Abstract
Fluctuations of interleukin-1β (IL-1β) and heparin-binding epidermal growth factor (HB-EGF) in endometrial receptivity were detected. Seventy-two patients receiving in vitro fertilization-embryo transfer (IVF-ET) for the first time in Yantaishan Hospital from July 2015 to September 2015 due to infertility were selected. The serum and follicular fluid of patients in ovulation-promoting cycle were collected; the levels of IL-1β and HB-EGF in serum and follicular fluid were detected via enzyme-linked immunosorbent assay (ELISA), and the levels of serum estradiol (E2), progesterone (P), follicle stimulating hormone (FSH) and luteinizing hormone (LH) were detected. The endometria in early follicular phase and middle luteal phase were collected, and the mRNA expression levels of IL-1β and HB-EGF were evaluated by reverse transcription quantitative polymerase chain reaction (RT-qPCR). Patients were divided into two groups, and the implantation group (n=33) and the non-implantation group (n=39), according to whether embryos were implanted and the general data. In IVF-ET, the levels of IL-1β and HB-EGF in follicular fluid and middle luteal phase, the level of serum IL-1β on human chorionic gonadotropin (HCG) day and embryo transfer (ET) day, the levels of E2, FSH and LH on HCG day in implantation group were obviously higher than those in non-implantation group (p<0.05); the level of P on ET day in implantation group was significantly higher than that in non-implantation group (p<0.05); the expression levels of IL-1β and HB-EGF in endometrium in middle luteal phase in implantation group were higher than those in non-implantation group (p<0.05); the expression levels of IL-1β and HB-EGF in endometrium were positively correlated with the levels of E2 and P, and endometrial thickness (p<0.05). IL-1β and HB-EGF may improve the endometrial receptivity to embryo, thus affecting the embryo implantation rate, through the synergistic action with E2 and P, so they may be the indexes of predicting the IVF-ET pregnancy outcome.
Keywords: endometrial receptivity, interleukin-1β, heparin-binding epidermal growth factor, in vitro fertilization-embryo transfer
Introduction
Endometrial receptivity refers to the receptivity of maternal endometrium to blastocysts. The complex process that the blastocysts in the activated state invade into the endometrium and interact with one another after the positioning, adhesion and implantation is known as embryo implantation (1). This state has a certain temporal and spatial specificity, so it is known as ‘implantation window’, which generally appears in the mid-secretive phase of endometrium on the 20th to 23rd day in menstrual cycle (namely the 7th-9th day after ovulation) (2). The endometrium in the embryo implantation window phase, on the one hand, creates a suitable micro-environment for the embryonic development and implantation, and, on the other hand, regulates embryos to reach the most suitable place for implantation under the guidance of cytokines (3). Although the extensive development of assisted reproductive technology has brought the gospel to many patients with infertility, and the relevant operation techniques have significantly optimized the number and quality of embryos, the embryo implantation rate is still as low as about 20–30% (4), and about two thirds of patients received failed embryo implantation repeatedly due to poor endometrial receptivity (5). Studies have found that a variety of cytokines and growth factors act synergistically in the mother-fetal crosstalk process, such as interleukin-1β (IL-1β) and heparin-binding epidermal growth factor (HB-EGF) (6,7). Therefore, the study on relevant factors involved in endometrial receptivity is expected to be a key to improving the success rate of embryo implantation. In this study, the contents of IL-1β and HB-EGF in serum, follicular fluid and endometrium of infertile patients at different time were detected, and their correlations with endometrial receptivity were investigated, so as to elucidate their values in predicting the endometrial receptivity in in vitro fertilization-embryo transfer (IVF-ET).
Patients and methods
Patient selection
A total of 72 patients who receiving IVF-ET for the first time in Department of Reproductive Medicine, Yantaishan Hospital (Yantai, China) from July 2015 to September 2015 due to infertility were selected. All patients had a menstrual cycle of 28–30 days, and did not receive steroid hormone therapy in the past three months. Controlled ovarian hyperstimulation was performed using the long protocol developed by our center, and the follicles, endometrial thickness and implantation were monitored via vaginal B ultrasound. Gonadotropin (Gn) was withdrawn when the diameter (d) of at least three dominant follicles were ≥16 mm in bilateral ovaries, d of two dominant follicles ≥17 mm and diameter of one dominant follicle ≥18 mm; human chorionic gonadotropin (HCG) was injected on that night, and the estradiol (E2), progesterone (P), follicle stimulating hormone (FSH) and luteinizing hormone (LH) in blood were detected; at 34–36 h after injection of HCG, oocytes were retrieved via vaginal B ultrasound. P (80 mg/day) was injected on the day of embryo transfer (ET), and β-HCG in blood was detected after 2 weeks. β-HCG >25 mIU/ml indicated the biochemical pregnancy, and pregnant women continued to receive the luteal support. At 5 weeks after transfer, patients were divided into implantation group (n=33) and non-implantation group (n=39) according to whether they had clinical pregnancy. P continued to be applied and was decreased gradually until 3 months after pregnancy. This study was approved by the Ethics Committee of Yantaishan Hospital, and the patients signed the informed consent.
Methods
General conditions
The age, menstrual history, body mass index (BMI), duration of infertility, causes of infertility, Gn dosage (n), number of retrieved oocytes (n), number of transferred embryos (n), number of high-quality embryos (n) and number of frozen embryos (n) were recorded. The weight of the patients in fasting state was weighed, and the height of each patient was also measured. BMI = weight/height2.
Collection of endometrial tissues
In the natural cycle, the ovulation was monitored via vaginal B ultrasound combined with urine test paper; the fasting blood was drawn in the early morning in middle luteal phase (namely the 5th-7th day after ovulation) for simulated ET and endometrial test. A small number of endometria were stored in the refrigerator at −80°C for detection. At the same time, the pathological examination was performed to confirm the mid-secretive phase of endometrium. Endometrial repair was performed in the early follicular phase (the 3rd day in menstrual period), and a small number of endometria were stored in the refrigerator at −80°C for detection.
Serum collection
Before receiving the IVF-ET, the fasting blood was collected on the 3rd day in the menstrual cycle (D3), day of injecting HCG (HCG day), day of oocyte retrieval (OR day) and the day of ET (ET day), the serum was separated via high-speed centrifugation at 1,000 × g at 4°C for 10 min and placed in the refrigerator at −20°C for detection. The levels of serum IL-1β (cat. no. EHC002b.96, Neobioscience, Shenzhen, China) and HB-EGF (cat. no. RJ12420, Renjiebio, Nanchang, China) on D3, HCG day, OR day and ET day were measured via double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). The E2, P, FSH and LH levels on D3, HCG day and ET day were measured via radioimmunoassay.
Collection of follicular fluid
When the follicular development reached the standard, appropriate sedation was performed at 30 min before ovulation, and the bladder was emptied; under the lithotomy position, oocytes were retrieved via culdocentesis under the guidance of vaginal B ultrasound; 3 ml follicular fluid not washed was retained (the contaminated fluid was discarded), and the supernatant was taken via high-speed centrifugation at 1,000 × g at 4°C for 10 min and placed at −20°C for detection. The levels of IL-1β and HB-EGF in follicular fluid (FF) on OR day were measured via double-antibody sandwich ELISA in strict accordance with the instructions. Three wells were repeated for each specimen, and the average was taken.
All the patients received transvaginal ultrasound to detect the embryonic development and endometrial thickness. The measurement errors were excluded, each index was measured 3 times, and the average was taken.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was determined as follows: Total RNA extraction, synthesis of the first cDNA chain and RT-qPCR. The procedures were according to the instructions of TRIzol kit (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA), cDNA kit (Toyobo Life Science, Osaka, Japan) and SYBR-Green PCR kit (code no. QPK-201, Toyobo Life Science), respectively. Amplification system: 12.5 µl SYBR mixture, 2 µl cDNA, 0.5 µl forward primer, 0.5 µl reverse primer (Table I), addition of ddH2O until 25 µl. Reaction procedure: 42°C for 20 min, 95°C ×5 min (95°C 15 sec, 55°C ×15 sec, 72°C ×20 sec) for 40 cycles, 95°C ×1 min, 55°C ×30 sec and 95°C ×30 sec. The experiment was repeated 3 times to reduce the error and bias, and β-actin gene was selected as the internal reference. The data were analyzed using the Real-time detector (ABI-7500; New York, NY, USA), and the relative expression levels of IL-1β and HB-EGF mRNA in endometrial tissues were presented using 2−ΔΔCq (8).
Table I.
Primer sequences.
| Molecule | Primer (5′3′) |
|---|---|
| IL-1β | F: AAT GAT GGC TTA TTA CAG TGG C |
| R: TGT AGT GGT GGT CGG AGA TT | |
| HB-EGF | F: ACA AGG AGG AGC ACG GGA AAA G |
| R: CGA TGA CCA GCA GAC AGA CAG ATG | |
| β-actin | F: CAC AAG TCA CAC TTC ACA |
| R: CTA TCA CCT CGT GCT CT |
F, forward; R, reverse; IL-1β, interleukin-1β; HB-EGF, heparin-binding epidermal growth factor.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 18.0 (SPSS Inc., Chicago, IL, USA) software was used for analysis. Measurement data in normal distribution are presented as mean ± SD. Student's t-test or post hoc test after one-way analysis of variance in SPSS were used to analyze the differences between the groups. Tukey's was the post hoc test used following one-way analysis of variance. Pearson's correlation analysis was performed for the correlation between indexes. P<0.05 was considered to indicate a statistically significant difference.
Results
Etiological analysis of infertile patients
Among the 72 patients, 25 cases (34.72%) suffered from infertility due to tubal factors (tubal inflammation, history of tubal ligation and congenital tubal abnormality), 15 cases (20.83%) due to pelvic factors, 6 cases (8.33%) due to ovulation disorders, 5 cases (6.94%) due to endometriosis, 14 cases (19.44%) due to male factors (sperm abnormality: Oligo-astheno-teratospermia, necrospermia and azoospermia), and 7 cases (9.72%) due to unknown causes. Tubal factors ranked first (Fig. 1).
Figure 1.
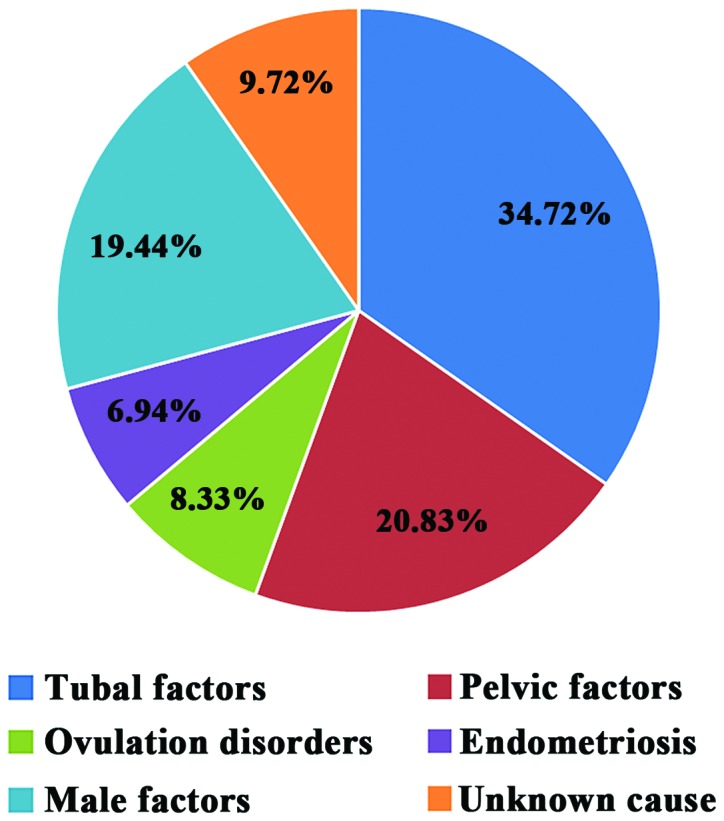
Pathogenesis of infertility patients.
Comparisons of general conditions between the two groups of patients
There were no significant differences in the age, duration of infertility, BMI, Gn dosage, number of retrieved oocytes, number of transferred embryos, number of high-quality embryos and number of frozen embryos between the two groups of patients (p>0.05). The baseline data were uniform and comparable (Table II).
Table II.
Comparisons of general conditions between implantation group and non-implantation group.
| Implantation group (n=33) | Non-implantation group (n=39) | t-value | P-value | |
|---|---|---|---|---|
| Age (years) | 30.12±4.32 | 29.56±4.51 | 0.81 | 0.31 |
| Duration of infertility (years) | 4.92±3.12 | 4.84±3.73 | 0.63 | 0.65 |
| BMI (kg/m2) | 22.01±2.45 | 21.71±2.14 | 0.54 | 0.69 |
| Gn dosage (n) | 37.76±8.31 | 38.22±9.17 | 0.71 | 0.62 |
| No. of retrieved oocytes (n) | 14.45±8.24 | 12.28±10.89 | 1.33 | 0.16 |
| No. of transferred embryos (n) | 2.14±0.53 | 2.03±0.65 | 1.20 | 0.24 |
| No. of high-quality embryos (n) | 8.41±4.63 | 8.32±4.58 | 0.98 | 0.27 |
| No. of frozen embryos (n) | 5.46±5.12 | 3.97±4.26 | 1.25 | 0.19 |
BMI, body mass index.
Comparisons of IL-1β and HB-EGF levels in serum and follicular fluid at each time-point between the two groups of patients
The HB-EGF levels changes are not significant in the early follicular phase, on HCG day, OR day and ET day of the two groups of patients (p>0.05). The level of HB-EGF in follicular fluid in implantation group was higher than that in non-implantation group, and the difference was statistically significant (t=2.76, p=0.01). There was no statistically significant difference in the IL-1β level on D3 between the two groups (p>0.05). The level of serum IL-1β on HCG day in implantation group was significantly increased compared with that on OR day or ET day (p<0.05). The level of serum IL-1β on HCG day in non-implantation group was higher than those on OR day and ET day (p<0.05), but it was still significantly lower than that in implantation group (p<0.05). The level of serum IL-1β on ET day in non-implantation group was significantly increased compared with that in implantation group (p<0.05). The level of IL-1β in follicular fluid in implantation group was significantly higher than that in non-implantation group (p<0.05). The level of IL-1β on OR day in implantation group was significantly higher than that on D3 (p<0.05) (Table III).
Table III.
IL-1β and HB-EGF levels in serum and follicular fluid of patients in the two groups.
| Serum | |||||
|---|---|---|---|---|---|
| Group | D3 | HCG day | OR day | ET day | FF |
| Implantation group (n=33) | |||||
| IL-1β (ng/ml) | 17.26±7.49 | 162.86±53.27 | 71.21±24.65 | 39.46±28.18 | 67.02±24.20 |
| HB-EGF (pg/ml) | 312.25±255.13 | 323.34±267.17 | 337.28±263.49 | 361.10±285.26 | 350.42±285.26 |
| Non-implantation group (n=39) | |||||
| IL-1β (ng/ml) | 15.32±7.37 | 82.86±35.34 | 33.91±14.53 | 60.46±25.12 | 39.08±18.26 |
| HB-EGF (pg/ml) | 272.28±195.06 | 288.43±207.62 | 295.29±213.87 | 285.14±205.83 | 230±285.26 |
IL-1β, interleukin-1β; HB-EGF, heparin-binding epidermal growth factor; D3, 3rd day in menstrual cycle; HCG day, day of injecting HCG; OR day, day of oocyte retrieval; ET day, day of embryo transfer; FF, follicular fluid.
Expression levels of IL-1β and HB-EGF in early follicular phase and middle luteal phase in the two groups of patients
The expression levels of IL-1β and HB-EGF in the early follicular phase in implantation group were significantly higher than those in non-implantation group, but the differences were not statistically significant (p<0.05). The expression levels of IL-1β and HB-EGF in endometrium in middle luteal phase in implantation group were higher than those in non-implantation group (p<0.05) (Table IV).
Table IV.
Expression levels of IL-1β and HB-EGF mRNA in early follicular phase and middle luteal phase in the two groups of patients.
| Group | Early follicular phase | t-value | P-value | Middle luteal phase | t-value | P-value |
|---|---|---|---|---|---|---|
| IL-1β mRNA expression level | ||||||
| Implantation group (n=33) | 0.35±0.18 | 1.45 | 0.09 | 0.92±0.20 | 6.41 | 0.01 |
| Non-implantation group (n=39) | 0.23±0.09 | 0.54±0.17 | ||||
| HB-EGF mRNA expression level | ||||||
| Implantation group (n=33) | 0.61±0.24 | 1.62 | 0.08 | 2.43±0.59 | 7.59 | 0.00 |
| Non-implantation group (n=39) | 0.50±0.17 | 1.12±0.58 | ||||
IL-1β, interleukin-1β; HB-EGF, heparin-binding epidermal growth factor.
Comparison of serum hormone levels at different time-points between the two groups of patients
The serum E2, P, FSH and LH levels in the two groups of patients on D3 had no statistically significant differences (p>0.05), and the basic endocrine level was consistent and comparable. The levels of E2, FSH and LH on HCG day in implantation group were significantly higher than those in non-implantation group (p<0.05), but there was no statistically significant difference in the P level (p>0.05). The P level on ET day in implantation group was obviously higher than that in non-implantation group (p<0.05), but there were no statistically significant differences in the levels of E2, FSH and LH (Table V).
Table V.
Comparison of serum hormone levels at different time-points between the two groups of patients.
| Group | E2 (pg/ml) | P (ng/ml) | FSH (mIU/ml) | LH (mIU/ml) |
|---|---|---|---|---|
| Implantation group (n=33) | ||||
| D3 | 56.12±10.23 | 0.65±0.46 | 4.28±1.47 | 3.13±1.39 |
| HCG day | 2893.52±1068.34 | 0.86±0.57 | 24.93±9.08 | 139.06±53.45 |
| ET day | 896.47±428.51 | 27.58±13.94 | 13.87±11.95 | 17.01±5.28 |
| Non-implantation group (n=39) | ||||
| D3 | 55.34±11.82 | 0.63±0.48 | 4.31±1.50 | 3.32±1.27 |
| HCG day | 1538.26±786.26 | 0.80±0.51 | 12.98±7.02 | 101.64±32.16 |
| ET day | 894.54±378.38 | 21.56±7.25 | 10.62±9.59 | 15.17±7.24 |
E2, estradiol; P, progesterone; FSH, follicle stimulating hormone; LH, luteinizing hormone; D3, 3rd day in menstrual cycle; HCG day, day of injecting HCG; ET day, day of embryo transfer.
Expression levels of IL-1β and HB-EGF in middle luteal phase in different endometrial thickness
There were 56 out of 72 patients with the endometrial thickness ≥9 mm (thick group), and 16 patients with the endometrial thickness <9 mm (thin group). The mRNA expression levels of IL-1β in middle luteal phase in the thick group and the thin group were 0.87±0.19 and 0.48±0.16, respectively. The expression of IL-1β mRNA in the thick group was significantly increased compared with that in the thin group (t=−2.68, P<0.01). The mRNA expression levels of HB-EGF in middle luteal phase in the thick group and the thin group were 1.91±0.86 and 0.90±0.28, respectively. The expression of HB-EGF mRNA in the thick group was significantly increased compared with that in the thin group (t=−3.12, P<0.05) (Fig. 2).
Figure 2.
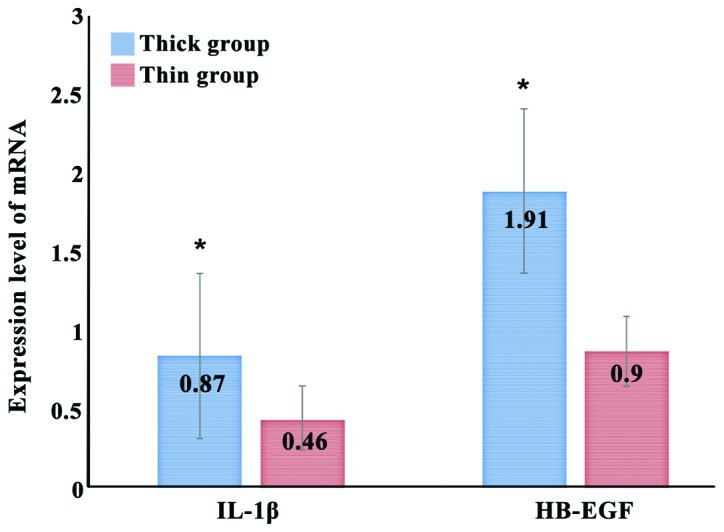
Expression levels of IL-1β and HB-EGF in middle luteal phase in different endometrial thickness. The mRNA expression level of IL-1β in the endometrium of the two groups in middle luteal phase has a statistically significant difference, *p<0.05 vs. intimal thickness <9 mm; the mRNA expression level of HB-EGF in the endometrium of the two groups in middle luteal phase has a statistically significant difference, *p<0.05 vs. intimal thickness <9 mm.
Correlation analysis of IL-1β and HB-EGF expression levels in endometrium in middle luteal phase with endometrial thickness and E2 and P
The expression level of IL-1β in the endometrium was positively correlated with the endometrial thickness and E2 and P levels (r=0.83, p<0.01; r=0.35, p<0.05; r=0.42, p<0.05). The expression level of HB-EGF was also positively correlated with the endometrial thickness and E2 and P (r=0.72, p<0.05; r=0.29, p<0.05; r=0.36, p<0.05) (Table VI).
Table VI.
Pearson's correlation analysis of IL-1β and HB-EGF expression levels in endometrium in middle luteal phase with endometrial thickness and E2 and P.
| Variables | Endometrial thickness (mm) | E2 (pg/ml) | P (ng/ml) |
|---|---|---|---|
| IL-1β in endometrium | |||
| r | 0.83 | 0.35 | 0.42 |
| p | 0.00 | 0.03 | 0.02 |
| HB-EGF in endometrium | |||
| r | 0.72 | 0.29 | 0.36 |
| p | 0.01 | 0.04 | 0.03 |
IL-1β, interleukin-1β; HB-EGF, heparin-binding epidermal growth factor; E2, estradiol; P, progesterone.
Discussion
Reasons for infertility in patients receiving IVF. Over the past 30 years, with the marketing of the assisted fertility technique and the vigorous development of derivative techniques, the assisted reproduction technique informtion has reached the majority of infertility patients. In terms of reasons for infertility, it was found in this study that the fallopian tube factor ranks first (34.72%), including the tubal non-specific inflammation, history of tubal ligation, and congenital abnormalities of fallopian tube; pelvic factors; the male factors (sperm abnormality: Oligo-astheno-teratospermia, necrospermia and azoospermia) are the main factors of intracytoplasmic sperm injection (ICSI); the endometriosis ranks second; ovulation disorders; and there are other causes of infertility, including chromosomal abnormalities, male ejaculatory disorders and genital malformations; and unknown causes (9). In this study, it was found that the number of patients receiving fresh ET due to ovulation disorders was small (8.33%). Patients with ovulation disorders are often patients with polycystic ovary syndrome (PCOS) and susceptible to ovarian hyper-stimulation syndrome (OHSS). Compared with fresh ET, the frozen ET has a higher live birth rate, and a lower abortion rate (10).
IL-1β and endometrial receptivity. IL-1 was first discovered in 1972 by Gray et al (11) from the mononuclear cell culture supernatant, which can promote the thymocyte proliferation, so it is named lymphocyte activation factor. After 7 years, it was officially renamed IL-1. IL-1 is a polypeptide consisting of two receptor agonists, IL-1α and IL-1β, and one IL-1 receptor antagonist (IL-1rα). IL-1 receptors have two subtypes, namely the type I (IL-1RtI) and type II (IL-1RtII). In recent years, the role of IL-1β in reproductive activity has attracted increasing attention and is thought to be associated with endometrial receptivity (12). IL-1β regulates the reproduction of women through systemic and local effects: 1) It directly acts on genital center through the blood circulation; 2) it induces local expression in ovarian, uterine and follicular fluid, and regulates the reproductive function by promoting the P secretion and affecting the activity of Na+/K+-ATPase (13,14). It is currently recognized that IL-1β in blood is derived from macrophages, but the theory of cell origin of IL-1β in follicular fluid is not clear, such as plasma ultrafiltrate or interstitial cells, follicular cell and granulocyte (15,16). In this study, it was found that the serum IL-1β level in women was significantly increased in follicular phase and before ovulation (HCG day). The document analysis showed that at IL-1β >80 pg/ml, the ET capacity was significantly increased (17); when the serum IL-1β concentration was increased, E2 was also significantly increased, and they had a positive correlation (r=0.35, p=0.03); E2 plays a vital role in follicular growth and development and endometrial growth; the change rate of serum IL-1β concentration in implantation group was similar to that of blood LH; the IL-1β concentration was gradually increased with oocyte maturation and reached the peak on the day before ovulation (HCG day), indicating that IL-1β may be involved in follicular growth, development, maturation and induced ovulation, which can improve the fertilization rate and embryo quality, thereby improving the implantation rate. This study showed that there was only a small amount of IL-1β in serum on D3, but its level was significantly increased in luteal phase after ovulation; the concentration of serum IL-1β was positively correlated with progesterone (P) (r=0.42, p=0.02), suggesting that the serum IL-1β is closely related to P (18). The low-concentration P promotes IL-1β gene expression, while the high-concentration of P inhibits the expression (19), but the critical concentration of P in regulating the IL-1β gene expression remains unclear and needs further study. Simón et al (20) found that IL-1β, IL-1RtI and IL-1rα can be detected in the human endometrium, endometrial fluid, embryo and endometrial-embryo interface, which can block the IL-1RtI in rat endometrium, and inhibit the gamete implantation. So far, how IL-1 regulates the endometrial tolerance is not yet clear. Some scholars believe that IL-1 increases the epithelial adhesion to the blastocyst by increasing the expression of epithelial cell adhesion molecules, thereby regulating the endometrial receptivity (21). IL-1 is found to be upregulated at 7 and 9 days in ovulation stage, consistent with the ‘implantation window’ stage. Moreover, IL-1β is involved in the immune tolerance in placental formation and promotes the growth of embryos via regulating the expression of NF-кB (22). In this study, it was found that IL-1 receptor antagonists act on the ‘implantation window’ stage of endometrium, terminating the embryo implantation process (22). In addition, studies have shown that (23) IL-1 may trigger angiogenesis, thus promoting the embryonic growth, through affecting the expression of HCG.
HB-EGF and endometrial receptivity. HB-EGF is one of the members of epidermal growth factor family and binds to the HB-EGF receptor on the surface of embryo and endometrium under the catalysis of heparan sulfate proteoglycan to play a role in mediating the ‘molecular crosstalk’ between embryo and intima (24). HB-EGF plays different roles in follicular development, ovulation, embryo implantation and early development (25), which has become a research hotspot in the field of reproduction. Studies have found that HB-EGF mRNA is expressed in the endometrium (mainly expressed in the luminal epithelium and glandular epithelium, and reaches the peak in the mid-secretive stage or at 20 days in menstrual cycle), early pregnancy villi, decidua and placenta in the entire menstrual cycle (26,27). The results of RT-qPCR in this study showed that the expression of HB-EGF mRNA in endometrium in middle luteal phase was significantly higher than that in early follicular phase (p<0.05), suggesting that the significantly increased HB-EGF mRNA in the endometrium in middle luteal phase may be closely related to the embryo implantation. Studies have revealed that HB-EGF may be maternal, which attracts blastocysts, promotes the development of blastocysts, adheres to the blastocyst and repairs endometrial function in the process of implantation (28). The results of this study showed that the relative expression of HB-EGF mRNA in implantation group in implantation window was significantly higher than that in non-implantation group (p<0.05). Maternal HB-EGF lacks causes the embryo implantation delay, and destroys the synchronous endometrial and embryonic development, leading to pregnancy failure (29). Thus, HB-EGF is expected to be one of the potential predictors of IVF-ET pregnancy outcome. In addition, it was found in this study that in the IVF cycle, the embryo implantation rate in patients with endometrial thickness of ≥9 mm was significantly higher than that with thickness <9 mm (p<0.05), and the results were consistent with the findings of Sher and Fisch (30). The expression level of HB-EGF was positively correlated with the endometrial thickness (r=0.72, p=0.01), indicating that HB-EGF mRNA may mediate the endometrial growth, thus affecting the IVF pregnancy outcome. It was found in this study that HB-EGF could be detected in serum throughout the menstrual cycle, but there were no statistically significant differences in the expression levels on D3, OR day and ET day (p>0.05). In view of many factors affecting the HB-EGF in serum, it is more important to evaluate the expression of HB-EGF in endometrium and follicular fluid, instead of evaluating the endometrial receptivity via serum HB-EGF. The cytokines in the microenvironment of follicular fluid will have direct effects on the development, maturation and discharge of oocytes and the embryogenesis after fertilization. It was found in this study that the level of HB-EGF in follicular fluid in implantation group was significantly higher than that in non-implantation group (p<0.05), indicating that the expression level of HB-EGF in follicular fluid is related to the embryo implantation and affects the outcome of pregnancy.
The expression of IL-1β and HB-EGF in endometrium show the periodic regularity; they are highly expressed in middle luteal phase and implantation group, but lowly expressed in early follicular phase and non-implantation group. Moreover, the expression levels of IL-1β and HB-EGF are positively correlated with the endometrial thickness, and levels of serum E2 and P, indicating that IL-1β and HB-EGF may improve the endometrial receptivity to embryo, thus affecting the embryo implantation rate, through the synergistic action with E2 and P, so they may be the indexes of predicting the IVF-ET pregnancy outcome.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are included in this published study.
Authors' contributions
HW contributed to the conception of the study and the performance of the statistical analysis with constructive discussions. GS helped with the collection of endometrial tissues. ML contributed significantly to data analysis and manuscript preparation; HF and HM performed the data analyses. LS contributed significantly to the data analysis and study preparation. All authors read and approved the final study.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Yantaishan Hospital (Yantai, China). Informed consents were signed by the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 2.Lim HJ, Dey SK. HB-EGF: A unique mediator of embryo-uterine interactions during implantation. Exp Cell Res. 2009;315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdez-Morales FJ, Gamboa-Domínguez A, Vital-Reyes VS, Cruz JC, Chimal-Monroy J, Franco-Murillo Y, Cerbón M. Changes in receptivity epithelial cell markers of endometrium after ovarian stimulation treatments: Its role during implantation window. Reprod Health. 2015;12:45. doi: 10.1186/s12978-015-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17:213–218. doi: 10.1093/humrep/17.1.213. [DOI] [PubMed] [Google Scholar]
- 5.Scalici J, Laughlin BB, Finan MA, Wang B, Rocconi RP. The trend towards minimally invasive surgery (MIS) for endometrial cancer: An ACS-NSQIP evaluation of surgical outcomes. Gynecol Oncol. 2015;136:512–515. doi: 10.1016/j.ygyno.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Huang HY, Wen Y, Kruessel JS, Raga F, Soong YK, Polan ML. Interleukin (IL)-1beta regulation of IL-1beta and IL-1 receptor antagonist expression in cultured human endometrial stromal cells. J Clin Endocrinol Metab. 2001;86:1387–1393. doi: 10.1210/jcem.86.3.7284. [DOI] [PubMed] [Google Scholar]
- 7.Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Kintiraki E, Papakatsika S, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones (Athens) 2015;14:211–223. doi: 10.14310/horm.2002.1582. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 11.Grey ST, Csizmadia V, Hancock WW. Differential effect of tumor necrosis factor-alpha on thrombomodulin gene expression by human monocytoid (THP-1) cell versus endothelial cells. Int J Hematol. 1998;67:53–62. doi: 10.1016/S0925-5710(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 12.Lédée N, Dubanchet S, Oger P, Meynant C, Lombroso R, Ville Y, Chaouat G. Uterine receptivity and cytokines: New concepts and new applications. Gynecol Obstet Invest. 2007;64:138–143. doi: 10.1159/000101737. [DOI] [PubMed] [Google Scholar]
- 13.Tobai H, Nishiya I. Nitric oxide mediates inhibitory effect of interleukin-1beta on estrogen production in human granulosa-luteal cells. J Obstet Gynaecol Res. 2001;27:53–59. doi: 10.1111/j.1447-0756.2001.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 14.Qinglei L, Jiang N, Shuling B, Lan Y. Effect of interleukin-1 β on progesterone production and sodium-potassium adenosine triphosphatase activity by rat luteal cell in vitro. J Reprod Med. 2000;9:88–91. [Google Scholar]
- 15.Austgulen R, Arntzen KJ, Vatten LJ, Kahn J, Sunde A. Detection of cytokines (interleukin-1, interleukin-6, transforming growth factor-beta) and soluble tumour necrosis factor receptors in embryo culture fluids during in-vitro fertilization. Hum Reprod. 1995;10:171–176. doi: 10.1093/humrep/10.1.171. [DOI] [PubMed] [Google Scholar]
- 16.Takacs P, Kauma S. The expression of interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor type I mRNA during preimplantation mouse development. J Reprod Immunol. 1996;32:27–35. doi: 10.1016/S0165-0378(96)00987-4. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez RR, Leavis P. Leptin upregulates beta 3-integrin expression and interleukin-1beta, upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine. 2001;16:21–28. doi: 10.1385/ENDO:16:1:21. [DOI] [PubMed] [Google Scholar]
- 18.Machelon V, Nome F, Durand-Gasselin I, Emilie D. Macrophage and granulosa interleukin-1 beta mRNA in human ovulatory follicles. Hum Reprod. 1995;10:2198–2203. doi: 10.1093/oxfordjournals.humrep.a136268. [DOI] [PubMed] [Google Scholar]
- 19.Díaz Flores M, Ortega-Camarillo C, Rosales-Torres AM, Baiza-Gutman LA, Hicks JJ. Nitric oxide as main effector in the interleukin-1 system in ovulation. Gac Med Mex. 2001;137:291–302. (In Spanish) [PubMed] [Google Scholar]
- 20.Simón C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1993;77:549–555. doi: 10.1210/jcem.77.2.8345061. [DOI] [PubMed] [Google Scholar]
- 21.Bourdiec A, Akoum A. Embryo implantation: Role of interleukin 1 family members. Med Sci (Paris) 2014;30:644–650. doi: 10.1051/medsci/20143006014. (In French) [DOI] [PubMed] [Google Scholar]
- 22.Geisert R, Fazleabas A, Lucy M, Mathew D. Interaction of the conceptus and endometrium to establish pregnancy in mammals: Role of interleukin 1β. Cell Tissue Res. 2012;349:825–838. doi: 10.1007/s00441-012-1356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourdiec A, Shao R, Rao CV, Akoum A. Human chorionic gonadotropin triggers angiogenesis via the modulation of endometrial stromal cell responsiveness to interleukin 1: A new possible mechanism underlying embryo implantation. Biol Reprod. 2012;87:66. doi: 10.1095/biolreprod.112.100370. [DOI] [PubMed] [Google Scholar]
- 24.Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev. 2009;76:1116–1127. doi: 10.1002/mrd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umata T. Mechanism for activation of heparin-binding EGF-like growth factor induced by stimuli. J UOEH. 2004;26:85–97. doi: 10.7888/juoeh.26.85. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 26.Birdsall MA, Hopkisson JF, Grant KE, Barlow DH, Mardon HJ. Expression of heparin-binding epidermal growth factor messenger RNA in the human endometrium. Mol Hum Reprod. 1996;2:31–34. doi: 10.1093/molehr/2.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jc.84.9.3355. [DOI] [PubMed] [Google Scholar]
- 28.Stavreus-Evers A, Koraen L, Scott JE, Zhang P, Westlund P. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil Steril. 2005;83:156–162. doi: 10.1016/j.fertnstert.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Armant DR, Wang J, Liu Z. Intracellular signaling in the developing blastocyst as a consequence of the maternal-embryonic dialogue. Semin Reprod Med. 2000;18:273–287. doi: 10.1055/s-2000-12565. [DOI] [PubMed] [Google Scholar]
- 30.Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–1076. doi: 10.1016/S0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]


