) (21), PI3K inhibitor (2 mg/ml; cat. no. 526559-5MGCN, Merck KGaA) or an equal volume of PBS at 37°C for 24 h.
ELISA
Blood was extracted from experimental rats on day 15 and sera was obtained via centrifugation (6,000 × g for 15 min) at 4°C. TNF-α, interleukin (IL)-1β, IL-17a and IL-8 concentrations in the serum of experimental rats were analyzed using ELISA kits (cat. nos. MTA00B, MLB00C, DY421 and P8000, respectively; all Bio-Rad Laboratories, Inc., Hercules, CA, USA), following the manufacturer's protocol. The results were analyzed using the 1775×Mark™ ELISA reader system at 450 nm (Bio-Rad Laboratories, Inc.).
Western blotting
Western blotting was performed to measure protein expression following a previously described method (22). Briefly, synovial fibroblasts (1×107) were lysed in a lysis buffer containing 1% phenylmethane sulfonyl fluoride (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for three cycles of freezing-thawing and subsequently centrifuged at 8,000 × g for 10 min at 4°C. Protein concentrations were measured using a BCA assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Proteins (20 µg) were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane, which was blocked with 5% (w/v) nonfat dry milk dissolved in Tris-buffered saline plus Tween-20 (TBST) solution for 2 h at 37°C. Membranes were subsequently incubated with primary rabbit anti-rat antibodies against TNF-α (1:1,000, cat. no. ab6671), IL-1β (1:1,000, cat. no. ab200478), IL-17a (1:1,000, cat. no. ab180904), IL-8 (1:1,000, cat. no. ab34100), PI3K (1:2,000, cat. no. ab1678), phosphorylated (p)-PI3K (1:1,000, cat. no. ab182651), AKT (1:1,000, cat. no. ab8805), pAKT (1:1,000, cat. no. ab64148), matrix metalloproteinase (MMP)-3 (1:1,000, cat. no. ab53015), MMP-9 (1:1,000, cat. no. ab38898), vascular endothelial growth factor (VEGF; 1:1,000, cat. no. ab39256), ADAMTS4 (1:1,000, cat. no. ab185722) and β-actin (1:1,000, cat. no. ab8226; all Abcam, Cambridge, UK) for 12 h at 4°C. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G monoclonal secondary antibodies (cat. no. PV-6001; OriGene Technologies, Inc., Beijing, China) were added for 24 h at 4°C. A Ventana Benchmark automated staining system was used to analyze protein expression (BX51; Olympus Corporation, Tokyo, Japan). Protein expression signals were analyzed using scanning densitometry with a Microtek ScanMaker 8700 (Beijing Zhongjing Electronic Technology Co., Ltd., Beijing, China) using ScanWizard 5 software (Shanghai Microtek Technology Co., Ltd., Shanghai, China). Band densities were analyzed using Quantity One v.4.62 (Bio-Rad Laboratories, Inc.).
Histopathological analysis
Rats with osteoarthritis were euthanized under pentobarbital anesthesia on day 14. Joints and articular cartilage were separated and fixed in 10% formalin for 10 min at room temperature. Paraffin-embedded joints and articular cartilages were sliced into 4-µm sections. Tissue sections were stained with hematoxylin and eosin for 30 min at room temperature for histological evaluation. Safranin O-fast green and Toluidine blue staining for 20 min at room temperature was used to evaluate proteoglycans in the cartilage matrix using a light microscope at a magnification, ×40.
Tissue preparation and histopathological evaluation
Tissue sections were fixed in 10% formalin at 37°C for 24 h, decalcified using Gooding and Stewart's fluid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and subsequently embedded in paraffin. Sections (4-µm thick) were stained with 0.05% Toluidine blue (pH 4.1) for 20 min at room temperature and the degree of osteonecrosis was evaluated using the modified Mankin scoring system (23). The Mankin scoring system was scored as follows: 0, normal; 1, irregular surface; 2, pannus; 3, absence of superficial cartilage layers; 4, slight disorganization; 5, fissure into the calcified cartilage layer; and 6, disorganization. Histopathological evaluation was performed by two independent blinded observers.
Statistical analysis
All data are expressed as the mean ± standard deviation. Each experiment was performed in triplicate. All data were analyzed using SPSS software ver. 19.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses were performed using one-way analysis of variance followed by Tukey's multiple comparison post hoc tests and P<0.05 was considered to indicate a statistically significant difference.
Results
Inflammatory factors are upregulated in the iodoacetate-induced osteoarthritis rat model
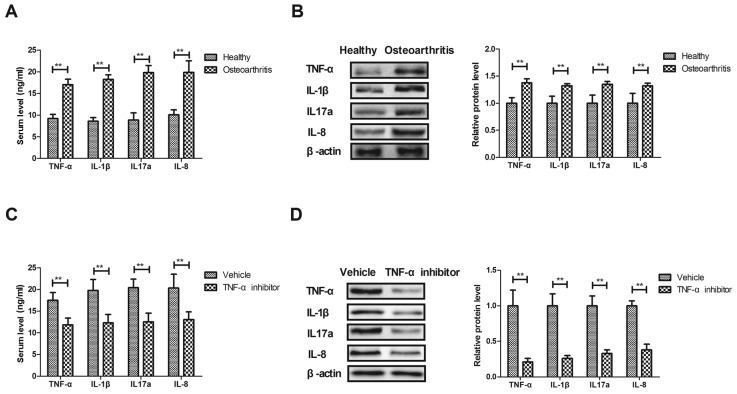
The expression of inflammatory factors was measured in the osteoarthritis rat model and compared with that of healthy rats. The results demonstrated that serum TNF-α, IL-1β, IL-17a and IL-8 expression was significantly higher in rats with osteoarthritis compared with healthy rats (P<0.01; Fig. 1A). Furthermore, TNF-α, IL-1β, IL-17a and IL-8 expression was significantly upregulated in synovial fibroblasts isolated from rats with osteoarthritis compared with healthy rats (P<0.01; Fig. 1B). By contrast, treatment with the TNF-α inhibitor significantly decreased TNF-α, IL-1β, IL-17a and IL-8 expression in the serum of rats with osteoarthritis (P<0.01; Fig. 1C). The TNF-α inhibitor also significantly decreased TNF-α, IL-1β, IL-17a and IL-8 expression in synovial fibroblasts isolated from rats with osteoarthritis (P<0.01; Fig. 1D). These results suggest that inflammatory factors are upregulated in the serum and synovial fibroblasts of rats with osteoarthritis.
Figure 1.
Inflammatory factor levels in a rat model of osteoarthritis. (A) Serum levels of TNF-α, IL-1β, IL-17a and IL-8 in rats with osteoarthritis compared with healthy rats, as determined by an enzyme-linked immunosorbent assay. (B) Levels of TNF-α, IL-1β, IL-17a and IL-8 expression in synovial fibroblasts isolated from rats with osteoarthritis or healthy rats, as measured by western blot analysis. (C) Serum levels of TNF-α, IL-1β, IL-17a and IL-8 in rats with osteoarthritis rat following treatment with TNF-α inhibitor or vehicle. (D) Levels of TNF-α, IL-1β, IL-17a and IL-8 expression in synovial fibroblasts from rats with osteoarthritis following treatment with TNF-α inhibitor or vehicle. Data are expressed as the mean ± standard deviation. **P<0.01. TNF-α, tumor necrosis factor α; IL, interleukin.
Effects of TNF-α on the expression of pro-inflammatory factors in synovial fibroblasts
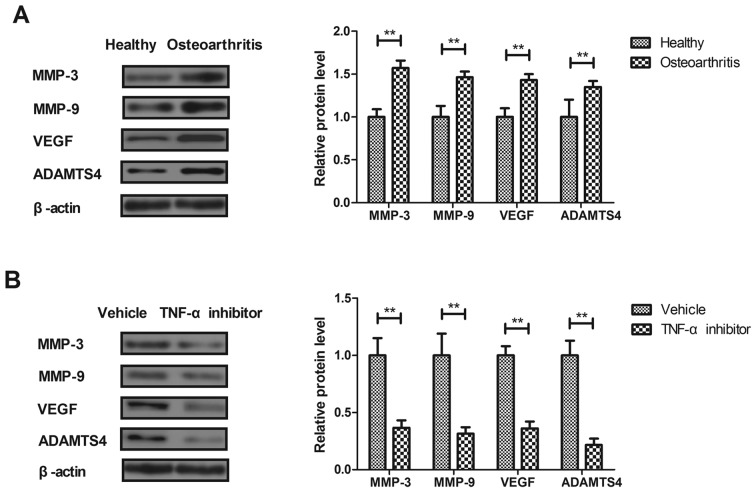
The effects of TNF-α on macrophage-regulated MMP levels were analyzed in the synovial fibroblasts of rats with osteoarthritis. MMP-3, MMP-9, VEGF and ADAMTS4 expression was significantly increased in the synovial fibroblasts of rats with osteoarthritis compared with healthy rats (P<0.01; Fig. 2A). Treatment with the TNF-α inhibitor significantly decreased MMP-3, MMP-9, VEGF and ADAMTS4 expression in synovial fibroblasts from rats with osteoarthritis compared with those from healthy rats (P<0.01; Fig. 2B). These results suggest that TNF-α inhibition may be used to treat osteoarthritis.
Figure 2.
Effect of the TNF-α inhibitor on the expression of inflammation-associated factors in synovial fibroblasts isolated from rats with osteoarthritis. (A) Levels of MMP-3, MMP-9, VEGF and ADAMTS4 expression in the synovial fibroblasts of rats with osteoarthritis and healthy rats, as determined by western blotting. (B) Levels of MMP-3, MMP-9, VEGF and ADAMTS4 expression in synovial fibroblasts taken from rats with osteoarthritis following treatment with TNF-α inhibitor or vehicle. Data are expressed as the mean ± standard deviation. **P<0.01. TNF-α, tumor necrosis factor α; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
TNF-α regulates the expression of inflammatory factors via the PI3K/AKT signaling pathway
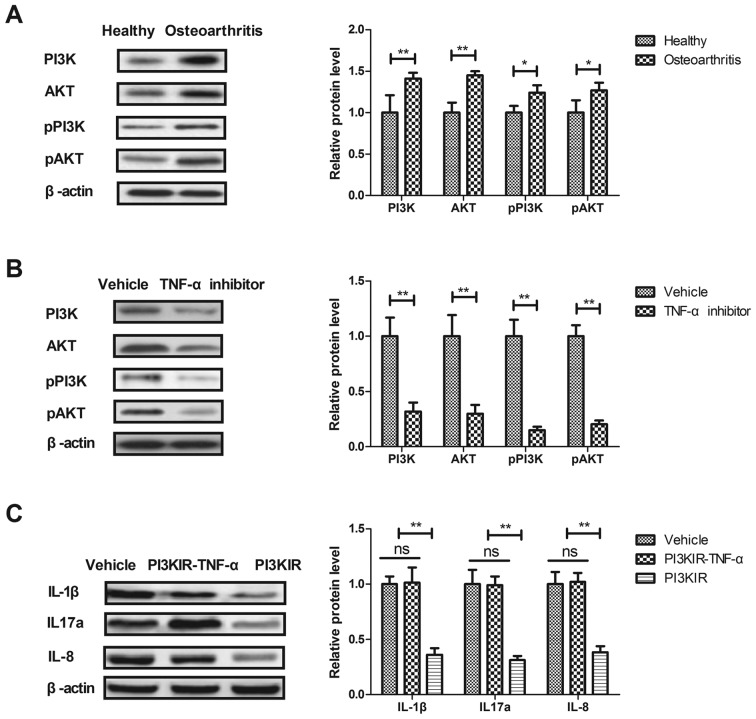
It has been reported that the PI3K/AKT signaling pathway is involved in inflammation during the progression of osteoarthritis (24). Therefore, the current study investigated the effects of TNF-α on the PI3K/AKT signaling pathway in synovial fibroblasts. PI3K and AKT expression and phosphorylation were significantly upregulated in synovial fibroblasts from rats with osteoarthritis compared with those from healthy rats (P<0.05; Fig. 3A). However, PI3K and AKT expression and phosphorylation were significantly decreased in the synovial fibroblasts of rats treated with the TNF-α inhibitor compared with those from rats treated with vehicle (P<0.01; Fig. 3B). The results of an in vitro assay revealed that the PI3K inhibitor significantly reversed the TNF-α-induced increase in IL-1β, IL-17a and IL-8 expression in synovial fibroblasts from rats with osteoarthritis (P<0.01; Fig. 3C). These results suggest that TNF-α regulates the expression of inflammatory factors in synovial fibroblasts via the PI3K/AKT signaling pathway.
Figure 3.
The TNF-α inhibitor regulates the expression of inflammatory factors via the PI3K/AKT signaling pathway. (A) Expression and phosphorylation levels of PI3K and AKT in synovial fibroblasts from rats with osteoarthritis and healthy rats were determined by western blotting. (B) Expression and phosphorylation levels of PI3K and AKT in the synovial fibroblasts from rats with osteoarthritis following treatment with TNF-α inhibitor or vehicle. (C) Effects of the PI3KIR on levels of the inflammatory factors IL-1β, IL-17a and IL-8 expression in synovial fibroblasts following treatment with TNF-α. Data are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01. ns, not significant; TNF-α, tumor necrosis factor α; PI3KR, PI3K inhibitor; PI3K/AKT, phosphoinositide 3-kinase/protein kinase B; IL, interleukin; p-, phosphorylated.
Treatment with TNF-α inhibitor helps to treat rats with iodoacetate-induced osteoarthritis
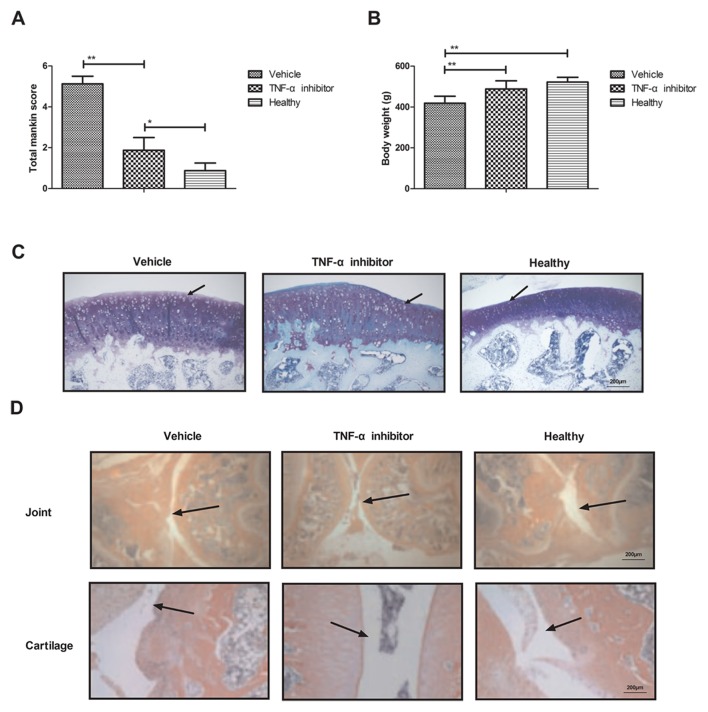
Finally, the effect of the TNF-α inhibitor on the iodoacetate-induced osteoarthritis rat model was assessed in vivo. The results indicated that mice treated with the TNF-α inhibitor experienced a significantly decreased total Mankin score compared with rats treated with vehicle (P<0.01; Fig. 4A), indicating that the TNF-α inhibitor ameliorates bone osteoarthritis. Furthermore, treatment with the TNF-α inhibitor significantly increased the body weight of rats compared with the vehicle group (P<0.01; Fig. 4B). Histological analyses indicated that treatment with the TNF-α inhibitor inhibited inflammatory cell infiltration (Fig. 4C) and decreased bone destruction in the joints and cartilage of rats with osteoarthritis (Fig. 4D). These results suggest that TNF-α inhibition may improve inflammation, inflammatory cell infiltration and bone destruction in a rat model of osteoarthritis.
Figure 4.
Therapeutic effects of the TNF-α inhibitor on rats with osteoarthritis. (A) Effects of the TNF-α inhibitor on the symptoms of osteoarthritis, as determined by the total Mankin score. (B) Effects of the TNF-α inhibitor on the body weights of rats with osteoarthritis. (C) Effects of the TNF-α inhibitor on inflammatory cell infiltration in the joints of rats with osteoarthritis. Arrows indicate inflammatory cells infiltration. (D) Effects of the TNF-α inhibitor on the destruction of joint and cartilage in osteoarthritis rat. Arrows indicate joint or cartilage morphology. Data are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01. TNF-α, tumor necrosis factor α.
Discussion
Synovial inflammation leads to structural damage and bone destruction in osteoarthritis and may decrease synovial function in osteoarthritis (25); therefore, alleviating synovial inflammation may be an effective method of treating patients with osteoarthritis (25). It has been demonstrated that the TNF-α inhibitor etanercept may alleviate pain in patients with moderate to severe osteoarthritis, suggesting that TNF-α may be an important pathological factor during the progression of osteoarthritis (26). The aim of the present study was to analyze the association between TNF-α and the PI3K/AKT signaling pathway in synovial fibroblasts from rats with osteoarthritis. The results indicated that treatment with TNF-α inhibitor decreased the expression of IL-1β, IL-17a and IL-8 by downregulating the PI3K/AKT signaling pathway in synovial fibroblasts taken from rats with osteoarthritis.
Previous studies have indicated that inflammatory cytokines are associated with synovial injury during the development of osteoarthritis (24,27,28). A previous study has also demonstrated that IL-1β and/or TNF-α induces the upregulation of MMP-1 and MMP-3 expression in chondrocyte subpopulations, which may be a pathogenic cause of osteoarthritis (29). The present study demonstrated that IL-1β and TNF-α are upregulated in synovial fibroblasts taken from rats with osteoarthritis. This is in accordance with the results of a previous study, which demonstrated that levels of IL-6 and IL-8 cytokines are upregulated in human osteoarthritis (30). Additionally, IL-17a expression is increased in inflammatory osteoarthritis, which may explain the non-response to anti-IL-17 therapy in subsets of patients with osteoarthritis (31). The present study indicated that IL-17a and IL-8 expression was upregulated in synovial fibroblasts taken from rats with osteoarthritis. Notably, TNF-α inhibition decreased levels of IL-1β, IL-17a and IL-8 in synovial fibroblasts isolated from rats with osteoarthritis (32–34).
Chen et al (35) indicated that treatment with the TNF-α inhibitor confers many benefits including the decrease of inflammation and pain for patients with osteoarthritis of the hand, who are refractory to analgesia. It has also been demonstrated that elevated VEGF levels may increase bone destruction in an in vivo model of osteoarthritis (33). Furthermore, it has been suggested that ADAMTS4 may be upregulated in osteoarthritis, which, in turn, increases the expression of the proinflammatory cytokine NF-κB (34). In the present study, VEGF and ADAMTS4 expression were upregulated in the synovial fibroblasts of rats with osteoarthritis, but were downregulated following treatment with TNF-α inhibitor. Taken together, these results suggest that treatment with TNF-α inhibitor may be beneficial in the treatment of osteoarthritis.
Inhibiting the PI3K/AKT signaling pathway may be developed as a promising method of treating patients with osteoarthritis (36,37). It has been reported that PI3K/AKT mediates the expression of TNF-α mRNA and NF-κB activation in calyculin A-treated primary osteoblasts (38). Notably, another study indicated that regulation of the PI3K/AKT signaling pathway may inhibit inflammation and the apoptosis of chondrocytes in a rat model of osteoarthritis (39). The present study demonstrated that treatment with TNF-α inhibitor downregulated levels of the inflammatory factors IL-1β, IL-17a and IL-8 via the PI3K/AKT signaling pathway. Treatment with the TNF-α inhibitor also decreased IL-1β, IL-17a and IL-8 levels by decreasing PI3K and AKT expression in synovial fibroblasts. It has been demonstrated that inhibiting TNF-α may decrease inflammation by downregulating the PI3K/AKT signaling pathway (39). These reports suggest that the PI3K/AKT signaling pathway may be a potential target for the treatment of osteoarthritis. The present study identified that treatment with a TNF-α inhibitor downregulated the PI3K/AKT signaling pathway in synovial fibroblasts isolated from rats with osteoarthritis. Previous studies have demonstrated that the activation of the NF-κB-mediated inflammation may be induced by TNF-α (40–42). However, the present study did not analyze the effects of TNF-α inhibitor on NF-κB, IL-6 and TGF-β levels. Further studies are required to assess the other mechanisms mediated by the TNF-α inhibitor.
In conclusion, the present study investigated the potential mechanisms mediated by TNF-α in a rat model of osteoarthritis induced by monosodium iodoacetate. The results indicate that the PI3K/AKT signaling pathway is an inflammatory pathway that may be mediated by TNF-α in osteoarthritis. The expression of inflammatory cytokines in synovial fibroblasts significantly decreased following treatment with TNF-α inhibitor. These results suggest that inhibiting the PI3K/AKT signaling pathway may contribute to the inhibition of inflammation in osteoarthritis and may therefore be developed as novel method of treating osteoarthritis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HoL performed the experiments. SX, YQ, HuL, and RZ prepared and analyzed experimental data. YL designed the experiment.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The Fourth Hospital affiliated to Harbin Medical University (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Onishi K, Utturkar A, Chang E, Panush R, Hata J, Perret-Karimi D. Osteoarthritis: A critical review. Crit Rev Phys Rehabil Med. 2012;24:251–264. doi: 10.1615/CritRevPhysRehabilMed.2013007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veenhof C, Huisman PA, Barten JA, Takken T, Pisters MF. Factors associated with physical activity in patients with osteoarthritis of the hip or knee: A systematic review. Osteoarthritis Cartilage. 2012;20:6–12. doi: 10.1016/j.joca.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Davis AM. Osteoarthritis year in review: Rehabilitation and outcomes. Osteoarthritis Cartilage. 2012;20:201–206. doi: 10.1016/j.joca.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Kuijt MT, Inklaar H, Gouttebarge V, Frings-Dresen MH. Knee and ankle osteoarthritis in former elite soccer players: A systematic review of the recent literature. J Sci Med Sport. 2012;15:480–487. doi: 10.1016/j.jsams.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Taylor R, Jr, Raffa RB, Pergolizzi JV., Jr Controlled release formulation of oxycodone in patients with moderate to severe chronic osteoarthritis: A critical review of the literature. J Pain Res. 2012;5:77–87. doi: 10.2147/JPR.S21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Role of interleukin-1 inhibitors in osteoarthritis: An evidence-based review. Drugs Aging. 2012;29:343–358. doi: 10.2165/11599350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1825–1834. doi: 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermann J, Lipp RW, Dunzinger A, Spreizer C, Schaffler G, Kvaternik H, Ofner P, Graninger W. Anti-TNF scintigraphy to assess TNF-α-associated joint inflammation in rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 2014;32:614. [PubMed] [Google Scholar]
- 9.Gross JB, Guillaume C, Gégout-Pottie P, Mainard D, Presle N. Synovial fluid levels of adipokines in osteoarthritis: Association with local factors of inflammation and cartilage maintenance. Biomed Mater Eng. 2014;24(Suppl 1):S17–S25. doi: 10.3233/BME-140970. [DOI] [PubMed] [Google Scholar]
- 10.Ballegaard C, Riis RG, Bliddal H, Christensen R, Henriksen M, Bartels EM, Lohmander LS, Hunter DJ, Bouert R, Boesen M. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: A cross-sectional study. Osteoarthritis Cartilage. 2014;22:933–940. doi: 10.1016/j.joca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Mancarella L, Addimanda O, Cavallari C, Meliconi R. Synovial inflammation drives structural damage in hand osteoarthritis: A narrative literature review. Curr Rheumatol Rev. 2016;13:43–50. doi: 10.2174/1573397112666160909105903. [DOI] [PubMed] [Google Scholar]
- 12.Han L, Song JH, Yoon JH, Park YG, Lee SW, Choi YJ, Nam SW, Lee JY, Park WS. TNF-α and TNF-β polymorphisms are associated with susceptibility to osteoarthritis in a Korean population. Korean J Pathol. 2012;46:30–37. doi: 10.4132/KoreanJPathol.2012.46.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko K, Sugitani M, Goto M, Murashima A. Tocilizumab and pregnancy: Four cases of pregnancy in young women with rheumatoid arthritis refractory to anti-TNF biologics with exposure to tocilizumab. Modern rheumatology. 2016;26:672–675. doi: 10.3109/14397595.2016.1140256. [DOI] [PubMed] [Google Scholar]
- 14.Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C, Qu Q. Effects of preventive administration of juanbi capsules on TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with knee osteoarthritis. J Tradit Chin Med. 2010;30:254–258. doi: 10.1016/S0254-6272(10)60052-0. [DOI] [PubMed] [Google Scholar]
- 15.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Olson SA, Furman BD, Kraus VB, Huebner JL, Guilak F. Reply to ‘Does progranulin account for the opposite effects of etanercept and infliximab/adalimumab in osteoarthritis?’ by Wei et al. J Orthop Res. 2016;34:15–16. doi: 10.1002/jor.23095. [DOI] [PubMed] [Google Scholar]
- 17.Martín G, Cañueto J, Santos-Briz A, Alonso G, Unamuno PD, Cruz JJ. Interstitial granulomatous dermatitis with arthritis associated with trastuzumab. J Eur Acad Dermatol Venereol. 2010;24:493–494. doi: 10.1111/j.1468-3083.2009.03428.x. [DOI] [PubMed] [Google Scholar]
- 18.Güler-Yüksel M, Allaart CF, Watt I, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Schaardenburg D, van Krugten MV, Dijkmans BA, Huizinga TW, Lems WF, Kloppenburg M. Treatment with TNF-α inhibitor infliximab might reduce hand osteoarthritis in patients with rheumatoid arthritis. Osteoarthritis Cartilage. 2010;18:1256–1262. doi: 10.1016/j.joca.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Golledge HD. Response to Roustan et al. Evaluating methods of mouse euthanasia on the oocyte quality: Cervical dislocation versus isoflurane inhalation: Animal welfare concerns regarding the aversiveness of isoflurane and its inability to cause rapid death. Lab Anim. 2012;46:358–359. doi: 10.1258/la.2012.012101. author reply 360. [DOI] [PubMed] [Google Scholar]
- 20.Barve RA, Minnerly JC, Weiss DJ, Meyer DM, Aguiar DJ, Sullivan PM, Weinrich SL, Head RD. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: Relevance to human disease. Osteoarthritis Cartilage. 2007;15:1190–1198. doi: 10.1016/j.joca.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013;33:1564–1573. doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurien BT, Scofield RH. Western blotting. Methods. 2006;38:283–293. doi: 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E, Piña-Oviedo S, et al. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009;60:3061–3071. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Zeng L, Zhang T, Liu J, Wang W. Tenuigenin prevents IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-κB signaling pathway. Inflammation. 2016;39:807–812. doi: 10.1007/s10753-016-0309-3. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Abu-Amer Y, O'Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58:49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, et al. Efficacy of direct injection of etanercept into knee joints for pain in moderate and severe knee osteoarthritis. Yonsei Med J. 2015;56:1379–1383. doi: 10.3349/ymj.2015.56.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Guan Y, Tian S, Wang Y, Sun K, Chen Q. Mechanical and IL-1β responsive miR-365 contributes to osteoarthritis development by targeting histone deacetylase 4. Int J Mol Sci. 2016;17:436. doi: 10.3390/ijms17040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z, Wang Y, Piao T, Liu J. Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation. 2016;39:543–549. doi: 10.1007/s10753-015-0278-y. [DOI] [PubMed] [Google Scholar]
- 29.Kunisch E, Kinne RW, Alsalameh RJ, Alsalameh S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and −3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int J Rheum Dis. 2016;19:557–566. doi: 10.1111/1756-185X.12431. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Gao SG, Zhang FJ, Luo W, Xue JX, Lei GH. Effects of osteopontin on the expression of IL-6 and IL-8 inflammatory factors in human knee osteoarthritis chondrocytes. Eur Rev Med Pharmacol Sci. 2014;18:3580–3586. [PubMed] [Google Scholar]
- 31.van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, Gerlag DM, Tak PP. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: Possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi: 10.1186/s13075-014-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, Roux C, Maugars Y, Mulleman D, Lukas C, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2015;74:1697–1705. doi: 10.1136/annrheumdis-2014-205348. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Q, Sun L, Li JJ, An CH. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC Musculoskelet Disord. 2014;15:437. doi: 10.1186/1471-2474-15-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: A review. Clin Exp Rheumatol. 2008;26:139–145. [PubMed] [Google Scholar]
- 35.Chen J, Crawford R, Xiao Y. Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem. 2013;114:245–249. doi: 10.1002/jcb.24362. [DOI] [PubMed] [Google Scholar]
- 36.Young SR, Gerard-O'Riley R, Harrington M, Pavalko FM. Activation of NF-kappaB by fluid shear stress, but not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone. 2010;47:74–82. doi: 10.1016/j.bone.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isozaki T, Kasama T, Takahashi R, Odai T, Wakabayashi K, Kanemitsu H, Nohtomi K, Takeuchi HT, Matsukura S, Tezuka M. Synergistic induction of CX3CL1 by TNF alpha and IFN gamma in osteoblasts from rheumatoid arthritis: Involvement of NF-kappa B and STAT-1 signaling pathways. J Inflamm Res. 2008;1:19–28. doi: 10.2147/jir.s4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu L, Zhang L, Zhu L, Yang D, Li Z, Qin K, Mi X. PI3K/Akt mediates expression of TNF-alpha mRNA and activation of NF-kappaB in calyculin A-treated primary osteoblasts. Oral Dis. 2008;14:727–733. doi: 10.1111/j.1601-0825.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen HW, Lin AH, Chu HC, Li CC, Tsai CW, Chao CY, Wang CJ, Lii CK, Liu KL. Inhibition of TNF-α-induced inflammation by andrographolide via down-regulation of the PI3K/Akt signaling pathway. J Nat Prod. 2011;74:2408–2413. doi: 10.1021/np200631v. [DOI] [PubMed] [Google Scholar]
- 40.Inam A, Shahzad M, Shabbir A, Shahid H, Shahid K, Javeed A. Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-κB, and iNOS levels. Phytomedicine. 2017;32:1–7. doi: 10.1016/j.phymed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Sanchavanakit N, Saengtong W, Manokawinchoke J, Pavasant P. TNF-α stimulates MMP-3 production via PGE2 signalling through the NF-κB and p38 MAPK pathway in a murine cementoblast cell line. Arch Oral Biol. 2015;60:1066–1074. doi: 10.1016/j.archoralbio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Kim JM, Cho HH, Lee SY, Hong CP, Yang Jw, Kim YS, Suh KT, Jung JS. Role of IRAK1 on TNF-induced proliferation and NF-κB activation in human bone marrow mesenchymal stem cells. Cell Physiol Biochem. 2012;30:49–60. doi: 10.1159/000339045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.