Abstract
The aim of this study was to synthesize and examine possible in vitro antioxidant effects of indole-based melatonin analogue compounds. As a part of our ongoing study nineteen indole hydrazide/hydrazone derivatives were synthesized, characterized and their in vitro antioxidant activity was investigated by three different assays: by evaluating their reducing effect against oxidation of a redox sensitive fluorescent probe, by examining their protective effect against H2O2-induced membrane lipid peroxidation and by determining their inhibitory effect on AAPH–induced hemolysis of human erythrocytes. The results indicated significant strong antioxidant activity for most of the compounds, when compared to melatonin.
Keywords: indole, hydrazone, hydrazide, melatonin, synthesis, antioxidant activity
1. Introduction
Antioxidants are molecules which can interact with free radicals and stop their chain reactions before important and essential molecules are damaged. As oxidative stress is an important part of many diseases, the use of antioxidants is intensively studied in medicinal chemistry, particularly as treatments for vital diseases such as stroke, cancer and neurodegenerative diseases. Free radicals are produced basically during cellular metabolism and some functional activities and have essential roles in cell signaling, apoptosis and gene expression. On the other hand, excessive free radical attack can damage DNA, proteins and lipids, resulting very important diseases. Antioxidants can decrease the oxidative damage by reacting with free radicals or by inhibiting their activity.
It is well known that indole derivatives, extensively present in natural compounds, are very important substances for their medicinal and biological aspects. Antioxidant effects of the indole ring- containing substance melatonin (MLT) have been well described and evaluated by Tan et al. [1]. It is a highly conserved molecule that it acts as a free radical scavenger and a broad-spectrum antioxidant [2]. Studies also showed the role of MLT and its derivatives in many physiological processes and therapeutic functions, such as the regulation of circadian rhythm and immune functions [3,4,5,6]. Melatonin is known to be a potent in vitro antioxidant as well as powerful in vivo radical scavenger. In in vitro conditions melatonin exhibited potent antioxidant activity in a linoleic acid emulsion system. It also showed potent superoxide radical scavenging activity, higher than either quercetin or BHT [7].
Recent research has proved that the indole ring in the MLT molecule is the reactive center dealing with oxidants due to its high resonance stability and very low activation energy barrier towards free radical reactions [8,9,10]. Indole is found to reduce cisplatin-induced reactive oxygen species formation [11] and scavenge hydroxyl radical directly [12]. It was shown that alkyl-substituted indole derivatives can trap ABTS+• and DPPH indicating that the alkyl group attached to indole is of importance for the antioxidant activity [13]. The hydrogen peroxide scavenging activity of melatonin showed that the scavenging activity augmented with increasing concentrations of melatonin. This result may be illustrative of melatonin's ability to inhibit lipid peroxidation [14]. A series of dihydroindenoindole derivatives containing methoxy, halogen, and hydroxyl groups was synthesized and showed effective inhibition against DPPH·, ABTS·+, DMPD·+, and superoxide anion radicals compared to standard antioxidants [15]. 2-Phenylindole derivatives significantly inhibited lipid peroxidation at 10-3 M concentration [16]. Indole-3-propionamide derivatives also exhibited important antioxidant activity compared to melatonin [17]. A large variety of synthetic compounds have been identified as potent in vitro antioxidants, yet many of these compounds have not provided great clinical benefits, and some produced side effects. In our earlier study [18] two sets of indole derivatives, with changes in the 5-methoxy and 2-acylaminoethyl groups of MLT were synthesized and tested for their in vitro antioxidant potency in the DPPH, superoxide dismutase and lipid peroxidation (LP) assays. With a few exceptions most of the compounds tested showed significant antioxidant activity at concentrations comparable with or much higher than that of MLT. These results prompted us to synthesize more indol-3-aldehyde hydrazone and hydrazide derivatives.
As a part of our ongoing study nineteen indole-based MLT analogue hydrazide/hydrazone derivatives were now synthesized and their antioxidant activity was investigated in vitro by three different assays: by evaluating their reducing effect against oxidation of a redox sensitive fluorescent probe, DCFH-DA, by investigating their protective effect against H2O2-induced membrane lipid peroxidation and by determining their inhibitory effect on 2,2’-azobis(2-amidinopropane hydrochloride) (AAPH) –induced hemolysis of human erythrocytes. Human erythrocytes were chosen as a biological model because they are readily available cells that are sensitive to oxidative damage. The results were compared with MLT. All the indole-based MLT analogue compounds except those previously synthesized (1a [19,20], 1j [21] and 1r [22]) were characterized on the basis of 1H- and 13C-NMR, mass and FT-IR spectra and elemental analysis.
2. Results and Discussion
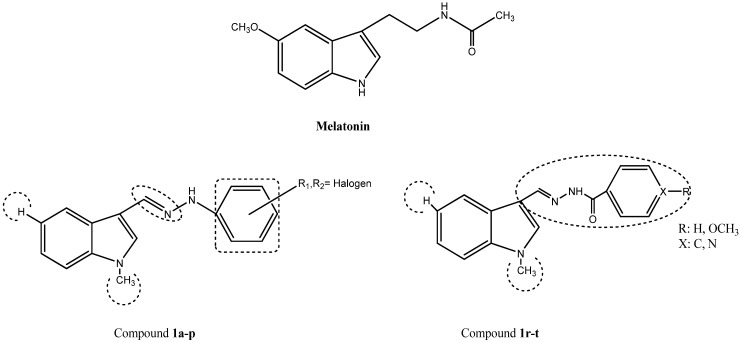
The present work aimed to synthesize, characterize and investigate the potential antioxidant effects of indole-based MLT analogue hydrazide/hydrazone derivatives by several in vitro test models. Based on MLT, N-acetyl-5-methoxytryptamine, a well-known antioxidant, free radical scavenger, and neuroprotectant, new indole imines were developed. Three parts of the MLT molecule were modified to develop new indole-based MLT analogue compounds. These modifications were done mainly on the acylamino group (Figure 1).
Figure 1.
Parts of the MLT molecule modified to develop new indole-based MLT analogue compounds.
These chemically significant modulations of the lead structure were made at three different points: the methoxy group replaced with hydrogen at the 5-position of the indole ring (modification I) and acetylaminoethyl side chain modified by formation of imine (modification II) and hydrogen replaced with methyl on nitrogen (modification III). Particular attention was dedicated to the role of the 5-methoxy group, which was eliminated. These modifications resulted in a set of compounds having different physical properties, lipophilicity and different substitution patterns on the indole nucleus. This helped to investigate the effect of substituents with different electronic and lipophilic properties on the antioxidant activity of new indole derivatives (Scheme 1).
Scheme 1.
Synthetic route to indole-based melatonin analogue hydrazide/hydrazone derivatives.
2.1. Effects of synthesized indole derivatives on cellular ROS
The protective effect of newly synthesized indole-based MLT analogue against DCFH-DA oxidation was determined in human erythrocytes that were preloaded with the fluorescent probe. In cells, DCFH-DA locates in the cytosol and reflects cellular ROS formation. Oxidation of the probe was screened at various time intervals up to 60 min. At 10 min incubation all the synthesized indole derivatives except 1r and 1s were found to have potent antioxidant activity, even higher than MLT itself. Among those synthesized indoles with changes in the aromatic side chain, p-halogenations were found to decrease antioxidant activity compared to o- and m- substitution of the same halogen atom (Figure 2). A significant further decrease in the antioxidant activity of p-halogenated compounds was observed at 60 min incubation indicating a possible oxido-reductive reaction took place in incubations with those derivates or a possible structural change in those analogues resulting in loss of their antioxidant effect (Figure 2). On the other hand m-halogenations in the aromatic side chain were observed to eliminate the time dependency of the antioxidant effectiveness. This can be seen from unchanged DCFH oxidation with 1b, 1h, 1k and 1n at 10 min and 60 min incubations. 1r and 1s had the weakest antioxidant activity (almost equal to MLT) among all tested compounds. This can be explained by their structural difference from the rest of the synthesized compounds, mainly by having no aromatic halogenations and having a carbonyl group on the side chain. The results also indicate a biphasic pattern in the antioxidant effect of MLT; higher effect in lower concentrations (1 µM) and higher concentrations (500–1000 µM) whereas lower antioxidant effect was observed in between (10, 100 µM) (Figure 2).
Figure 2.
Oxidation of DCFH via ROS in erythrocytes after the incubation with various concentrations of MLT or 10 µM synthesised indole derivatives for 10 and 60 min. Values are mean ±SD of three individual experiments. Values above the bars are % control values.
2.2. Inhibitory effect of synthesized indole derivatives on hydrogen peroxide-induced peroxidation of human erythrocyte membranes
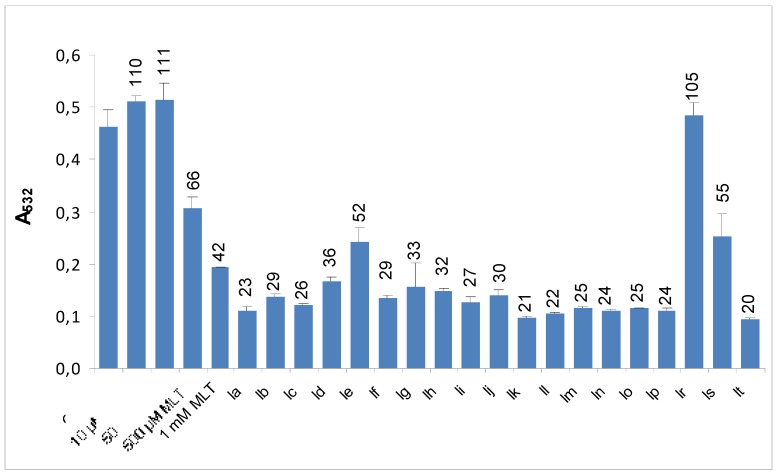
Once ROS are formed, one of the subsequent detrimental outcomes is peroxidation and oxidative destruction of polyunsaturated fatty acids (PUFA) in cell membranes. The process is initiated by an oxidizing radical that is capable of abstracting one hydrogen atom from PUFA. After several rearrangement and oxidation reactions, generated lipid hydroperoxides decompose to a wide range of products, mainly small molecule alkanes and aldehydes. Among those aldehydes, malondialdehyde (MDA), assayed by the thiobarbituric acid (TBA) assay, is the most widely used biomarker of oxidative damage to lipids. In the present study we investigated protective effect of synthesized indole derivatives on H2O2-induced MDA formation in erythrocyte membranes. Figure 3 shows the inhibitory effect of MLT and the synthesized indole derivatives on H2O2-induced peroxidation of human erythrocytes in vitro.
Figure 3.
Effects of various concentrations of MLT and 10 μM synthesised indole derivatives on H2O2-induced lipid peroxidation in erythrocyte membranes. MDA values were determined as an endproduct of lipid peroxidation. Values are mean ±SD of three individual experiments. Values above the bars are % control values.
MLT, a well known antioxidant, was used as a reference control for comparison purposes. The results obtained in this model were in accordance with data from ROS-mediated DCFH oxidation assay; all analogues except 1r and 1s were effective in protecting erythrocyte membranes from the attack of H2O2 and further lipid peroxidation (Figure 3). By combining those findings lack of protective effect of 1r and 1s in H2O2-induced erythrocyte membrane LP might be explained by the lack of their ROS scavenging ability. Furthermore we did additional experiments with four of the selected synthesised indole derivatives (1c, 1f, 1j and 1t) in order to see the concentration dependency of their protective effect against H2O2-induced erythrocyte membrane LP. As presented in Figure 4 several of the compounds that were found to have higher protective effect against H2O2-induced erythrocyte membrane LP than MLT at same concentration (10 μM) were found to have same effect even at their lower concentrations (Figure 4 - 1f, 1t). These finding indicate that several of the newly synthesized indole derivatives may exert their antioxidant effect even at lower concentrations which make them promising candidates as antioxidant drugs.
Figure 4.
Effects of various concentrations of 1c, 1f, 1j and 1t on H2O2-induced LP. Values are mean ±SD of three individual experiments. * p < 0.05, ** p < 0.01, *** p < 0.005 significantly different from control.
2.3. The antioxidant effect of synthesized indole derivatives on AAPH-induced oxidative hemolysis
The hemolysis of erythrocytes induced by free radicals is a good model system to study both oxidative damage and protection by antioxidants. Thermal decomposition of AAPH results in free radicals which attack the erythrocyte membranes to induce LP [23]. Once LP chain reaction starts the RBC membranes are quickly damaged, leading to hemolysis. On the other hand if antioxidants are added to red blood cells (RBCs) they would react with the radicals and inhibit hemolysis. Hemolysis does not start at the beginning of the reaction because the endogenous antioxidants of erythrocytes protect the membrane against oxidative damage induced by AAPH. Hemolysis takes place after the endogenous antioxidants are depleted thoroughly, generating an inhibition period (tlag) [23]. Oxidative hemolysis of the erythrocytes was screened for 5 h. As can be seen in Table 1, presenting the quantitative indice (tlag) obtained from this assay, MLT was found to have highest antioxidant activity among all models tested in the present study. This protective effect of MLT against AAPH-induced oxidative hemolysis indicates free radical scavenging effect of MLT which was previously shown by Zhao et al. [24]. All tested MLT analogues in this model was found to have either equal or higher tlag values compared to MLT. Similar to the findings from other models that we used 1s was found to have the least antioxidant effect where 1r did not exert any radical scavenging effect at all. These findings may suggest that aromatic halogenation increases the free radical scavenging and therefore antioxidant effect of indole derivatives.
Table 1.
Quantitative indicator measured from AAPH-induced erythrocyte hemolytic curves. Values are the means of data obtained from three separate curves. Data represent mean of three different curves for each compound. tlag; lag time before the starting of hemolysis.
| Substrate | tlag (min) |
|---|---|
| AAPH | 97 |
| 10 μM MLT | 128 |
| 1 MM MLT | >300 |
| 10 μM 1a | 160 |
| 10 μM 1b | 137 |
| 10 μM 1d | 160 |
| 10 μM 1g | 160 |
| 10 μM 1j | 160 |
| 10 μM 1k | 160 |
| 10 μM 1l | 160 |
| 10 μM 1n | 131 |
| 10 μM 1p | 160 |
| 10 μM 1r | 97 |
| 10 μM 1s | 124 |
| 10 μM 1t | 124 |
Like other indole derivatives and tryptophan metabolites, MLT has inherent redox properties due to the presence of an electron-rich aromatic ring system, which allows the indoleamine to easily function as an electron donor [25]. A number of oxygen-centered radicals and other reactive species have been shown to be capable of oxidizing MLT in various experimental systems. It is possible that making the indole ring more stable electronically helped to act as a better electron donor. Introduction of an imine group in to the side chain increased the stability of the indole molecule by helping the delocalization of the electrons. This might help to have high free radical scavenging activity. Also according to Reiter [26] MLT scavenges the radicals most likely via electron donation, thereby neutralizing the radicals and generating nitrogen centered radical, the indolyl (or melatonyl) cation radical. These results suggest a new approach for the in vitro antioxidant activity properties and structure activity relationship of 1 and 3 substituted indole ring regarding to antioxidant activity.
3. Experimental
3.1. Material and methods
Uncorrected melting points were determined with a Büchi SMP-20 apparatus. The 1H- and 13C- NMR spectra were measured with a Varian 400 MHz instrument using TMS internal standard and DMSO-d6 as solvent. ESI Mass spectra were determined on a Waters Micromass ZQ. FT-IR spectra were recorded on a Jasco 420 Fourier Transform apparatus. Elemental analyses were performed using a CHNS-932 instrument (LECO). All spectral analysis was performed at the Central Laboratory of the Faculty of Pharmacy, Ankara University. Chromatography was carried out using Merck silica gel 60 (230–400 mesh ASTM). The chemical reagents used in synthesis were purchased from Sigma (Germany) and Aldrich (USA).
3.2. Chemistry
The target imines were derived from 1-methyl-1H-indole-3-carboxaldehyde and appropriate hydrazine or hydrazide derivatives using simple reaction strategies. For the synthesis of compounds 1a–p a methodology similar to that of Kidwai et al. [27] has been adopted. The hydrazones 1r and 1s were also prepared from the reaction of equimolar amounts of hydrazide with 1-methyl-1H-indole-3-carboxaldehyde in the presence of ethanol. Finally N,N’-bis-(1-methylindole-3-ylmethylene)-hydrazine derivatives were synthesized using hydrazine hydrate with 1-methyl-1H-indole-3-carboxaldehyde in the presence of ethanol. All the new compounds (except 1a [19,20], 1j [21] and 1r [22]) were characterized on the basis of their spectral and analytical data.
3.3. General procedure for the synthesis of compounds 1a–p
1-Methyl-1H-indole-3-carboxaldehyde (1 mmol) and phenyl hydrazine or its derivatives (1.3 mmol) in EtOH (10 mL) was heated for 30 min on the hot water bath in the presence of CH3COONa (0.4 g). On cooling, the precipitate was collected washed with cold EtOH and recristallized from EtOH to give 1a–p with 44 to 82% yield.
1-Methylindole-3-carboxaldehyde (2-fluorophenyl)hydrazone (1b). Yield 65.2%, m.p. 130–131 ºC; 1H-NMR: δ 3.78 (3H,s), 6.76 (1H, m), 7.09 (2H, m), 7.24 (2H, m), 7.46 (2H, m), 7.62 (1H, s, azomethine-CH), 8.23 (1H, d), 8.31 (1H, s) 9.72 (1H, s, hydrazine-NH); 13C-NMR: δ 33.11, 110.74, 112.30, 113.78, 115.37, 115.54, 117.90, 121.03, 122.42, 123.09, 125.23, 125.73, 132.38, 135.00, 137.97, 148.39 (azomethine-C), 150.76; ESI MS m/z 268 (M+1, %100), 269 (M+2); Anal. Calcd. for C16H14N3F: C, 71.89%; H, 5.28%; N, 15.72%. Found: C, 71.35%; H, 4.48%; N, 15.76%. FT-IR (KBr) cm-1 1580 (C=N, azomethine stretch), 3295 (N-H stretch).
1-Methylindole-3-carboxaldehyde (3-fluorophenyl)hydrazone (1c). Yield 49%, m.p. 144–145 ºC; 1H-NMR: δ 3.80 (3H,s), 6.46 (1H, m), 6.80 (2H, m), 7.24 (3H, m), 7.48 (1H, d), 7.66 (1H, s), 8.11 (1H, s, azomethine-CH), 8.23 (1H, d), 10.16 (1H, s, hydrazine-NH); 13C-NMR: δ 33.22, 98.19, 104.27, 108.20, 110.76, 112.21, 121.04, 122.30, 123.09, 125.21, 131.35, 132.35, 136.25, 138.16, 148.78 (azomethine-C), 162.96, 165.35; ESI MS m/z 268 (M+1, %100), 269 (M+2); Anal. Calcd. for C16H14N3F: C, 71.89%; H, 5.27%; N, 15.72%. Found: C, 69.30%; H, 5.05%; N, 15.13%. FT-IR (KBr) cm-1 1582 (C=N, azomethine stretch), 3318 (N-H stretch).
1-Methylindole-3-carboxaldehyde (4-fluorophenyl)hydrazone (1d). Yield 68.9%, m.p. 150–151 ºC; 1H-NMR: δ 3.80 (3H,s), 7.04 (4H, m), 7.23 (2H, m), 7.47 (1H, d), 7.61 (1H, s), 8.08 (1H, s, azomethine-CH), 8.24 (1H, d,), 9.83 (1H, s, hydrazine-NH); 13C-NMR: δ 33.28,110.68, 112.47, 112.88, 116.36, 120.88, 122.39, 123.03, 125.24, 131.86, 135.26, 138.14, 143.58 (azomethine-C), 154.75, 157.05: ESI MS m/z 268 (M+1, %100), 269 (M+2); Anal. Calcd. for C16H14N3F: C, 71.89%; H, 5.28%; N, 15.72%. Found: C, 71.31%; H, 5.24%; N, 15.19%. FT-IR (KBr) cm-1 1570 (C=N, azomethine stretch), 3331 (N-H stretch).
1-Methylindole-3-carboxaldehyde (2,4-difluorophenyl)hydrazone (1e). Yield 68.8%, m.p. 137–138 ºC; 1H-NMR: δ 3.78 (3H,s), 7.02 (1H, m), 7.18 (3H, m), 7.45 (2H, m), 7.62 (1H, s), 8.22 (1H, d), 8.30 (1H, s, azomethine-CH), 9.66 (1H, s, hydrazine-NH); ESI MS m/z 286 (M+1, %100), 287 (M+2); Anal. Calcd. for C16H13N3F2: C, 67.36%; H, 4.59%; N, 14.73%. Found: C, 66.99%; H, 4.60%; N, 14.68%. FT-IR (KBr) cm-1 1598 (C=N, azomethine stretch), 3340 (N-H stretch).
1-Methylindole-3-carboxaldehyde (2,5-difluorophenyl)hydrazone (1f). Yield 70.5%, m.p. 156–157 ºC; 1H-NMR: δ 3.78 (3H,s), 6.37 (1H, m), 6.59 (2H, dd), 7.17 (1H, dd), 7.22 (2H, m), 7.45 (1H, d), 7.67 (1H, s, azomethine-CH), 8.11 (1H, s), 8.17 (1H, d), 10.40 (1H, s, hydrazine-NH); 13C-NMR: δ 33.35, 92.55, 94.49, 110.82, 111.86, 121.21, 122.22, 123.16, 125.16, 132.93, 137.40, 138.17, 149.21 (azomethine-C), 162.93, 165.33; ESI MS m/z 286 (M+1, %100), 287 (M+2); Anal. Calcd. for C16H13N3F2:C, 67.36%; H, 4.59%; N, 14.73%. Found: C, 66.41%; H, 4.48%; N, 14.55%. FT-IR (KBr) cm-1 1591 (C=N, azomethine stretch), 3433 (N-H stretch).
1-Methylindole-3-carboxaldehyde (3,5-difluorophenyl)hydrazone (1g). Yield 44.9%, m.p. 120–121 ºC; 1H-NMR: δ 3.81 (3H, s), 6.45 (1H, m), 7.15 (2H, m), 7.25 (2H, m), 7.49 (1H, d), 7.70 (1H, s, azomethine-CH), 8.18 (1H, d), 8.36 (1H, s), 10.03 (1H, s, hydrazine-NH); ESI MS m/z 286 (M+1, %100); Anal. Calcd. for C16H13N3F2: C, 67.36%; H, 4.59%; N, 14.72%. Found: C, 67.76%; H, 4.61%; N, 14.52%. FT-IR (KBr) cm-1 1591 (C=N, azomethine stretch), 3332 (N-H stretch).
1-Methylindole-3-carboxaldehyde (2-chlorophenyl)hydrazone (1h). Yield 82.3%, m.p. 134–135 ºC; 1H-NMR: δ 3.82 (3H,s), 6.73 (1H, m), 7.20-7.32 (4H, m), 7.50 (2H, dd), 7.67 (1H, s, azomethine-CH), 8.24 (1H, d), 8.47 (1H, s) 9.43 (1H, s, hydrazine-NH); 13C-NMR: δ 33.35, 110.83, 112.17, 113.84, 116.24, 119.06, 121.14, 122.39, 123.15, 125.23, 128.83, 129.93, 132.72, 138.21, 138.94, 142.69 (azomethine-C), 150.76; ESI MS m/z 284 (M+, %100), 286 (M+2); Anal. Calcd. for C16H13N3Cl: C, 67.72%; H, 4.97%; N, 14.81%. Found: C, 67.75%; H, 4.36%; N, 14.09%. FT-IR (KBr) cm-1 1592 (C=N, azomethine) stretch), 3328 (N-H stretch).
1-Methylindole-3-carboxaldehyde (3-chlorophenyl)hydrazone (1i). Yield 78.4%, m.p. 135–136 ºC; 1H- NMR: δ 3.78 (3H,s), 6.46 (1H, m), 6.55 (1H, dd, 6.92 (1H, dd), 7.00 (1H, m), 7.20 (3H, m), 7.47 (1H, d), 7.64 (1H, s), 8.08 (1H, s, azomethine-CH), 8.18 (1H, d), 10.08 (1H, s, hydrazine-NH); 13C-NMR: δ 33.34, 110.70, 111.19, 112.14, 117.50, 121.04, 122.20, 123.11, 125.21, 131.42, 132.42, 134.44, 136.49, 138.15, 148.13 (azomethine-C), 162.96, 165.35; ESI MS m/z 284 (M+, %100), 286 (M+2); Anal. Calcd. for C16H14N3Cl: C, 67.72%; H, 4.97%; N, 14.81%. Found: C, 67.57%; H, 4.81%; N, 14.77%. FT-IR (KBr) cm-1 1592 (C=N, azomethine stretch), 3302 (N-H stretch).
1-Methylindole-3-carboxaldehyde (2,5-dichlorophenyl)hydrazone (1k). Yield 78.3%, m.p. 150–151 ºC; 1H-NMR: δ 3.83 (3H,s), 6.76 (1H, dd), 7.26 (3H, m), 7.34 (1H, d), 7.45 (1H, d), 7.52 (1H, d), 7.74 (1H, s, azomethine-CH), 8.14 (1H, d), 8.52 (1H, s), 9.68 (1H, s, hydrazine-NH); 13C-NMR: δ 33.41, 111.01, 111.76, 112.80, 114.83, 118.23, 121.30, 121.94, 123.24, 125.20, 131.36, 133.28, 138.23, 140.37, 143.73 (azomethine-C), 162.93, 165.33; ESI MS m/z 318 (M+, %100), 320 (M+2), 322 (M+4); Anal. Calcd. for C16H13N3Cl2: C, 59.97%; H, 4.12%; N, 13.21%. Found: C, 59.97%; H, 4.18%; N, 13.23%. FT-IR (KBr) cm-1 1592 (C=N, azomethine) stretch), 3315 (N-H stretch).
1-Methylindole-3-carboxaldehyde (3,4-dichlorophenyl)hydrazone (1l). Yield 89.3%, m.p. 160–161 ºC; 1H-NMR: δ 3.80 (3H, s), 6.95 (1H, dd), 7.15 (1H, d), 7.24 (2H, m), 7.42 (1H, d), 7.49 (1H, d), 7.68 (1H, s, azomethine-CH), 8.10 (1H, s), 8.18 (1H, d), 10.25 (1H, s, hydrazine-NH); 13C-NMR: δ 33.36, 110.85, 11.95, 112.30, 112.72, 118.56, 121.12, 122.18, 123.16, 125.16, 131.61, 132.13, 132.75, 137.18, 138.16, 146,70 (azomethine-C), 154,07, 156.38, 168.66; ESI MSm/z 318 (M+, %100), 320 (M+2); Anal. Calcd. for C16H13N3Cl2: C, 60.39%; H, 4.12%; N, 13.21%. Found: C, 60.16%; H, 4.16%; N, 12.96%. FT-IR (KBr) cm-1 1593 C=N (azomethine) stretch band, 3433 N-H stretch band.
1-Methylindole-3-carboxaldehyde (3,5-dichlorophenyl)hydrazone (1m). Yield 80%, m.p. 161–162 ºC; 1H-NMR: δ 3.81 (3H, s), 6.77 (1H, t), 6.95 (2H, d), 7.25 (2H, m), 7.49 (1H, d), 7.72 (1H, s, azomethine-CH), 8.14 (2H, d and s), 10.35 (1H, s, hydrazine-NH); 13C-NMR: δ 34.41, 110.06, 110.94, 111.77, 116.75, 121.24, 122.04, 123.22, 125.17, 133.07, 135.31, 137.92, 138.19, 148,74 (azomethine-C); ESI MS m/z 318 (M+, %100), 320 (M+2); Anal. Calcd. for C16H13N3Cl2: C, 60.39%; H, 4.12%; N, 13.21%. Found: C, 60.26%; H, 3.80%; N, 13.18%. FT-IR (KBr) cm-1 1586 (C=N, azomethine stretch), 3312 (N-H stretch).
1-Methylindole-3-carboxaldehyde (2-bromophenyl)hydrazone (1n). Yield 65.8%, m.p. 152–153 ºC; 1H-NMR: δ 3.82 (3H,s), 6.68 (1H, m), 7.23 (2H, m), 7.33 (1H, m), 7.53 (3H, m), 7.66 (1H, s, azomethine-CH), 8.23 (1H, d), 8.49 (1H, s) 9.15 (1H, s, hydrazine-NH); 13C-NMR: δ 33.33, 106.22, 110.80, 112.13, 114.32, 119.79, 121.12, 122.33, 123.13, 125.24, 129.34, 132.70, 133.12, 138.20, 139.68, 143.68 (azomethine-C), 150.76; ESI MS m/z 328 (M+, %100), 330 (M+2, %100), 331 (M+3); Anal. Calcd. for C16H14N3Br: C, 58.55%; H, 4.30%; N, 12.80%. Found: C, 58.67%; H, 4.28%; N, 12.92%. FT-IR (KBr) cm-1 1589 C=N (azomethine) stretch band, 3322 N-H stretch band.
1-Methylindole-3-carboxaldehyde (3-bromophenyl)hydrazone (1o). Yield 49%, m.p. 165–166 ºC; 1H- NMR: δ 3.81 (3H,s), 6.81 (1H, d), 6.99 (1H, d), 7.16-7.28 (4H, m), 7.49 (1H, d), 7.67 (1H, s), 8.10 (1H, s, azomethine-CH), 8.18 (1H, d), 10.07 (1H, s, hydrazine-NH); 13C-NMR: δ 33.35, 110.83, 111.07, 112.15, 114.14, 120.41, 121.06, 122.20, 123.13, 125.24, 131.75, 132.44, 136.55, 138.17, 148.29 (azomethine-C); ESI MS m/z 328 (M+, %100), 330 (M+2, %100), 331 (M+3); Anal. Calcd. for C16H14N3Br: C, 58.55%; H, 4.41%; N, 12.80%. Found: C, 58.78%; H, 4.42%; N, 12.75%. FT-IR (KBr) cm-1 1593 (C=N, azomethine stretch), 3436 (N-H stretch).
1-Methylindole-3-carboxaldehyde (4-bromophenyl)hydrazone (1p). Yield 68.6%, m.p. 195–196 ºC; 1H-NMR: δ 3.80 (3H,s), 7.00 (2H, d), 7.24 (2H, m), 7.36 (2H, d), 7.65 (1H, s), 8.10 (1H, s, azomethine-CH), 8.22 (1H, d,), 10.18 (1H, s, hydrazine-NH); 13C-NMR: δ 33.34,108.71, 110.76, 112.30, 113.93, 120.99, 122.41, 123.09, 125.20, 132.38, 136.03, 138.15, 146.02 (azomethine-C); ESI MS m/z 328 (M+, %100), 330 (M+2, %100), 331 (M+3); Anal. Calcd. for C16H14N3Br: C, 58.55%; H, 4.30%; N, 12.80%. Found: C, 58.16%; H, 4.53%; N, 12.51%. FT-IR (KBr) cm-1 1589 (C=N, azomethine stretch), 3440 (N-H stretch).
3.4. General procedure for the synthesis of compounds 1r–s
A solution of 1-methyl-1H-indole-3-carboxaldehyde (0.5 mmol) and anisic acid hydrazide or izonicotinic acid hydrazide (0.5 mmol) in EtOH (50 mL) was heated for 2.5 h on a hot water bath. On cooling, the precipitate was collected washed with cold EtOH to give 1r–1s with 15 to 25% yield.
N-(4-methoxybenzoyl)-N’-(1-methylindolyl-3-methylene)-hydrazine (1s). Yield 15.3%, m.p. 254–255 ºC; 1H-NMR: δ 3.835 and 3.838 (6H, brs, NCH3 and OCH3), 7.04 (2H, d), 7.20 (1H, t), 7.27 (1H, t), 7.50 (1H, d), 7.80 (1H, s), 7.92 (1H, d,) 8.31 (1H d), 8.58 (1H, s, azomethine-CH), 11.39 (1H, brs, hydrazine-NH); 13C-NMR: δ 33.47, 56.09, 110.87, 111.51, 114.33, 121.27, 122.83, 123.35, 125.44, 126.74, 129.98, 134.40, 138.23, 144.53 (azomethine-C), 162.39 (C=O), 162.63, 167.63; ESI MS m/z 308 (M+1), 330 (M+Na, 100%); Anal. Calcd. for C18H17N3O2Br: C, 70.34%; H, 5.75%; N, 13.67%. Found: C, 70.07%; H, 5.59%; N, 13.63%. FT-IR (KBr) cm-1 1608 (C=N, azomethine stretch), 3194 (NH-CO stretch).
3.5. General procedure for the synthesis of compound 1t
A solution of 1-methyl-1H-indole-3-carboxaldehyde (2 mmol) and hydrazine hydrate (1 mmol) in EtOH (25 mL) was heated for 4 h on a hot water bath. On cooling, the precipitate was collected washed with cold EtOH to give N,N’-bis-(1-methylindole-3-ylmethylene)hydrazine (1t) in 34% yield. m.p. 233–234 ºC; 1H-NMR: δ 3.85 (6H, s), 7.27 (4H, tt), 7.52 (2H, d), 7.89 (2H, s), 8.36 (2H, d), 8.87 (2H, s, azomethine-CH); 13C-NMR: δ 33.62, 1MS mass m/z 315 (M+1, %100), 316 (M+2); Anal. Calcd. for C20H18N4: C, 76.40%; H, 5.77%; N, 17.82%. Found: C, 76.41%; H, 5.47%; N, 17.75%. FT-IR (KBr) cm-1 1571 (C=N, azomethine stretch).
3.6. In Vitro Antioxidant Activities
3.6.1. Erytrocyte Isolation
Blood samples were collected into heparinized tubes. The samples were centrifuged for 15 min at 3,000 rpm at +4 ºC. After removing the plasma and the buffy coat, RBCs were washed in equal volume of cold NaCl (0.155 mol/L) for three times. Following the third saline wash, supernatants were removed and packed RBCs were obtained.
3.6.2. Estimation of Reactive Oxygen Species by DCFH-DA
For estimation of ROS inside erythrocytes DCFH-DA was used as a probe. In cellular systems non fluorescent probe DCFH-DA readily crosses the cell membrane and undergoes hydrolysis by intracellular estrases to nonfluorescent 2',7'-dichlorofluorescin (DCFH). DCFH is then rapidly oxidized in the presence of reactive oxygen species to highly fluorescent 2′,7′- dichlorofluorescein (DCF) [28]. In our study, 1% erythrocyte suspension was incubated at 37 ºC in phosphate buffer (50 mM, pH 7.4) and DCFH-DA (20 μM) for an hour. At the end of incubation period, the erythrocyte suspension was washed with PBS three times, resuspended in PBS, pipetted onto a black 96-well plate and various concentrations of melatonin or its derivates were added into the wells. The production of fluorescent DCF was measured using a multiplate spectrofluorometer (excitation wavelength = 488 nm, emission wavelength = 525 nm) [29].
3.6.3. Measurement of H2O2-induced lipid peroxidation levels
Lipid peroxidation was assesed by the determination of malondialdehyde (MDA) levels using the method of Gutteridge et al. [30] and Quinlan et al. [31] based on the reaction of MDA with thiobarbituric acid (TBA) at 95 ºC. In the TBA test reaction, MDA and TBA react to form a pink adduct with an absorption maximum at 532 nm. The reaction was performed at pH 2–3 at 95 ºC for 15 min. Erythrocytes were resuspended in phosphate buffer (50 mM, pH 7.4) with 7.8 mM azide and different concentrations of melatonin or its derivates were added. The samples were preincubated for 30 min at 37 ºC, added 5 mM H2O2 and incubated for 2 h at 37 ºC. The sample was mixed with 28% (w/v) trichloroacetic acid to precipitate the protein. The precipitate was pelleted by centrifigation and an aliquot of supernatant was reacted with 1% (w/v) TBA in a boiling water-bath for 15 min. After cooling, the absorbance was read at 532 nm.
3.6.4. Determination of Erythrocyte Hemolysis
Erythrocytes were resuspended in phosphate saline (PBS: 150 mM NaCl, 1 mM Na2PO4 and 1.9 mM NaH2PO4, pH 7.4) at a 20% (v/v) suspension. Erythrocytes at 5% (v/v) suspension in PBS were incubated with 75 mM AAPH in the presence of different concentrations of melatonin or its derivates for 5 h at 37 ºC in a gently shaking water bath. Aliquots were taken out from this mixture at appropriate intervals and centrifuged 2000 rpm for 5 min to obtain supernatant. The absorbance of the supernatant was determined spectrophotometrically at 540 nm [32]. The percentage of hemolysis at different incubation intervals was compared with that of complete hemolysis. For reference, erythrocytes were treated with distilled water and the absorbance for the hemolysate at 540 nm was used as 100% hemolysis. Every experiment was repeated three times and the lag time of hemolysis (tlag) was determined.
3.6.5. Statistical analysis
Unpaired t test was performed to evaluate the significance of the differences between groups. p < 0.05 was accepted as significant.
4. Conclusions
In general all the synthesized indole derivatives were found to have potent antioxidant activity, even higher than MLT itself, according to the results of three in vitro antioxidant experiments revealing differences in their relative potencies probably related to electronic distribution. No significant antioxidant activity was observed in two compounds 1r, 1s. These compounds were the isonicotinic (1r) and anisic acid (1s) hydrazides of indole 3-aldehydes and they have no halogen atoms in their structure that makes them different from the rest of the synthesized compounds. Structural investigation of the rest of the active compounds showed that having o- and m- halogenated aromatic side chain increase the antioxidant activity (such as compounds 1b, 1c, 1m, 1k and 1l). These are the most promising compounds that should be kept in mind for designing new MLT-based indole derivatives for our ongoing study. These results suggest a new approach for the in vitro antioxidant activity properties and structure activity relationships of 1,3-disubstituted indole rings. Lack of a methoxy group in the 5 position did not affect the antioxidant capacity of the new indole derivatives. In fact, the in vitro assays showed that lack of a methoxy group, introduction of a methyl group at the nitrogen in the indole ring and a halogenated aromatic side chain resulted in much more active compounds than MLT itself. This may be due to increased stability of the indole ring and delocalization of the electrons to help to scavenge free radicals by forming stable indolyl cation radicals.
Acknowledgements
This work was supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) Research and Development Grant 109S099.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Tan D.X., Chen L.D., Poeggeler B., Manchester L.C., Reiter R.J. Melatonin: a potent endogenous hydroxyl radical scavenger. Endocr. J. 1993;1:57–60. [Google Scholar]
- 2.Sreejith P., Beyo R.S., Divya L., Vijayasree A.S., Manju M., Oommen O.V. Triiodothyronine and melatonin influence antioxidant defense mechanism in a teleost Anabas testudineus (Bloch): in vitro study. Indian J. Biochem. Biophys. 2007;44:164–168. [PubMed] [Google Scholar]
- 3.Guerrero J.M., Reiter R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002;2:167–179. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 4.Ates-Alagoz Z., Coban T., Suzen S. A Comparative Study: Evaluation of Antioxidant Activity of Melatonin and Some Indole Derivatives. Med. Chem. Res. 2005;14:169–179. doi: 10.1007/s00044-005-0132-0. [DOI] [Google Scholar]
- 5.Suzen S., Buyukbingol E. Anti-Cancer Activity Studies of Indolalthiohydantoin (PIT) on certain cancer cell lines. Farmaco. 2000;55:246–248. doi: 10.1016/S0014-827X(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 6.Suzen S., Buyukbingol E. Evaluation of Anti-HIV Activity of 5-(2-phenyl-3’- Indolyl)-2-thiohydantoin. Farmaco. 1998;53:525–527. doi: 10.1016/S0014-827X(98)00053-6. [DOI] [PubMed] [Google Scholar]
- 7.Gulcin I., Buyukokuroglu M.E., Oktay M., Kufrevioglu O.I. On the in vitro antioxidative properties of melatonin. J. Pineal Res. 2002;33:167–171. doi: 10.1034/j.1600-079X.2002.20920.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan D.X., Reiter R.J., Manchester L.C., Yan M.T., El-sawi M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 9.Bozkaya P., Dogan B., Suzen S., Nebioglu D., Ozkan S.A. Determination and investigation of electrochemical behaviour of 2-phenylindole derivatives: discussion on possible mechanistic pathways. Can. J. Anal. Sci. Spec. 2006;51:125–139. [Google Scholar]
- 10.Suzen S., Demircigil T., Buyukbingol E., Ozkan S.A. Electroanalitical Evaluation and Determination of 5-(3’-indolyl)-2-thiohydantoin Derivatives by Voltammetric studies: possible relevance to in vitro metabolism. New J. Chem. 2003;27:1007–1011. doi: 10.1039/b300160c. [DOI] [Google Scholar]
- 11.Suzen S., Ateş-Alagoz, Demircigil T., Ozkan S.A. Synthesis and Analytical Evaluation by Voltammetric Studies of Some New Indole-3-propionic acid Derivatives. Farmaco. 2001;56:835–840. doi: 10.1016/S0014-827X(01)01150-8. [DOI] [PubMed] [Google Scholar]
- 12.Kruk I., Aboul-Enein H.Y., Michalska T., Lichszteld K., Kubasik-Kladna K., Olgen S. In vitro scavenging activity for reactive oxygen species by N-substituted indole-2-carboxylic acid esters. Luminescence. 2007;22:379–386. doi: 10.1002/bio.974. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F., Zai-Qun L. Indole and its alkyl-substituted derivatives protect erythrocyte and DNA against radical-induced oxidation. J. Biochem. Mol. Toxicol. 2009;23:273–279. doi: 10.1002/jbt.20289. [DOI] [PubMed] [Google Scholar]
- 14.Gulcin I., Buyukokuroglu M.E., Kufrevioglu O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003;34:278–281. doi: 10.1034/j.1600-079X.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 15.Talaz O., Gulcin İ., Goksu S., Saracoglu N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg. Med. Chem. 2009;17:6583–6589. doi: 10.1016/j.bmc.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 16.Suzen S., Bozkaya P., Coban T., Nebioglu D. Investigation of in vitro antioxidant behaviour of some 2-phenylindole derivatives: discussion on possible antioxidant mechanisms and comparison with melatonin. J. Enzyme Inh. Med. Chem. 2006;21:405–411. doi: 10.1080/14756360500381210. [DOI] [PubMed] [Google Scholar]
- 17.Ates-Alagoz Z., Coban T., Suzen S. A comparative study: evaluation of antioxidant activity of melatonin and some indole derivatives. Med. Chem. Res. 2005;14:169–179. doi: 10.1007/s00044-005-0132-0. [DOI] [Google Scholar]
- 18.Gurkok G., Coban T., Suzen S. Melatonin analogue new indole hydrazide/hydrazone derivatives with antioxidant behavior: Synthesis and discussion on structure activity relationships. J. Enzym. Inh. Med. Chem. 2009;24:506–515. doi: 10.1080/14756360802218516. [DOI] [PubMed] [Google Scholar]
- 19.Wieland H., Konz W., Mittasch H. Toad positions VII. Constitution of bufotenin and bufotenidine. Justus Liebig Ann. Chem. 1934;513:1–25. doi: 10.1002/jlac.19345130102. [DOI] [Google Scholar]
- 20.Bulatova N.N., Suvorov N.N. Indole derivatives XXXVI. Reaction of 3-beta-nitrovinylindoles with nucleophilic reagents. Khim. Geterosikli. Soedin. 1969;5:813–17. [Google Scholar]
- 21.Baker J.W., Happold F.C., Walker N. The tryptophanase-tryptophan reaction: 7. Further evidence regarding the mechanism of the enzymic degradation of tryptophan to indole: criticism of the theory that β-o-aminophenylacetaldehyde is the indole-forming intermediate. Biochem. J. 1946;40:420–426. doi: 10.1042/bj0400420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L., Xinhua H., Zhibing Z., Yan L., Beifen S. Preparation of aryl hydrazide compounds as immunosupressives. WO/2007/036083. PCT Int. Appl. 2007
- 23.Niki E., Komuro E., Takahashi M., Urano S., Ito E., Terao K.J. Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. Biol. Chem. 1988;263:19809–19814. [PubMed] [Google Scholar]
- 24.Zhao F., Liu Z.-Q., Wu D. Antioxidative effect of melatonin on DNA and erythrocytes against free radical-induced oxidation. Chem. Phys. Lipids. 2008;151:77–84. doi: 10.1016/j.chemphyslip.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Suzen S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. In: Khan M.T.H., editor. Topics in Heterocyclic Chemistry. Vol. 11. Springer; Berlin, Heidelberg, Germany: 2007. pp. 145–178. [Google Scholar]
- 26.Allegra M., Reiter R.J., Tan D.X., Gentile C., Tesoriere L., Livrea M.A. The chemistry of melatonin's interaction with reactive species. J. Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079X.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 27.Kidwai M., Negi N., Gupta S.D. Synthesis and antifertility activity of 1,5-diaryl-3 (3'-indolyl)formazans. Chem. Pharm. Bull. (Tokyo) 1994;42:2363–2364. doi: 10.1248/cpb.42.2363. [DOI] [PubMed] [Google Scholar]
- 28.Lautraite S., Bigot-Lasserre D., Bars R., Carmichael N. Optimisation of cell-based assays for medium throughput screening of oxidative stress. Toxicol. In Vitro. 2003;17:207–220. doi: 10.1016/S0887-2333(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 29.Puntarulo S., Cederbaum A.I. Production of Reactive Oxygen Species by Microsomes Enriched in Specific Human Cytochrome P450 Enzymes. Free Radic. Biol. Med. 1998;24:1324–1330. doi: 10.1016/S0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 30.Gutteridge J.M., Quinlan G.J., Clark I., Halliwell B. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim. Biophys. Acta. 1985;835:441–447. doi: 10.1016/0005-2760(85)90113-4. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan G.J., Halliwell B., Moorhouse C.P., Gutteridge J.M. Action of lead(II) and aluminium (III) ions on iron-stimulated lipid peroxidation in liposomes, erythrocytes and rat liver microsomal fractions. Biochim. Biophys. Acta. 1988;962:196–200. doi: 10.1016/0005-2760(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z.Q., Luo X.Y., Sun Y.X., Chen Y.P., Wang Z.C. Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochim. Biophys. Acta. 2002;1572:58–66. doi: 10.1016/S0304-4165(02)00281-7. [DOI] [PubMed] [Google Scholar]