Abstract
The direct aerobic coupling reaction of arenes with olefins was successfully achieved by the use of Pd(OAc)2/molybdovanadophosphoric acid (HPMoV) as a key catalyst under 1 atm of dioxygen. This catalytic system could be extended to the coupling reaction of various substituted benzenes with olefins such as acrylates, aclrolein, and ethylene through the direct aromatic C-H bond activation.
Keywords: direct coupling, oxidative coupling, arenes, molybdovanadophosphoric acid
1. Introduction
The transition-metal-catalyzed arylation of olefins with aryl halides or triflates, which referred to as the Mizoroki-Heck reaction, is an important and a highly versatile C-C bond transformation for the synthesis of arene derivatives [1,2]. However, this existing methodology does not avoid the formation of undesired waste salts arising from the use of aryl halides and bases. Therefore, the development of the direct oxidative couplings of arenes with olefins to afford alkenyl arenes without formation of any salts is highly desired, especially from the environmental and industrial point of view [3,4,5,6,7].
The first waste-free oxidative coupling of arenes was achieved by Fujiwara and Moritani by the stoichiometric reaction of benzene with styrene-palladium chloride dimer in AcOH to give stilbenes in 24% yield [8]. Thereafter, the same group presented the catalytic aerobic oxidative Mizoroki-Heck type reaction by using Pd(OAc)2 combined with Cu(OAc)2 under elevated oxygen pressure (50 atm) [9]. After several modified versions of this prototype reactions were reported [10,11,12], several practical advances in the development of the catalytic method was evident in recent years [13,14,15]. For instance, Kitamura and Fujiwara reported that the use of a stoichiometric amount of tert-butyl hydroperoxide as the oxidant in the Pd(OAc)2 combined with benzoquinone as catalysts led to a significant development in more active reoxidation system [13]. Furthermore, Jacobs and coworkers found that the addition of small amounts (4–20 mol %) of Mn(OAc)3 or benzoic acid are effective for the Pd(II)-catalyzed oxidative Mizoroki-Heck type reaction under 8 atm of O2 [14]. This methodology has been recently extended to the Pd (II)-catalyzed C-H bond alkenylation of anilides and heteroarenes such as pyrroles and indoles [15,16,17].
In this review, we summarize recent progress from our laboratory in terms of the aerobic oxidative coupling reaction of arenes with olefins such as acrylates [18,19], α,β-unsaturated aldehydes [20,21] as well as ethylene [22] under ambient dioxygen atmosphere by using Pd(OAc)2/molybdovanado-phosphoric acid (HPMoV) as a key catalyst system.
2. Results and Discussion
2.1. Oxidative Mizoroki-Heck type coupling reaction of arenes with acrylates
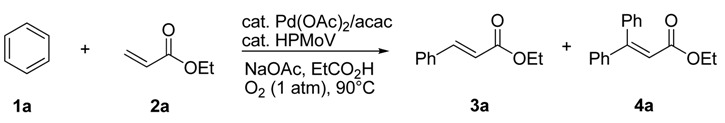
The reaction of benzene (1a) with ethyl acrylate was chosen as a model reaction and carried out under various reaction conditions (Equation 1). The results are shown in Table 1.
 |
(1) |
Table 1.
Oxidative coupling of benzene (1a) with ethyl acrylate (2a) catalyzed by Pd(II)/HPMoV. a
| Entry | HPMoV | Base | Yield/% b | ||
|---|---|---|---|---|---|
| 3a | 4a | 5 | |||
| 1 | H4PMo11VO40·30H2O | NaOAc | 74 | 13 | 4 |
| 2c | H4PMo11VO40·30H2O | NaOAc | 6 | 81 | ndd |
| 3 | H4PMo11VO40·30H2O | None | 6 | ndd | 4 |
| 4 e | H4PMo11VO40·30H2O | NaOAc | 53 | 2 | 8 |
| 5 e,f | H4PMo11VO40·30H2O | NaOAc | 75 | 16 | 1 |
| 6g | H4PMo11VO40·30H2O | NaOAc | 51 | 5 | 16 |
| 7h | H4PMo11VO40·30H2O | NaOAc | 20 | nd d | nd d |
| 8 | None | NaOAc | 9 | nd d | 1 |
| 9 | H5PMo10V2O40·28H2O | NaOAc | 73 | 14 | 5 |
| 10 | H6PMo9V3O40·30H2O | NaOAc | 70 | 13 | 5 |
| 11 | H7PMo8V4O40·28H2O | NaOAc | 62 | 4 | 5 |
| 12 | H3PMo12O40·30H2O | NaOAc | 43 | 1 | 4 |
| 13 | H4PMo11VO40·30H2O | LiOAc | 66 | 5 | 7 |
| 14 | H4PMo11VO40·30H2O | CsOAc | 13 | nd d | 1 |
| 15 | H4PMo11VO40·30H2O | KOAc | 11 | nd d | nd d |
| 16 | H4PMo11VO40·30H2O | NH4OAc | 12 | nd d | nd d |
| 17 i | H4PMo11VO40·30H2O | NaOAc | 74 | 8 | 6 |
| 18 j | H4PMo11VO40·30H2O | NaOAc | 10 | Nd d | 3 |
a A mixture of 1a (30 mmol) and 2a (1.5 mmol) was reacted in the presence of Pd-catalyst (0.1 mmol, 6.7 mol %), HPMoV (0.02 mmol, 1.3 mol %), base (0.08 mmol, 5.3 mol %) and acetylacetone (0.1 mmol, 6.7 mol %) in EtCOOH (5 mL) at 90 °C for 2.5 h. b GC yield based on 2a used. c Reaction time was 5 h. d Not detected by GC. e The reaction was performed in the absence of acetylacetone. f Pd(acac)2 was used as catalyst. g 1a (6 mmol) was used. h AcOH was used instead of EtCOOH. i The reaction was performed under air (1 atm) for 5 h. j The reaction was performed under argon (1 atm).
The coupling reaction of 1a (30 mmol) and 2a (1.5 mmol) catalyzed by Pd(OAc)2 (0.1 mmol, 6.7 mol %) combined with H4PMo11VO40·30H2O (HPMo11V1, 0.02 mmol, 1.3 mol %) proceeded smoothly in the presence of small amounts of acetylacetone (0.1 mmol, 6.7 mol %) and NaOAc (0.08 mmol, 5.3 mol %) under 1 atm of O2 in propionic acid at 90 °C for 2.5 h to give ethyl cinnamate (3a) and ethyl β-phenylcinnamate (4a) in 74% and 13% yields, respectively. In this reaction, ethyl 3-propionyloxyacrylate (5) derived from 2a and propionic acid was obtained in 4% as by-products (entry 1). The product distribution of 3a and 4a was controlled by the reaction time. When the reaction time was prolonged to 5 h, 4a was formed in good yield (81%) as a major adduct (entry 2). Removing of NaOAc or acetylacetone from the present catalytic system resulted in the decrease of the coupling products, 3a and 4a (entries 3 to 4). It is considered that acetylacetone would be functioned as a ligand in the catalyst system, and the addition of acetylacetone is not necessary when Pd(acac)2 was employed in place of Pd(OAc)2 (entry 5). The use of excess amount of 1a is favorable and when the amount of 1a was reduced from 30 mmol to 6 mmol, the yield of 3a decreased to 51% and the yield of 5 was increased from 4% to 16% (entry 6). As for a choice of a solvent in this reaction, the use of acetic acid instead of propionic acid resulted in considerable decrease of the yields of 3a and 4a (entry 7). The yield of coupling products, 3a and 4a, was markedly influenced by the combination of Pd(OAc)2 with molybdovanadophosphoric acids (HPMoV) which serve as reoxidation catalysts of the reduced Pd(0) to Pd(II) during the reaction course. Thus, the reaction was sluggish in the absence of HPMoV (entry 8). The performance of various heteropoly acid including HPMoV as reoxidation catalysts was affected by the vanadium content in the heteropolyacid (entries 9 to 12). The reaction by the use of 12-molybdophosphoric acid (HPMo12) not involving V ion brought about 3a in low yield (52%) (entry 12). In addition, the effect of several alkali metal acetates as a base, was examined. As a result, NaOAc was found to be the best base in the present catalytic system (entry 1 vs. entries 13–16). One of the most important feature of this method is that the reaction smoothly proceeded under 1 atm of O2. Moreover, it is useful from the practical synthetic viewpoint that the reaction tolerated under air in place of pure O2, and 3a was obtained in 74% yield for 5 h (entry 17). However, the reaction in the absence of dixoygen, namely under Ar (1 atm), was difficult to take place (entry 18).
Various arenes (1) were allowed to react with acrylates 2 under the optimized reaction conditions. These results are summarized in Table 2. Benzene (1a) reacted smoothly with several acrylates 2b-2e to give the corresponding cinnamates 3b-3d in 68–73% yields with concomitant formation of β-phenylcinnamate in 11–16% yields (entries 1 to 3). The arylation of 4-phenyl-2-butenone (2e) under these reaction conditions gave 1,1-diphenyl-3-butenone (3e) in 93% yield (entry 4). Reaction of various arenes with ethyl acrylate (2a) was also carried out under optimized reaction conditions (entries 4-10). The reaction of toluene (1b) with 2a afforded the coupling products 3f in 70% total yield as a isomeric mixture consisting of o-3f : m-3f : p-3f = 15:41:44 (entry 5).
Table 2.
Oxidative coupling of various arenes 1 and acrylates 2 catalyzed by Pd(II)/HPMoV. a
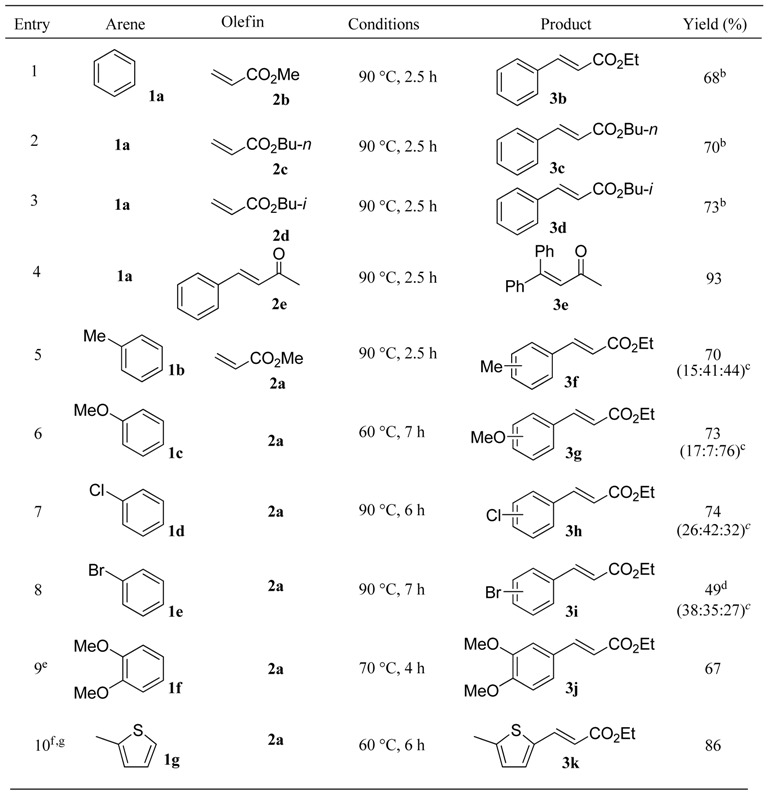 |
a A mixture of arene 1 (30 mmol) and olefins 2 (1.5 mmol) was reacted in the presence of Pd-catalyst (0.1 mmol, 6.7 mol %), H4PMo11VO40·30H2O (0.02 mmol, 1.3 mol %), NaOAc (0.08 mmol, 5.3 mol %) and acetylacetone (0.1 mmol, 6.7 mol %) in EtCOOH (5 mL). b β-methylcinnamates are formed in 11–16% yields as by-products. c Ratio of o:m:p. d Ethyl cinnamate was obtained in 5%. e Arene (5 mmol) was used. g Arene (3 mmol) was used. h 5 wt % Pd(OAc)2/C was used instead of Pd(OAc)2.
Anisole (1c) was subjected to react with 2a at 60 °C to give a mixture of coupling products 3g in 73% total yield (entry 6). The reaction of chlorobenzene (1d) and bromobenzene (1e) with 2a gave the corresponding oxidative coupling products, 3h and 3i exclusively, in 74% and 49% yields, respectively. It is noteworthy that the reaction of 1d and 1e gave oxidative coupling products exclusively, in preference to the products based on the Mizoroki-Heck reaction (entries 7 and 8). The reaction of 1,2-dimethoxybenzene (1f) with 2a afforded ethyl 3,4-dimethoxycinnamate (3j) in 67% yield (entry 9). Unfortunately, the reaction of 2-methyl-thiophene (1g) with 2a under the above-mentioned reaction conditions did not take place at all. Alternatively, Pd(OAc)2 supported on active carbon, Pd(OAc)2/C, was efficient for the coupling of 1g with 2a to give the corresponding coupling product, 3k, in 86% yield (entry 10).
In this reaction, reoxidation step is important to determine the turn-over number (TON) of the present reaction, which was markedly affected by the choice of HPMoV catalysts. Therefore, the reaction of 1a (45 mmol) and 2a (3 mmol) was carried out in the presence of Pd(OAc)2 (0.03 mmol), HPMoV (0.02 mmol), NaOAc (0.08 mmol), acetylacetone (0.03 mmol), and EtCOOH (5 mL) under O2 (1 atm) at 90 °C for 12 h. The results of TONs for the formation of 3a and 4a by using various HPMoV under these conditions were as follows: 19 for H3PMo12O40·30H2O, 76 for H4PMo11VO40·30H2O, 10 for H5PMo10V2O40·28H2O, 8 for H6PMo9V3O40·30H2O, and 6 for H7PMo8V4O40·28H2O.
We previously reported that combined catalysts of Pd(OAc)2 with HPMo11V1 and HPMo12 as the reoxidation catalyst was the most active for the Pd-catalyzed oxidative coupling of benzene to biphenyl under O2 (1 atm) [23]. In the present reaction, the best TON (121) was observed by the reaction in the presence of Pd(OAc)2 combined with a 1:1 mixture of HPMo11V1 and HPMo12 under these reaction conditions.
To obtain mechanistic information on the present reaction, the initial rate of the reaction of benzene 1a with 2a was compared with that of benzene-d6 (1a-d) with 2a under the same conditions as entry 1 in Table 1. It was found that a kinetic isotope effect for the coupling reaction was evident and the rate of the reaction of 1a with 2a was four times faster than that of benzene-d6 (1a-d) with 2a, i.e., kH/kD ≈ 4. The same results (kH/kD ≈ 4) were obtained by the reaction of a 1:1 mixture of 1a and 1a-d with 2a under these conditions. These facts indicate that the cleavage of the C-H bond of the 1a is the rate-determining step in a sequence of reactions.
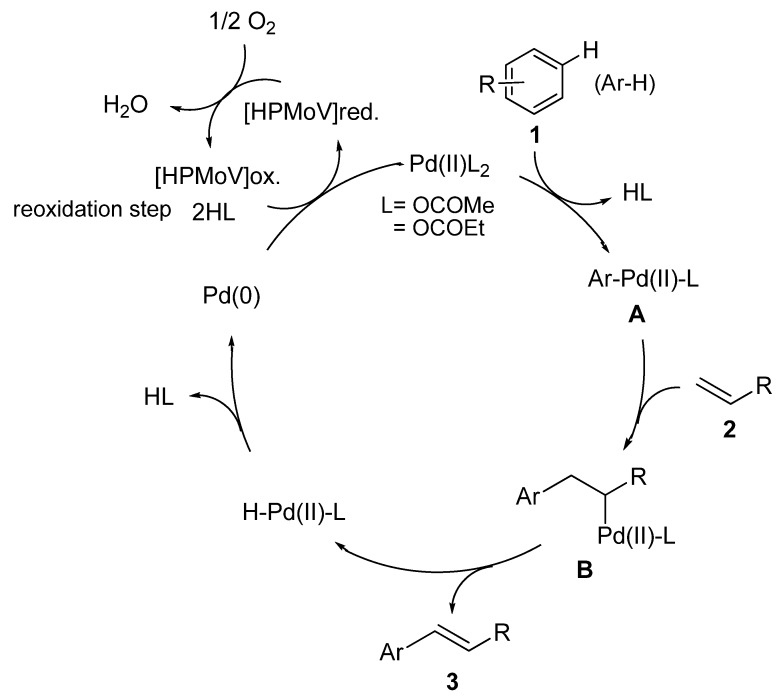
Although a detailed reaction mechanism remains to be further elucidated, it is possible to explain the reaction rationally by a pathway similar to that proposed by Kitamura and Fujiwara [13]. A plausible reaction path is shown in Scheme 1. First, electrophilic attack of a Pd(II) to arene 1 would initiate the reaction, leading to a σ-aryl-palladium (II) intermediate (A), which is considered to be the rate-determining step by judging from the labeled experiment (vide supra). The insertion of olefins like acrylate 2 to σ-aryl-palladium (II) intermediate (A) takes place to give σ-alkyl-palladium (II) intermediate B. Subsequently, β -hydride elimination of the B gives the desired coupling products 3 along with Pd-H intermediate. The Pd-H intermediate is reduced to Pd(0), and the resulting Pd(0) species is reoxidized by [HPMoV]ox. to generate initial Pd(II) catalyst. The [HPMoV]red. is easily oxidized to [HPMoV]ox. with atomospheric dioxygen.
Scheme 1.
A possible reaction path for the coupling of arene with acrylate.
2.2. Oxidative Mizoroki-Heck type coupling of benzenes with α,β-unsaturated aldehydes
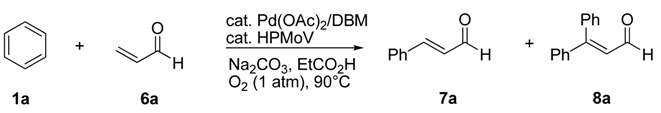
The oxidative coupling reaction of benzene (1a) with α,β-unsaturated aldehydes such as acrolein (6a) was also achieved by slight modifications of the reaction conditions as reported in section 2.1, which leads to cinnamaldehyde (7a) and β-phenyl cinnamaldehyde (8) as products (Equation 2).
 |
(2) |
The results of the reaction of 1a with 6a under various conditions are summarized in Table 3. The best result for the formation of cinnamaldehyde (7a) was attained by the reaction conditions as shown in Table 3, entry 1. Namely, a mixture of 1 (30 mmol) and 6a (1.5 mmol) was allowed to react under the influence of Pd(OAc)2 (0.1 mmol, 6.7 mol %), H4PMo11VO40·26H2O (HPMo11V1, 0.02 mmol, 1.3 mol %), Na2CO3 (0.05 mmol, 3.4 mol %), and dibenzoylmethane (DBM) (0.1 mmol, 6.7 mol %) under O2 (1 atm) in propionic acid (5 mL) at 90 °C for 1.5 h, giving 7a in 59% yield along with 8 (5%). In the present reaction, a trace amount of cinnamic acid was detected by GC. Similar to the reaction of 1a and acrylate 2a (see Section 2.1), prolonged reaction time resulted in dicoupling product 8a as a major product rather than 3 (entries 1–4). The maximum yield of 7a was attained in 1.5 h (entries 1). The best yield of 7a was obtained when the reaction was carried out by using the excess amount of 1a (30 mmol) to 6a (1.5 mmol). However, the yield of 7a was still good to high when the amount of 1 was reduced to 20 mmol (entry 5). The addition of DBM as a ligand was effective in the present reaction and the yield of 7a in the absence of a DBM ligand resulted in a decrease of the yield of 7a to 33% (entry 6). As for a ligand, the use of benzoylacetone led to a similar result as that of DBM (entry 7). When acetylacetone (acacH) which is an effective ligand in the reaction of 1a and 2a (section 2.1) was employed instead of DBM, longer reaction time is needed to obtain the same catalytic activity (entries 8 and 9). The addition of the base is indispensible in the reaction and the reaction in the absence of Na2CO3 resulted in a considerable decrease of the yield of 7a and 8a, and several unidentified products (probably oligomers of 6a) were formed (entry 10). A slight decrease of the yield of 7a was observed when NaOAc was added as a base (entry 11).
Table 3.
Oxidative coupling of benzene (1a) with acrolein (6a) catalyzed by Pd(II)/HPMoV. a
| Entry | HPMoV | Ligand | Time/h | Yield/% b | |
|---|---|---|---|---|---|
| 7a | 8a | ||||
| 1 | H4PMo11VO40·26H2O | DBM | 1.5 | 59 | 5 |
| 2 | H4PMo11VO40·26H2O | DBM | 1 | 26 | nd d |
| 3 | H4PMo11VO40·26H2O | DBM | 2 | 45 | 18 |
| 4 | H4PMo11VO40·26H2O | DBM | 3 | 17 | 40 |
| 5c | H4PMo11VO40·26H2O | DBM | 1.5 | 53 | 6 |
| 6 | H4PMo11VO40·26H2O | None | 1.5 | 33 | nd d |
| 7 | H4PMo11VO40·26H2O | benzoylacetone | 1.5 | 52 | 6 |
| 8 | H4PMo11VO40·26H2O | acacH | 1.5 | 48 | 1 |
| 9 | H4PMo11VO40·26H2O | acacH | 2 | 54 | 8 |
| 10e | H4PMo11VO40·26H2O | acacH | 2 | 3 | nd d |
| 11f | H4PMo11VO40·26H2O | acacH | 2 | 44 | 2 |
| 12 | H5PMo10V2O40·28H2O | DBM | 1.5 | 54 | 6 |
| 13 | H6PMo10V3O40·30H2O | DBM | 1.5 | 47 | 6 |
| 14 | H7PMo8V4O40·28H2O | DBM | 1.5 | 35 | 1 |
| 15 | H5PW10V2O40·27H2O | DBM | 1.5 | ndd | nd d |
| 16 | H3PMo12O40·30H2O | DBM | 1.5 | 24 | nd d |
a A mixture of 1a (30 mmol) and 6a (1.5 mmol) was reacted in the presence of Pd(OAc)2 (0.1 mmol, 6.7 mol %), HPMoV (0.02 mmol, 1.3 mol %), Na2CO3 (0.05 mmol, 3 mol %) and ligand (0.1 mmol, 6.7 mol %) under O2 (1 atm) in EtCOOH (5 mL). b GC yield based on 6a used. c 1a (20 mmol) was used. d Not detected by GC. e The reaction was performed in the absence of Na2CO3. f NaOAc (0.08 mmol) was used instead of Na2CO3.
Furthermore, the effect of the HPMoV was examined under the reaction conditions as entry 1, Table 3 (entries 12–16). The best result was obtained by using H4PMo10VO40·26H2O to afford 7a in 59% yield as shown in entry 1. Increase of the V content in the HPMoV was found to bring about a decrease of the yields of 7a (entries 12–14). Moreover, vanadotungstphosphoric acid (H5PW10V2O40·27H2O) did not catalyze the present coupling reaction at all (entry 15). H3PMo12O40·30H2O not containing a vanadium atom was less efficient catalyst than HPMoV (entry 16).
In this reaction, acrolein (6a) was rapidly consumed when the reaction started, and the coupling product 7a was reached maximum after 1.5 h, and gradually decreased because of further coupling with 1a leading to dicoupling product 8a. To confirm this sequential pathway for the formation of 8a, an independent reaction of 7a with 1 under these conditions gave 8a in 61% yield.
Several substituted benzenes were reacted with 2 under varying conditions and the results are summarized in Table 4.
Table 4.
Oxidative coupling of arenes 1 with acrolein (6a) catalyzed by Pd(II)/HPMoV. a
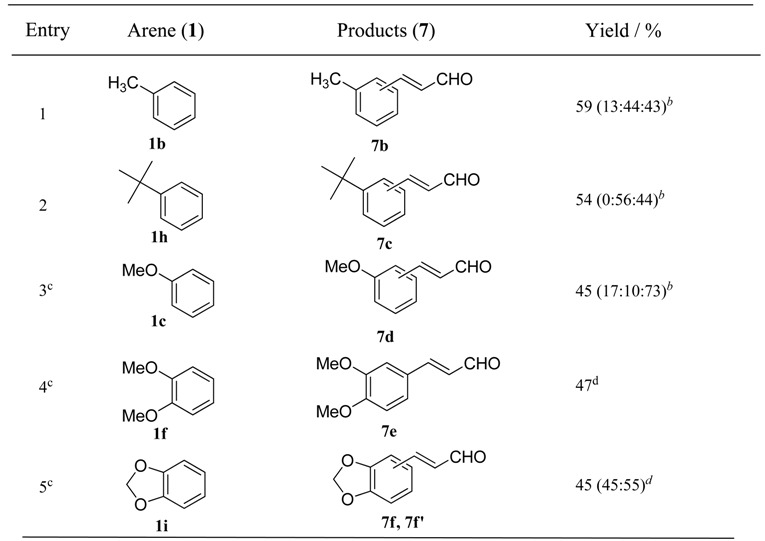 |
a A mixture of 1 (30 mmol) and 6a (1.5 mmol) was reacted in the presence of Pd(OAc)2 (0.1 mmol, 6.7 mol %), H4PMo11VO40·26H2O (0.02 mmol, 1.3 mol %), Na2CO3 (0.05 mmol, 3 mol %) and DBM (0.1 mmol, 6.7 mol %) under O2 (1 atm) in EtCOOH (5 mL) at 90 °C, 1.5 h. b Ratio of o-:m-:p-isomer. c 1 (10 mmol) was reacted with 6a (1.5 mmol) by Pd(OAc)2 (0.1 mmol, 6.7 mol %), H4PMo11VO40·26H2O (0.02 mmol, 1.3 mol %), Na2CO3 (0.05 mmol, 3 mol %) and acacH (0.1 mmol, 6.7 mol %) under O2 (1 atm) in EtCOOH (5 mL) at 90 °C, 1 h. d 2,3-Dimethoxycinnamaldehyde was obtained 2% yield as by-product. e Ratio of 2,3-methylenedioxy cinnamaldehyde (7f) to 3,4-methylenedioxy cinnamaldehyde (7f’).
The reaction of toluene (1b) with 6a afforded a mixture of structural isomers 7b (o:m:p = 13:44:43) in 59% yield along with small amounts of dicoupling products (entry 1). In the reaction with t-butylbenzene (1h) having bulky alkyl group, m- and p-products were obtained and o-product was not detected at all (entry 2). Anisole (1c) was subjected to react with 6a to give a mixture of the corresponding coupling products 7d (o:m:p = 17:10:73) in 45% total yield (entry 3). The reaction of 1,2-dimethoxybenzene (1f) with 6a proceeded in high regioselectivity to produce 3,4-dimethoxycinnamaldehyde (7e) (45%), and the yield of the isomer, 2,3-dimethoxycinnamaldehyde, was found to be less than 2% (entry 4). In contrast, the reaction of 1,2-methylenedioxybenzene (1i) with 6a led to a 45:55 mixture of 2,3- and 3,4-methylenedioxycinnnamaldehydes (7f and 7f’) in 45% yield (entry 5).
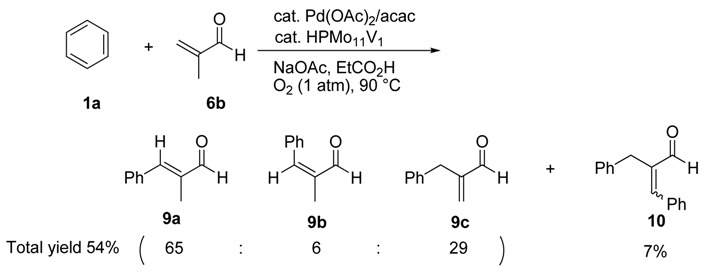
The oxidative coupling of methacrolein (6b) with 1a was carried out under the conditions as entry 1, Table 3 (Equation 3).
 |
(3) |
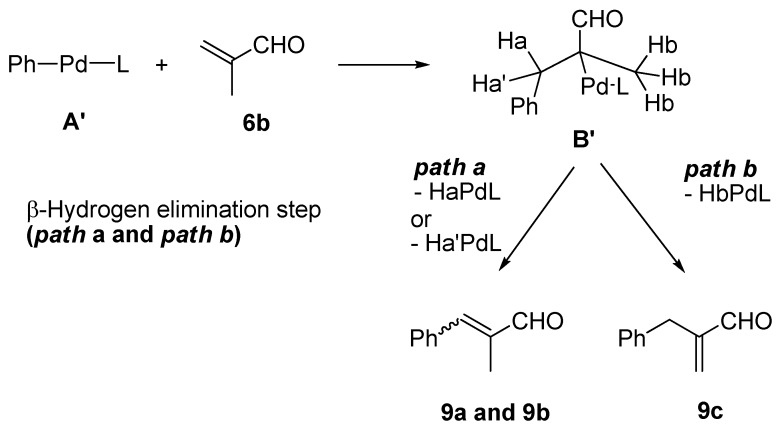
In this reaction, a mixture of three isomeric coupling products consisting of 9a, 9b and 9c were obtained in 54% yield (9a:9b:9c = 65:6:29) along with the dicoupling product 10 (7%). Based on the reaction mechanism as illustrated in Scheme 1, the reaction would proceed through a phenyl-palladium intermediate (B’) formed by the insertion of phenyl-palladium σ-complex (A’) to 6b. Therefore, 9a, 9b and 9c can be formed by the following β-Hydrogen elimination step via path a (9a and 9b: elimination of Ha or Ha’) and path b (9c: elimination of Hb) (Scheme 2) [12].
Scheme 2.
Formation of 9a, 9b, and 9c from phenyl-palladium intermediate (B’).
2.3. Oxidative Mizoroki-Heck type coupling of benzenes with ethylene
Synthesis of styrene by the oxidative arylation of ethylene with benzene under mild reaction conditions is also an attractive in organic synthesis, because the styrene is produced by the Friedel-Crafts reaction of benzene with ethylene followed by dehydrogenation of the resulting ethylbenzene. We performed the aerobic oxidative coupling reaction of benzene (1a) with ethylene (11) catalysed by the Pd(OAc)2/H4PMo11VO40·15H2O system, and styrene (12) was obtained as a major adduct. The results under various reaction conditions are summarized in Table 5.
 |
(4) |
Table 5.
Oxidative coupling of benzene (1a) with ethylene (11) catalyzed by Pd(II)/HPMoV. a
| entry | HPMoV | Base | TONb (μmol) | |||
|---|---|---|---|---|---|---|
| 12 | 13 | 14 | 15 | |||
| 1 | H4PMo11VO40·15H2O | NaOAc | 20 (202) | 5.2 (25) | 5.0 (50) | 3.0 (30) |
| 2c | H4PMo11VO40·15H2O | NaOAc | 4.6 (46) | ndd | 8.3 (82) | 4.5 (45) |
| 3e | H4PMo11VO40·15H2O | NaOAc | 7.5(75) | 0.9 (4) | 6.0 (59) | 3.9 (39) |
| 4f | H4PMo11VO40·15H2O | NaOAc | 14 (138) | 4.8 (24) | 3.8 (38) | 4.9 (49) |
| 5 | H4PMo11VO40·15H2O | None | 1.6 (16) | ndd | 2.0 (20) | ndd |
| 6 | H4PMo11VO40·15H2O | LiOAc | 9.1 (90) | 2.6 (13) | 2.9 (29) | 2.9 (29) |
| 7 | H4PMo11VO40·15H2O | KOAc | 5.9 (59) | 0.8 (4) | 1.7 (17) | 1.4 (14) |
| 8g | H4PMo11VO40·15H2O | Na2CO3 | 19 (188) | 9.7 (48) | 5.2 (52) | 7.1 (71) |
| 9h | H4PMo11VO40·15H2O | NaOAc | 14 (142) | 4.0 (20) | 3.4 (33) | -i |
| 10j | H4PMo11VO40·15H2O | NaOAc | 12 (119) | ndd | 19 (190) | -i |
| 11k | H4PMo11VO40·15H2O | NaOAc | 5.3 (53) | ndd | 24 (243) | -i |
| 12l | H4PMo11VO40·15H2O | NaOAc | 14 (137) | 4.9 (24) | 5.2 (52) | -i |
| 13 | H5PMo10V2O40·28H2O | NaOAc | 16 (154) | 4.2 (21) | 4.4 (44) | 3.8 (38) |
| 14 | H6PMo9V3O40·30H2O | NaOAc | 17 (166) | 7.7 (38) | 2.9 (29) | 4.0 (40) |
| 15 | H7PMo8V4O40·28H2O | NaOAc | 19 (192) | 4.9 (2.4) | 6.4 (64) | 3.6 (36) |
| 16 | H3PMo12O40·30H2O | NaOAc | 22 (223) | 11 (53) | 2.9 (29) | 0.3 (3) |
| 17m | H3PMo12O40·30H2O | NaOAc | 42 (420) | 4.4 (22) | 3.5 (35) | ndd |
| 18m,n | H3PMo12O40·30H2O | NaOAc | 100 (1003) | 67 (335) | 3.9 (39) | ndd |
| 19 | H3PMo11WO40·27H2O | NaOAc | 24 (239) | 12 860) | 3.0 (30) | ndd |
| 20 | H3PMo10W2O40·29H2O | NaOAc | 23 (228) | 7.8 (39) | 2.8 (28) | ndd |
| 21 | H4SiMo12O40·27H2O | NaOAc | 22 (218) | 7.8 (39) | 2.9 (29) | ndd |
| 22 | H5PW10V2O40·27H2O | NaOAc | 1.5 (15) | trace | ndd | ndd |
a A mixture of benzene (1a), Pd(OAc)2 (10 μmol), H4PMo11VO40·15H2O (10.3 mg, ca. 5 μmol), NaOAc (25 μmol), dibenzoylmethane (DBM) (30 μmol) was allowed to react under 0.9 atm of etyhylene (11) and 1.6 atm of air in EtCOOH (2 mL) at 90 °C for 8 h. b Turnover number (TON) based on Pd(OAc)2 used. c The reaction was performed in the absence of dbm. d Not detected by GC. e Dbm (10 μmol) was used. f Acetylacetone was used instead of dbm. g Na2CO3 (13 μmol) was used. h The reaction was performed under ethylene/air = 0.5 atm/1 atm. i Not determined. j The reaction was performed under ethylene/air = 3.6 atm/6.4 atm. k The reaction was performed under ethylene/air = 5.4 tm/9.6 atm. l The reaction was performed under ethylene/air = 0.9 atm/29.1 atm. m 1a (120 μmol) was reacted with 0.9 atm of 11 in the presence of Pd(OAc)2 (10 μmol), HPMo11V1 (6.8 μmol), NaOAc (32 μmol), and dbm (120 μmol) in EtCOOH (8 mL) under 1.6 atm of air using a 120 mL autoclave. n The reaction was performed at 120 °C.
The reaction of benzene (1a) (30 mmol) was performed with a mixed gas of ethylene (11) (0.9 atm, ca. 2.0 mmol) and air (1.6 atm) in the presence of Pd(OAc)2 (10 μmol, ca. 0.5 mol % based on 11), H4PMo11VO40·15H2O (10.3 mg, ca. 5 μmol), NaOAc (25 μmol), dibenzoylmethane (DBM) (30 μmol) and propionic acid (2 mL) at 90 °C for 8 h. As a result, styrene (12) was obtained in 202 μmol corresponding to 20 turnover numbers of Pd (TON = 20) with concomitant formation of trans-stilbene (13) in 25 μmol (TON = 5.2), and vinyl propionate (14) in 50 μmol (TON = 5.0), and phenol (15) in 30 μmol (TON = 3.0) (entry 1). Similar to the result reported in the reaction of benzene with acrylates and acrolein, the addition of ligand such as DBM brought about the improvement of the catalytic activity. The amount of DBM was reduced from 30 μmol to 10 μmol, the yield of 12 was decreased to 75 μmol (TON = 7.5) (entry 3). The reaction in the absence of DBM gave 12 in low yield and the catalyst was deactivated by forming the Pd black during the reaction course. As a ligand, acetylacetone (acacH) showed the similar catalytic activity for the formation of 12 (entry 4). The addition of the base is necessary to achieve the reaction and the reaction was sluggish in the absence of NaOAc (entry 5). The use of other base such as LiOAc or KOAc instead of NaOAc resulted in the lower TON of Pd for the formation of 12 (entries 6 and 7). Alternatively, Na2CO3 can also be used as an efficient base in the present reaction (entry 8).
The reaction was markedly influenced by the concentrations of ethylene and air, and the coupling reaction of 1a with 11 was conducted under several varying ethylene/air pressures (entriy 1 vs. entries 9–12). Under the reaction of ethylene/air = 0.9 atm/1.6 atm, TON of Pd for 12 attained maximum (TON = 20) (entry 1). Thus, when the reaction of was performed under lower pressure of ethylene (ethylene/air = 0.5 atm/1 atm), the yield of 12 was decreased to 142 μmol (TON = 14) (entry 9). Furthermore, the amount of vinyl propionate 14 was increased with increasing of ethylene pressure. The yields of 14 was 190 μmol (TON = 19) and 243 μmol (TON = 24) when the reaction was carried out under ethylene/air = 3.6 atm/6.6 atm and 5.4/9.6 atm, respectively (entries 10–11). The reaction under high pressure of air (29.1 atm) with 0.9 atm of ethylene resulted in slightly lower catalytic activity (TON = 14) (entry 12).
As mentioned in the previous section, the role of HPMoV is important as the reoxidation system of the reduced Pd(0) catalyst Therefore, the reaction was performed by the use of HPAs under these conditions. In this reaction, HPAs having various V contents such as H5PMo10V2O40·28H2O, H6PMo9V3O40·30H2O and H7PMo8V4O40·28H2O can be used as reoxidation catalysts and afforded 12 in comparable yields with that of H4PMo11VO40·15H2O (entries 13 to 15). It was found that H3PMo12O40·30H2O not including V ion was found to serve a good reoxidation catalyst (entries 16–18) and the highest total TON of Pd for the formation of 12 and 13 was reached to 167, under the optimum reaction conditions (entry 18). In addition, various heteropoly acids such as H4PMo11WO40·27H2O, H3PMo10W2O40·29H2O, and H4SiMo12O40·27H2O showed similar catalytic activities (entries 19–21). However, the heteropoly acid without molybdenum ion such as H5PW10V2O40·27H2O was found to be inactive for the present reaction (entry 22). It is noteworthy that phenol (15) was not produced in the reaction using heteropoly acids not including V ion. By judging of these results, it is considered that molybdenum ion in the heteropoly acids is an essential component to promote the reoxidation of the reduced Pd(0) to Pd(II) for the present reaction.
3. Experimental
3.1. General procedure for oxidative coupling of benzene (1a) with ethyl acrylate (2a) (entry 1, Table 1)
A solution of Pd(OAc)2 (0.1 mmol), H4PMo11V1O40·30H2O (HPMo11V1) (46.7 mg, ca. 0.02 mmol), NaOAc (0.08 mmol), acetylacetone (0.1 mmol), benzene (1a) (30 mmol) and ethyl acrylate (2a) (1.5 mmol) in propionic acid (5 mL) was placed in a round bottom flask (30 mL) equipped with a balloon filled with O2, and the mixture was allowed to react under stirring at 90 °C for 2.5 h. The reaction gave ethyl cinnamate (3a), β-phenylcinnamate (4a) and 3-propionylacrylate (5) in 74%, 14% and 5% yields, respectively. All yields were determined by GLC analysis using nonane as internal standard. The products were characterized by 1H- and 13C-NMR and GC-MS, respectively.
3.2. General procedure for oxidative coupling of benzene (1) with ethylene (11) (entry 1 in Table 5)
Caution: In order to avoid an explosion of a mixture of ethylene and air, the reaction was carried out at the ethylene concentration deviated from explosion limits ranging in 3.1 to 32%. To a 50 mL stainless steel autoclave equipped with a magnetic stir bar were placed Pd(OAc)2 (10 μmol), H4PMo11VO40·15H2O (10.4 mg, ca. 5 μmol), NaOAc (25 μmol), dibenzoylmethane (DBM) (30 μmol), and benzene (1a) (30 mmol) and propionic acid (2 mL). To the autoclave was introduced with 0.9 atm of ethylene gas (11) and 1.6 atm of air, and the mixture was stirred at 90 °C for 8 h. All yields were detected by GLC analysis using dodecane as internal standard. The products were identified through a comparison of these analytical data with those of authentic samples.
4. Conclusions
In conclusion, we have shown the first direct oxidative coupling of benzenes with acrylates, α,β-unsaturated aldehydes, and ethylene under atmospheric dioxygen and air by using Pd(OAc)2 combined with HPMoV. In this catalytic system, addition of ligand such as acetylacetone (acacH) and dibenzoylmethane (DBM) was effective to suppress the Pd(0) black precipitation. All the presented oxidative coupling reactions in this review uses molecular oxygen as terminal oxidant. Therefore, these reactions provide very useful organic transformations from environmental and economical points of view.
Acknowledgments
We acknowledge that these studies were carried out in collaboration with co-workers at Kansai University listed in the references. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas “Advanced Molecular Transformation of Carbon Resources”, KAKENHI (No. 13853008 and 15036265) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and “High-Tech Research Center” Project for Private Universities: matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, 2005–2009. We also thank Nippon Inorganic Colour & Chemical Co. Ltd. for a gift of heteropoly acids.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Mizoroki T., Mori K., Ozaki A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971;44:581. doi: 10.1246/bcsj.44.581. [DOI] [Google Scholar]
- 2.Heck R.F., Nolley J.P., Jr. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972;37:2320–2322. doi: 10.1021/jo00979a024. [DOI] [Google Scholar]
- 3.Gooßen L., Gooßen K. Waste-minimized Mizoroki-Heck Reactions. In: Oestreich M., editor. The Mizoroki-Heck Reaction. Wiley; Chichester, UK: 2009. pp. 163–178. [Google Scholar]
- 4.Trepohl V.T., Oestreich M. Palladium-catalyzed Arylation Reactions Of Alkenes (Mizoroki-Heck Reaction And Related Processes) In: Ackermann L., editor. Modern Arylation Methods. Wiley-VCH; Weinheim, Germany: 2009. pp. 221–269. [Google Scholar]
- 5.Leeuwen P.W.N.M., de Vries J.G. Palladium-catalyzed Oxidative Vinylation. In: Dyker G., editor. Handbook of C-H Transformation. Volume 1. Wiley-VCH; Weinheim, Germany: 2005. pp. 203–212. [Google Scholar]
- 6.Fujiwara Y., Kitamura T. Fujiwara reaction: Palladium-catalyzed hydroarylations of alkynes. In: Dyker G., editor. Handbook of C-H Transformation. Volume 1. Wiley-VCH; Weinheim, Germany: 2005. pp. 194–202. [Google Scholar]
- 7.Jia C., Kitamura T., Fujiwara Y. Catalytic functionalization of arenes and alkanes via C-H bond activation. Acc. Chem. Res. 2001;34:633–639. doi: 10.1021/ar000209h. [DOI] [PubMed] [Google Scholar]
- 8.Moritani I., Fujiwara Y. Aromatic substitution of styrene–palladium chloride complex. Tetrahedron Lett. 1967:1119–1122. doi: 10.1016/S0040-4039(00)90648-8. [DOI] [Google Scholar]
- 9.Fujiwara Y., Moritani I., Danno S., Asano R., Teranishi S. Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate. J. Am. Chem. Soc. 1969;91:7166–7169. doi: 10.1021/ja01053a047. [DOI] [PubMed] [Google Scholar]
- 10.Shue R.S. Catalytic coupling of aromatics and olefins by homogeneous palladium (II) compounds under oxygen. Chem. Commun. :1510–1511. doi: 10.1039/c29710001510. [DOI] [Google Scholar]
- 11.Maruyama O., Yoshidomi M., Fujiwara Y., Taniguchi H. Pd(II)-Cu(II)-catalyzed synthesis of mono- and dialkenyl-substituted five menbdered aromatic heterocycles. Chem. Lett. :1229–1230. [Google Scholar]
- 12.Tsuji J., Nagashima H. Palladium-catalyzed oxidative coupling of aromatic compounds with olefins using tert-butyl perbenzoate as a hydrogen acceptor. Tetrahedron. 1984;40:2699–2702. doi: 10.1016/S0040-4020(01)96888-7. [DOI] [Google Scholar]
- 13.Jia C., Lu W., Kitamura T., Fujiwara Y. Highly efficient Pd-catalyzed coupling of arenes with olefins in the presence of tert-butyl hydroperoxide as oxidant. Org. Lett. 1999;1:2097–2100. doi: 10.1021/ol991148u. [DOI] [Google Scholar]
- 14.Dams M., de Vos D.E., Celen S., Jacobs P.A. Toward waste-free production of Heck products with a catalytic palladium system under oxygen. Angew. Chem. Int. Ed. 2003;42:3512–3515. doi: 10.1002/anie.200351524. [DOI] [PubMed] [Google Scholar]
- 15.Boele M.D.K., van Strijdonck G.P.F., de Vries A.H.M., Kamer P.C.J., de Vries J.G., van Leeuwen P.W.N.M. Selective Pd-catalyzed oxidative coupling of anilides with olefins through C-H bond activation at room temperature. J. Am. Chem. Soc. 2002;124:1586–1587. doi: 10.1021/ja0176907. [DOI] [PubMed] [Google Scholar]
- 16.Beck E.M., Grimster N.P., Hatley R., Gaunt M.J. Mild aerobic oxidative palladium(II) catalyzed C-H bond functionalization: regioselective and switchable C-H alkenylation and annulations of pyrroles. J. Am. Chem. Soc. 2006;128:2528–2529. doi: 10.1021/ja058141u. [DOI] [PubMed] [Google Scholar]
- 17.Grimster N.P., Gauntlett C., Godfrey C.R.A., Gaunt M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. 2005;44:3125–3129. doi: 10.1002/anie.200500468. [DOI] [PubMed] [Google Scholar]
- 18.Yokota T., Tani M., Sakaguchi S., Ishii Y. Direct coupling of benzene with olefin catalyzed by Pd(OAc)2 combined with heteropolyoxometalate under dioxygen. J. Am. Chem. Soc. 2003;125:1476–1477. doi: 10.1021/ja028903a. [DOI] [PubMed] [Google Scholar]
- 19.Tani M., Sakaguchi S., Ishii Y. Pd(OAc)2-catalyzed oxidative coupling of benzenes with olefins in the presence of molybdovanadophosphoric acid under atmospheric dioxygen and air. J. Org. Chem. 2004;69:1221–1226. doi: 10.1021/jo035568f. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T., Sakaguchi S., Ishii Y. Oxidative coupling of benzenes with α,β-unsaturated aldehydes by the Pd(OAc)2/molybdovanadophosphoric acid/O2 system. J. Org. Chem. 2005;70:5471–5474. doi: 10.1021/jo050534o. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T., Sakaguchi S., Ishii Y. Oxidative Coupling Of Benzenes with A,B-Unsaturated Aldehydes By Pd(Oac)2/Hpmov/O2 System. In: Roberts S.M., Whittall J., editors. Catalysis for Fine Chemical Synthesis. Volume 5. Wiley; Chichester, UK: 2007. pp. 275–278. [Google Scholar]
- 22.Yamada T., Sakakura A., Sakaguchi S., Obora Y., Ishii Y. Oxidative arylation of ethylene with benzene catalyzed by Pd(OAc)2/heteropoly acid/O2 system. New J. Chem. 2008;32:738–742. doi: 10.1039/b716425d. [DOI] [Google Scholar]
- 23.Yokota T., Sakaguchi S., Ishii Y. Aerobic oxidation of benzene to biphenyl using a Pd(II)/molybdovanadophosphoric acid catalytic system. Adv. Synth. Catal. 2002;344:849–854. doi: 10.1002/1615-4169(200209)344:8<849::AID-ADSC849>3.0.CO;2-Q. [DOI] [Google Scholar]