Abstract
Single electron transfer (SET)-photochemical decarboxylation of free carboxylic acids was performed in a polar solvent using several arenes such as phenanthrene, naphthalene, 1-methylnaphthalene, biphenyl, triphenylene, and chrysene in the presence of various electron acceptors such as 1,2-, 1,3-, and 1,4-dicyanobenzenes, methyl 4-cyanobenzoate, and 1,4-dicyanonaphthalene. The decarboxylation reaction was influenced by the arenes, electron acceptors, and solvent. The best result was achieved by the photoreaction using biphenyl and 1,4-dicyanonaphthalene in aqueous acetonitrile.
Keywords: photochemical decarboxylation, single electron transfer, free carboxylic acid
1. Introduction
Decarboxylation of carboxylic acids (RCO2H) is one of the most important functional group transformations in organic synthesis, because carboxylic acids are found widely in Nature and are also commercially available. This transformation is usually performed by radical methods such as the Kolbe electrolysis and the Barton decarboxylation. The Kolbe electrolysis involves the anodic oxidation of a carboxylate ion (RCO2–) in which alkyl radicals are formed via the decarboxylation of carboxy radicals [1]. This reaction has been successfully utilized for C-C bond formation; however, it is limited in scope to producing the dimer (R-R) or the solvent adduct (ROH or ROR’) via further oxidation. The most efficient method is the Barton decarboxylation that involves the formation of alkyl radicals by homolysis of thiohydroxamic esters under either thermal or photochemical conditions [2,3]; however, this requires the esterification of carboxylic acids to thiohydroxamic esters as precursors. In addition to these methods, free carboxylic acids are also known to undergo decarboxylation via photoinduced electron transfer (PET) in the presence of various electron acceptors such as iminium salts [4], acridine [5], phthalimide [6,7], 1-cyanonaphthalene [8], and tetracyanobenzene [9] or heterogeneous photocatalysts such as TiO2 [10]. However, the carboxylic acids used in these cases have been limited to aryl, vinyl, and aryloxy acetic acids, which are relatively easy to oxidize. Thus, the generation of alkyl radicals from free carboxylic acids under mild conditions is still desired.
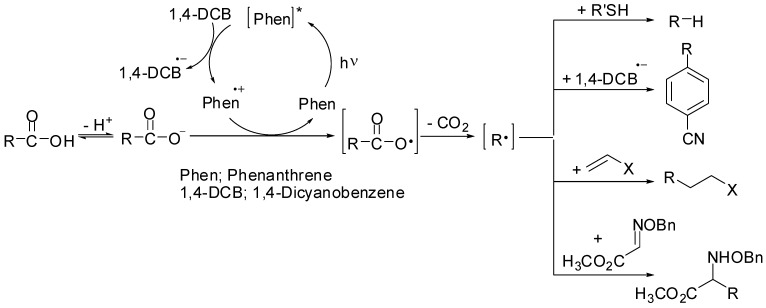
We have recently reported that the decarboxylation of free carboxylic acids by the photogenerated cation radical of phenanthrene (Phen) in a redox-photosensitized reaction system [11,12,13] produced alkyl radicals (Scheme 1) [14,15,16,17]. This process is promoted by a single electron transfer (SET) from the carboxylate ion to the cation radical of Phen formed by PET with 1,4-dicyanobenzene (1,4-DCB). This leads to the formation of the carboxy radical, which is then decarboxylated to produce an alkyl radical. The generated alkyl radicals react with the thiol [14], the anion radical of 1,4-DCB [15], alkenes [16], and oxime ether [17] to yield the corresponding reduction, substitution, and addition products, respectively. Thus, this photoreaction was proven to provide an efficient method for generating alkyl radicals from free aliphatic carboxylic acids under mild conditions. In this study, we endeavored to investigate the effects of solvent, electron acceptor, and arene on the photochemical decarboxylation.
Scheme 1.
Decarboxylation of free carboxylic acid with the photogenerated cation radical of phenanthrene.
2. Results and Discussion
2.1. Solvent Effect
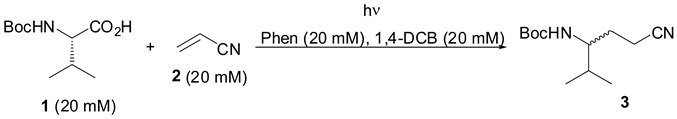
Initially, photodecarboxylative addition of N-Boc-l-valine 1 (Boc = tert-butoxycarbonyl) to acrylonitrile (2) in a variety of solvents was examined, as shown in Table 1. Excitation under an argon atmosphere of aqueous acetonitrile solutions (CH3CN/H2O = 9:1) containing Phen (20 mM), 1,4-DCB (20 mM), 1 (20 mM), and 2 (20 mM) with a 100-W high-pressure mercury lamp through a Pyrex filter (>300 nm) for 6 h at room temperature afforded 3 as a racemic mixture in 85% yield, along with recovery of Phen and 1,4-DCB (>90%) (entry 1 in Table 1). Catalytic amounts of Phen and 1,4-DCB (5 or 1 mM) under the same conditions required a long irradiation time (12 or 15 h) to produce a good yield of 3 (entries 2 and 3). The use of dry acetonitrile, DMF, methanol, and DMSO in place of aqueous acetonitrile as a solvent decreased the yield of 3 (entries 4–7), and less polar solvents such as 1,4-dioxane, THF, and benzene failed to produce the adduct 3, with near-quantitative recovery of 1 (entries 8–10). Thus, the photochemical decarboxylation required a polar medium for forming the cation radical of Phen, and a higher yield of 3 was observed in aqueous acetonitrile.
Table 1.
Solvent effect on photoreaction of 1 and 2 via decarboxylation.
| Entry | Solvent | Yield of 3 / %a |
| 1 | CH3CN/H2O = 9:1 | 85 |
| 2b | 77 | |
| 3c | 60 | |
| 4 | CH3CN | 53 |
| 5 | DMF | 68 |
| 6 | CH3OH | 32 |
| 7 | DMSO | 13 |
| 8 | 1,4-Dioxane | 0 |
| 9 | THF | 0 |
| 10 | Benzene | 0 |
a Isolated yield. b Conditions: Phen (5 mM), 1,4-DCB (5 mM), 12 h.cConditions: Phen (1 mM), 1,4-DCB (1 mM), 15 h.
2.2. Dependence on Electron Acceptor and Arene
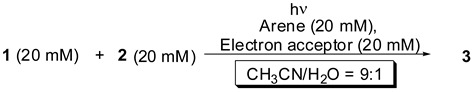
Next, the photoreaction of 1 with 2 was examined using various electron acceptors and arenes (Table 2). Photodecarboxylation with 1,2-dicyanobenzene, 1,3-dicyanobenzene, methyl 4-cyano-benzoate, or 1,4-dicyanonaphthalene (1,4-DCN) as an electron acceptor in the presence of Phen occurred readily in aqueous acetonitrile (entries 1–4). In the case of methyl 4-cyanobenzoate, its low ability as an electron acceptor caused a decrease in the yield of 3 (entry 3). When a variety of arenes such as naphthalene, 1-methylnaphthalene, and biphenyl were used with 1,4-DCB, the adduct 3 was also obtained in a yield similar to that obtained with Phen and 1,4-DCB (entries 5–7); however, the use of pyrene (entry 8) did not produce 3. This result indicated that a lower oxidation potential of the arene than that of the carboxylate ion resulted in no occurrence of SET between the cation radical of the arene and the carboxylate ion. In fact, the oxidation potential of an aliphatic carboxylate ion such as the hexanoate ion (+1.16 V vs. SCE in acetonitrile) [18] is similar to that of pyrene (+1.16 V vs. SCE in acetonitrile), yet the cation radical of pyrene did not oxidize the carboxylate ion in acetonitrile [19]. Thus, the decarboxylation was dependent on the oxidation potential of the arene. Similar photoreactions using arenes in the presence of 1,4-DCN also led to the formation of the adduct 3 in a yield similar to that obtained with Phen and 1,4-DCN (entries 9–11), except in the case of pyrene (entry 12). The use of 1,4-DCN as an electron acceptor led to the competitive absorption of arene and 1,4-DCN at 313 nm; however, the yield of 3 similar to that obtained with 1,4-DCB was observed. It is noteworthy that the yield of 3 was improved by using biphenyl and 1,4-DCN (entry 11).
Table 2.
Photoreaction of 1 and 2 using several arenes and electron acceptors in aqueous acetonitrile.
| Entry | Arene | Oxidation potential of arene / Va | Electron acceptor | Yield of 3 / %b |
| 1 | Phenanthrene | + 1.50 | 1,2-DCB | 73 |
| 2 | 1,3-DCB | 80 | ||
| 3 | Methyl 4-cyanobenzoate | 64 | ||
| 4 | 1,4-DCN | 82 | ||
| 5 | Naphthalene | + 1.70 | 1,4-DCB | 77 |
| 6 | 1-Methylnaphthalene | + 1.43 | 73 | |
| 7 | Biphenyl | 87 | ||
| 8 | Pyrene | + 1.16 | 0 | |
| 9 | Naphthalene | 1,4-DCN | 75 | |
| 10 | 1-Methylnaphthalene | 80 | ||
| 11 | Biphenyl | 93 | ||
| 12 | Pyrene | 0 |
aOxidation potentials vs. SCE in acetonitrile. Values compiled in Ref. [20]. bIsolated yield.
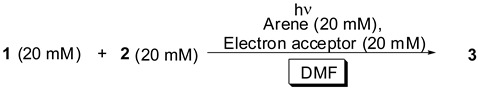
The other arenes such as triphenylene and chrysene did not dissolve in aqueous acetonitrile, but dissolved in DMF, which led us to investigate the photoreaction using arenes with 1,4-DCB or 1,4-DCN in DMF. Table 3 shows that the photoreaction of 1 using triphenylene and chrysene in the presence of 1,4-DCB in DMF proceeded (entries 1 and 2), and, in the case of chrysene, a high yield of 3 was observed (entry 2). The use of naphthalene and 1-methylnaphthalene slightly decreased the yield of 3, compared with that obtained in aqueous acetonitrile (entries 3 and 4). In particular, the use of biphenyl resulted in a low yield of 3 (entry 5). As reported earlier [21], the photoexcited 1,4-DCB reacted with DMF to yield a substituted product. The higher absorption of chrysene than 1,4-DCB at 313 nm prevented the photoreaction of 1,4-DCB with DMF to produce a higher yield of 3; however, absorption of biphenyl did not occur at 313 nm, which produced photochemically excited 1,4-DCB, and this led to a low yield of 3. Interestingly, the use of pyrene yielded the adduct 3 in the presence of 1,4-DCB or 1,4-DCN in DMF (entries 6 and 12), in contrast to the results obtained in aqueous acetonitrile. This indicates that the DMF-induced increase and decrease, respectively, of the oxidation potentials for pyrene and the carboxylate ion of 1 caused the SET between the cation radical of pyrene and the carboxylate ion of 1 to form the radical via decarboxylation. On the other hand, the use of 1,4- DCN in DMF significantly decreased the yield of 3 (entries 7–11), except in the case of chrysene. In addition, the recovery of 1,4-DCN was very low (10–30%). As with 1,4-DCB, these results could be attributed to the photoreaction of 1,4-DCN with DMF preventing decarboxylation, although the reaction of the photoexcited 1,4-DCN and DMF has not been reported. A detailed study of this photoreaction of 1,4-DCN with DMF is now in progress in our laboratory and will be reported in separate publications. Thus, decarboxylation was influenced by the arene, electron acceptor, and solvent.
Table 3.
Photoreaction of 1 and 2 using several arenes and electron acceptors in DMF.
| Entry | Arene | Oxidation potential of arene / Va | Electron acceptor | Yield of 3 / %b |
| 1 | Triphenylene | + 1.55 | 1,4-DCB | 44 |
| 2 | Chrysene | + 1.35 | 80 | |
| 3 | Naphthalene | 71 | ||
| 4 | 1-Methylnaphthalene | 68 | ||
| 5 | Biphenyl | 7 | ||
| 6 | Pyrene | 27 | ||
| 7 | Triphenylene | 1,4-DCN | 19 | |
| 8 | Chrysene | 74 | ||
| 9 | Naphthalene | 10 | ||
| 10 | 1-Methylnaphthalene | 14 | ||
| 11 | Biphenyl | 6 | ||
| 12 | Pyrene | 22 |
a Oxidation potentials vs. SCE in acetonitrile. Values compiled in Ref. [20]. bIsolated yield.
3. Experimental Section
3.1. General
The melting point was measured on a hot stage apparatus and is uncorrected. The IR spectrum was recorded on a JASCO FT/IR-620 spectrometer. 1H- and 13C-NMR spectra were recorded on a JEOL JNM-AL500 (500 and 125 MHz) spectrometer for solutions in CDCl3 containing tetramethylsilane as an internal standard. The high resolution mass spectrum (HRMS) was obtained on a JEOL JMS-700T mass spectrometer. The light source was a Riko UV-100HA 100-W high-pressure mercury arc. Dicyanobenzenes and arenes were recrystallized from hexane and EtOAc.
3.2. General Procedure for the Photoreaction of N-Boc-l-Valine (1) to Acrylonitrile (2): Preparation of N-tert-butoxycarbonyl-4-amino-4-isopropylbutanenitrile (3)
An aqueous CH3CN solution (CH3CN 54 mL, H2O 6 mL) of 1 (1.2 mmol, 20 mM), Phen (1.2 mmol, 20 mM), and 1,4-DCB (1.2 mmol, 20 mM) in four Pyrex vessels (18 mm × 180 mm) was purged with argon for 10 min, and 2 (1.2 mmol, 20 mM) was added under argon atmosphere. The mixture was irradiated with a 100-W high-pressure mercury lamp for 6 h. The solvent was then evaporated, and the resulting residue was dissolved in ether and washed with water, dried over Na2SO4, and concentrated under reduced pressure to yield the adduct 3. This product was isolated by column chromatography on a silica gel using hexane and EtOAc as eluents and by preparative HPLC using a GPC column. White solid; m.p. 76 °C; IR (KBr, cm-1) 3383, 2964, 2249, 1680, 1512; 1H-NMR: δ 4.34 (d (br), 0.75H), 4.03 (s (br), 0.10H), 3.48-3.43 (m, 1H), 2.46–2.34 (m, 2H), 1.91 (m, 1H), 1.71 (m, 1H), 1.62 (m, 1H), 1.44 (s, 9H), 0.94–0.90 (m, 6H); 13C-NMR: δ 155.9, 119.8, 79.6, 55.2, 32.3, 29.2, 28.3, 19.1, 17.8, 14.6; HRMS (FAB) calcd for (M+H)+ C12H23N2O2: 227.1761, found: 227.1738.
4. Conclusions
In conclusion, we have found that several arenes and electron acceptors could be used in the SET-photochemical decarboxylation of free carboxylic acids. The photoreaction proceeded smoothly in a polar solvent and was highly dependent on the oxidation potential of the arenes used. The highest yield of 3 was achieved by using biphenyl and 1,4-DCN in aqueous acetonitrile. Further investigation of the application of this photoreaction is currently in progress.
Acknowledgements
This work was supported by Mitsubishi Gas Chemical Company Award in Synthetic Organic Chemistry, Japan.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Schaefer H.J. Recent contributions of Kolbe electrolysis to organic synthesis. Top. Curr. Chem. 1990;152:91–151. [Google Scholar]
- 2.Barton D.H.R., Herve Y., Potier P., Thierry J. Manipulation of the carboxyl groups of α-amino-acids and peptides using radical chemistry based on esters of N-hydroxy-2-thiopyridone. Tetrahedron. 1988;44:5479–5486. doi: 10.1016/S0040-4020(01)86053-1. [DOI] [Google Scholar]
- 3.Crich D., Quintero L. Radical chemistry associated with the thiocarbonyl group. Chem. Rev. 1989;89:1413–1432. doi: 10.1021/cr00097a001. [DOI] [Google Scholar]
- 4.Kurauchi Y., Nobuhara H., Ohga K. Electron-transfer-initiated photoreactions of 1-methyl-2-phenyl-1-pyrrolinium perchlorate with α-heteroatom-substituted alkanoate anions. Bull. Chem. Soc. Jpn. 1986;59:897–905. doi: 10.1246/bcsj.59.897. [DOI] [Google Scholar]
- 5.Brimage D.R.G., Davidson R.S., Steiner P.R. Use of heterocyclic compounds as photo-sensitizers for the decarboxylation of carboxylic acids. J. Chem. Soc. Perkin Trans. I. 1973:526–529. doi: 10.1039/p19730000526. [DOI] [Google Scholar]
- 6.Oelgemoller M., Griesbeck A.G. Photoinduced electron transfer chemistry of phthalimdes: an efficient tool for C-C bond formation. J. Photochem. Photobio. C. 2002;3:109–127. doi: 10.1016/S1389-5567(02)00022-9. [DOI] [Google Scholar]
- 7.Yoon U.C., Jin Y.X., Oh S.W., Park C.H., Park J.H., Campana C.F., Cai X., Duesler E.N., Mariano P.S. A synthetic strategy for the preparation of cyclic peptide mimetics based on SET-promoted photocyclization processes. J. Am. Chem. Soc. 2003;125:10664–10671. doi: 10.1021/ja030297b. [DOI] [PubMed] [Google Scholar]
- 8.Libman J. Light induced charge transfer processes. The photochemical behavior of 1-cyanonaphthalene in the presence of phenylacetic acid derivatives. J. Am. Chem. Soc. 1975;97:4139–4141. doi: 10.1021/ja00847a051. [DOI] [Google Scholar]
- 9.Tsujimoto K., Nakao N., Ohashi M. Electron-donating behavior of aliphatic carboxylic acids in the photoreaction with 1,2,4,5-tetracyanobenzene. J. Chem. Soc., Chem. Commun. 1992:366–367. doi: 10.1039/c39920000366. [DOI] [Google Scholar]
- 10.Fujishima A., Rao T.N., Tryk D.A. Titanium dioxide photocatalysis. J. Photochem. Photobio. C. 2000;1:1–21. doi: 10.1016/S1389-5567(00)00002-2. [DOI] [Google Scholar]
- 11.Pac P., Nakasone A., Sakurai H. Photosensitized electron-transfer reaction of electron donor-acceptor pairs by aromatic hydrocarbons. J. Am. Chem. Soc. 1977;99:5806–5808. doi: 10.1021/ja00459a049. [DOI] [Google Scholar]
- 12.Majima T., Pac C., Nakasone A., Sakurai H. Redox-photosensitized reactions. 7. Aromatic hydrocarbon-photosensitized electron-transfer reactions of furan, methylated furans, 1,1-diphenylethylene, and indene with p-dicyanobenzene. J. Am. Chem. Soc. 1981;103:4499–4508. doi: 10.1021/ja00405a035. [DOI] [Google Scholar]
- 13.Ohashi M., Nakatani K., Maeda M., Mizuno K. Photoinduced tandem three-component coupling of propanedinitrile, 2,5-dimethylhexa-2,4-diene, and cyanoarenes. J. Org. Chem. 2008;73:8348–8351. doi: 10.1021/jo801624q. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimi Y., Itou T., Hatanaka M. Decarboxylative reduction of free aliphatic carboxylic acids by photogenerated cation radical. Chem. Commun. 2007:5244–5246. doi: 10.1039/b714526h. [DOI] [PubMed] [Google Scholar]
- 15.Itou T., Yoshimi Y., Morita T., Tokunaga Y., Hatanaka M. Decarboxylative photosubstitution of dicyanobenzenes with aliphatic carboxylate ions. Tetrahedron. 2009;65:263–269. doi: 10.1016/j.tet.2008.10.054. [DOI] [Google Scholar]
- 16.Yoshimi Y., Masuda M., Mizunashi T., Nishikawa K., Maeda K., Koshida N., Itou T., Morita T., Hatanaka M. Inter- and intramolecular addition reactions of electron-deficient alkenes with alkyl radicals, generated by SET-photochemical decarboxylation of carboxylic acids, serve as a mild and efficient method for the preparation of γ-amino acids and macrocyclic lactones. Org. Lett. 2009;11:4652–4655. doi: 10.1021/ol9019277. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimi Y., Kobayashi K., Kamakura H., Nishikawa K., Haga Y., Maeda K., Morita T., Itou T., Okada Y., Hatanaka M. Addition of alkyl radicals, generated from carboxylic acids via photochemical decarboxylation, to glyoxylic oxime ether: a mild and efficient route to α-substituted α-aminoesters. Tetrahedron Lett. 2010;51:2332–2334. doi: 10.1016/j.tetlet.2010.02.112. [DOI] [Google Scholar]
- 18.Galicia M., Gonzalez F.J. Electrochemical oxidation of tetrabutylammonium salts of aliphatic carboxylic acids in acetonitrile. J. Electrochem. Soc. 2002;149:D46–D50. doi: 10.1149/1.1450616. [DOI] [Google Scholar]
- 19.The oxidation potentials of carboxylate ions (RCO2–) show a slight dependence on R. Thus, the value of oxidation potential for the carboxylate ion of 1 might be similar to that of the hexanoate ion: Billing, R.; Zakharova, G.V.; Atabekyan, L.S.; Hennig, H. Luminescence quenching of [UO2F2]2- in aqueous solution by anions. J. Photochem. Photobio. A. 1991;59:163–174. [Google Scholar]
- 20.Murov S.L., Carmichael I., Hug G.L. “Handbook of Photochemistry”. Marcel Dekker; New York, NY, USA: 1993. [Google Scholar]
- 21.Tsuji M., Higashiyama K., Yamauchi T., Kubo H., Ohmiya S. Photosubstitution reaction of cyanoaromatics with aliphatic amides. Heterocycles. 2001;54:1027–1032. [Google Scholar]