Supplemental Digital Content is available in the text
Keywords: meta-analysis, metastatic castration-resistant prostate cancer, tasquinimod
Abstract
Background:
Tasquinimod is an oral quinoline-3-carboxamide derivative for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Tasquinimod has antiangiogenic, immunomodulatory, and antimetastatic properties, but it is also associated with toxicities, including skeletal pain, digestive disorders, fatigue, insomnia, and mental disorders. We aimed to perform a meta-analysis to evaluate the efficacy, safety, and long-term survival for tasquinimod in patients with mCRPC.
Methods:
Searches were carried out in PubMed, Embase, and the Cochrane Library. Eligible articles included randomized clinical trials (RCTs) comparing systemic or combination therapy (excluding primary or secondary androgen deprivation therapy, bone protective agents, or radionuclides) with placebo in men with mCRPC.
Results:
Three RCTs were selected for final evaluation. The pooled results from the 3 studies indicated that tasquinimod was associated with good radiologic progression-free survival (rPFS) in mCRPC. For adverse effects (AEs), the results of meta-analysis indicated that patients with mCRPC who received tasquinimod had obvious anemia (risk ratio (RR) 1.35, 95% confidence interval (CI) 1.06–1.73, P = .02), back pain (RR: 1.57, 95% CI: 1.01–2.47, P = .05), pain in the extremities (RR: 1.90, 95% CI: 1.14–3.17, P = .01), insomnia (RR: 1.50, 95% CI: 1.03–2.17, P = .03), vomiting (RR: 1.52, 95% CI: 1.04–2.21, P = .03), and peripheral edema (RR: 1.52, 95% CI: 1.03–2.23, P = .03).
Conclusions:
Tasquinimod is associated with better rPFS in mCRPC. The toxicity of tasquinimod requires further investigation, it is not recommended for routine clinical use.
1. Introduction
In most western countries, prostate cancer is the second leading cause of cancer-related death in men, and the most common nonskin cancer among men in northern European countries and the United States.[1–3] The data of GLOBOCAN2012 show that the estimated number of new cases worldwide was 1,095,000, with an estimated 307,000 deaths.[4] Despite the fact that most men treated for localized prostate cancer are cured, 20% of patients will experience recurrence of the disease.[5] Androgen deprivation therapy is commonly used for men with recurrent, progressive, or metastatic prostate cancer that is androgen sensitive,[6] but almost all of these patients will eventually develop castration-resistant prostate cancer (CRPC) despite low levels of testosterone.
Recently, the therapeutic options for metastatic CRPC (mCRPC) has increased, including immunotherapy (sipuleucel-T), chemotherapeutics (docetaxel and cabazitaxel), hormonal therapies (abiraterone acetate and enzalutamide), and bone-targeted therapies (denosumab and radium-223).[7–12] However, the efficacy of these treatments is not good and they characteristically cause many adverse effects (AEs). For this reason, the development of new drugs for CRPC is ongoing. A study found that angiogenesis play a vital role in the process of prostate cancer development. Other findings have revealed that microvascular density is substantially higher in metastatic prostate cancer tissue and the expression level of vascular endothelial growth factor is associated with the degree of cancer differentiation.
Tasquinimod is an oral quinoline-3-carboxamide derivative with antiangiogenic, immunomodulatory, and antimetastatic properties.[13–16] Preclinically, the growth of prostate cancer in clinical tumors from patients with localized or metastatic, androgen receptor (AR)-positive or AR-negative, AR wild-type or mutant, as well as prostate-specific antigen (PSA)-positive or PSA-negative cancers, can be strongly inhibited by tasquinimod in vivo, for a range of phenotypes and genotypes.[17] The molecular target of tasquinimod is S100A9 (MRP-14), a suitable therapeutic target in oncology that, as suggested by tumor growth is impaired in S100A9 knockout mouse models.[18] Tasquinimod binds to histone deacetylase,[4] a potential target, as has been described in various in vitro studies, which may result in reductions in stress-mediated hypoxia signaling and angiogenesis induction in the tumor microenvironment.[19] These data suggested a novel mechanism for tasquinimod efficacy in prostate cancer, which is different from other agents that have been evaluated to date.
Since the publication of randomized phase II and III studies of maintenance therapy with tasquinimod in patients with mCRPC, several AEs of tasquinimod have been reported, including skeletal pain, digestive disorders, fatigue, insomnia, and mental disorders. The aim of this study was to determine whether tasquinimod therapy improves patient outcomes and to perform a pooled analysis evaluating the risk ratio (RR) for AEs of tasquinimod in patients with mCRPC.
2. Materials and methods
2.1. Search strategy
This meta-analysis was carried out in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA). We carried out a search of the PubMed, Embase, and Cochrane Library databases covering all papers published up to March 2018. The language was restricted to English, and we used search terms that were a combination of subject headings and free text words, with the keywords “prostatic neoplasms” and “tasquinimod.” All eligible studies were retrieved, and their references were cross-searched to identify additional suitable studies. We have included the most complete published report when the data published by the same investigators overlapped (see Supplemental List, for the detailed search strategy terms).
No ethical approval and consent from patients are required as all analyses were based on previous published studies.
2.2. Study selection
Studies were included in our meta-analysis if they met all of the following criteria: tumors were mCRPC, confirmed by pathological or histological examination; the study was a randomized controlled trial (RCT) and evaluation was conducted of the association between efficiency of tasquinimod and survival; and inclusion of complete information was included of hazard ratios (HRs) with their 95% confidence interval (CI). The main reasons for excluding studies were if they were: case reports, editorial, reviews, animals studies, or conference abstracts without original data; duplicates of previous publications.
2.3. Screening and data extraction
In this study, data extraction and quality assessment were done independently by 2 investigators (PG, XL), and agreement on all items. For each study, we extracted the following characteristics: first author surname, year of publication, country, number of patients, HRs with their 95% CI, and AEs indicators, including the number of patients with cancer pain, decreased appetite, nausea, fatigue, constipation, anemia, asthenia, decreased weight, back pain, pain in extremities, arthralgia, diarrhea, insomnia, vomiting, and peripheral edema of all grades. If the above data were not reported, items were designated as “NR (not reported).”
2.4. Risk of bias assessment
We applied the Cochrane Collaboration tool for assessing risk of bias, including the following items: generation of randomization sequence, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias. This tool mainly evaluates the risk of bias based on six domains of bias, and determination is made according to “low risk of bias,” “unclear risk of bias,” or “high risk of bias” for each indicator (see Supplemental Figure 1, which describes the risk of bias assessment).
2.5. Outcomes
In our study, the primary outcome was radiologic progression-free survival (rPFS), and the secondary efficacy endpoint was overall survival (OS). HR and its 95%CI were used to estimating the effect of tasquinimod on survival in mCRPC patients. RRs and their 95% CIs were used to estimate the adverse effects of tasquinimod on survival in patients with mCRPC.
2.6. Statistical analysis
Two separate analyses were performed for articles reporting extended rPFS with the use of tasquinimod and AEs of tasquinimod. We only used the sources for HRs and RRs that were given explicitly in the published studies, and we used a Mantel–Haenszel random-effect model to weight and pool the estimates of HRs and RRs. Chi-squared tests and the I2 statistic were used to quantify heterogeneity.[20,21] A funnel plot test was used to assess publication bias.[22,23] Statistical significance was considered to be when the 95%CI for the HR did not include 1 (equivalent to P < .05). All P values were 2-sided.
In our meta-analysis, all statistical analyses were conducted using STATA 13 statistical software (Stata Corp., College Station, TX) and Review Manager 5.3.
3. Results
3.1. Search results
The results of the search strategy are described in Fig. 1. With our retrieval strategy, a total of 61 studies were found. Upon further screening, 10 reports were excluded as they were duplicate publications, 2 were excluded for having no overall text, and 4 were excluded because they were animal and not human studies. After excluding duplicate studies, screening the titles and abstracts, and reading the full texts, 3 studies were selected for the final evaluation; the characteristics of these studies are summarized in Table 1.[24–26] The total number of patients was 1568. All 3 studies were dealing with rPFS, but OS results were not significant in 2 in the studies of final evaluation because no safety concerns were raised.
Figure 1.
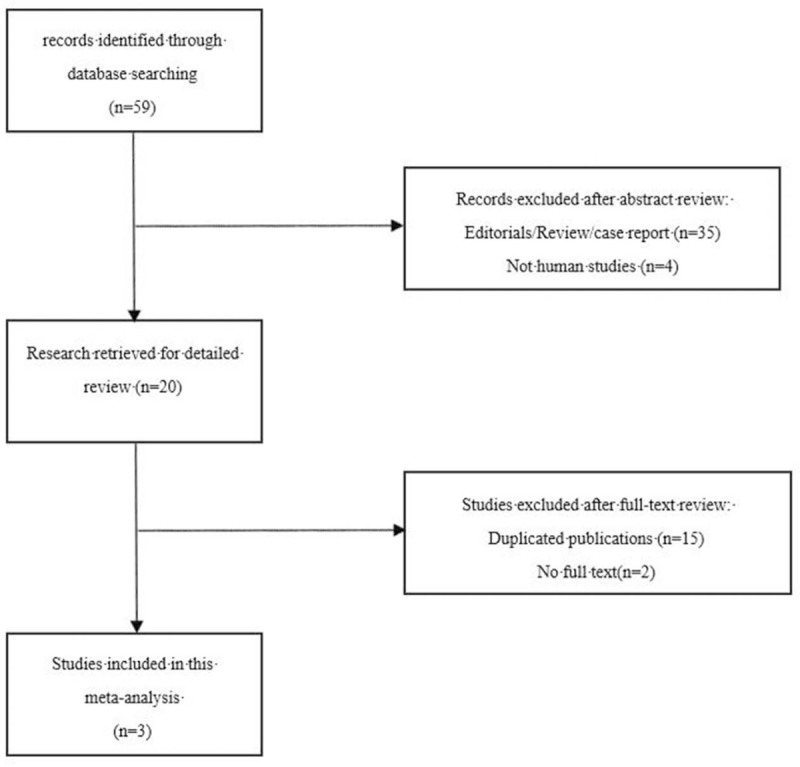
Flow chart of search strategy.
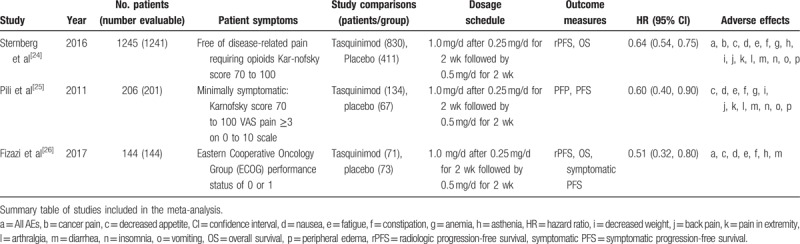
Table 1.
Main characteristics and results of the eligible studies.
3.2. Meta-analysis results
The main results of the meta-analysis are listed in Table 2. The pooled results from the 3 included studies indicated that tasquinimod was associated with good rPFS in mCRPC (HR: 0.62, 95% CI: 0.54–0.72, P < .001, I2 = 0.0%) (Fig. 2).
Table 2.
Main meta-analysis results.

Figure 2.
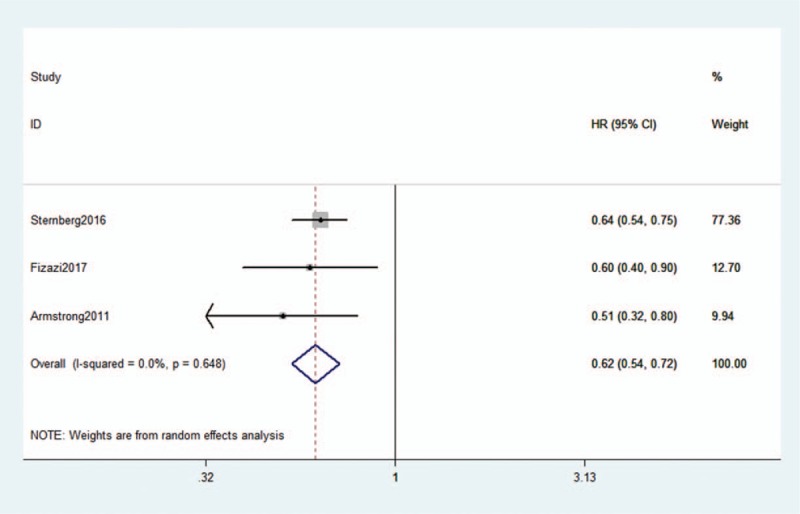
Forest plot of hazard ratio for the association of tasquinimod and radiologic progression-free survival.
The RRs and 95% CI: for all aspects of AEs are listed in Table 3. Tasquinimod was associated with AEs in mCRPC. The results of meta-analysis indicated that patients with mCRPC who received treatment with tasquinimod had AEs that included obvious anemia (RR: 1.35, 95% CI: 1.06–1.73, P = .02, I2 = 0.0%), back pain (RR: 1.57, 95% CI: 1.01–2.47, P = .05, I2 = 30.0%), pain in extremity (RR: 1.90, 95% CI: 1.14–3.17, P = .01, I2 = 24.0%), insomnia (RR: 1.50, 95% CI: 1.03–2.17, P = .03, I2 = 0.0%), vomiting (RR: 1.52, 95% CI: 1.04–2.21, P = .03, I2 = 0.0%), and peripheral edema (RR: 1.52, 95% CI: 1.03–2.23, P = .03, I2 = 0.0%). Other AEs demonstrated no significant association with tasquinimod (Fig. 3 ).
Table 3.
Meta-analysis results of AEs.
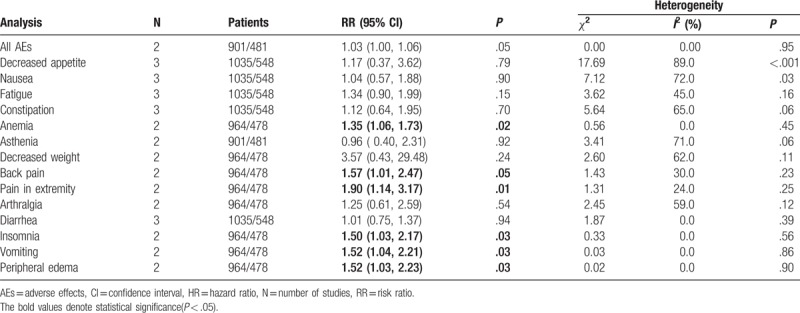
Figure 3.
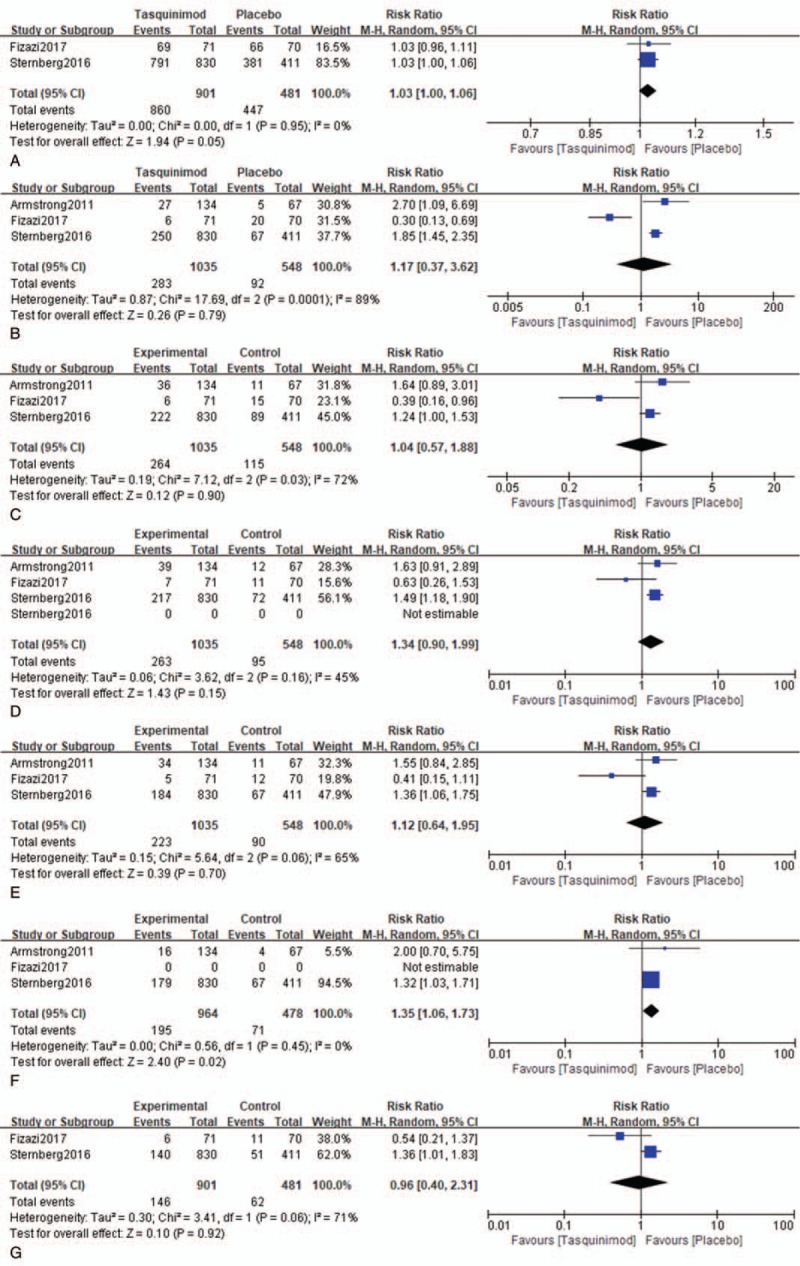
Forest plot of hazard ratio for the association of tasquinimod and AEs. A = All AEs, B = decreased appetite, C = nausea, D = fatigue, E = constipation, F = anemia, G = asthenia, H = decreased weight, I = back pain, J = pain in extremity, K = arthralgia, L = diarrhea, M = insomnia, N = vomiting, O = peripheral edema.
Figure 3 (Continued).
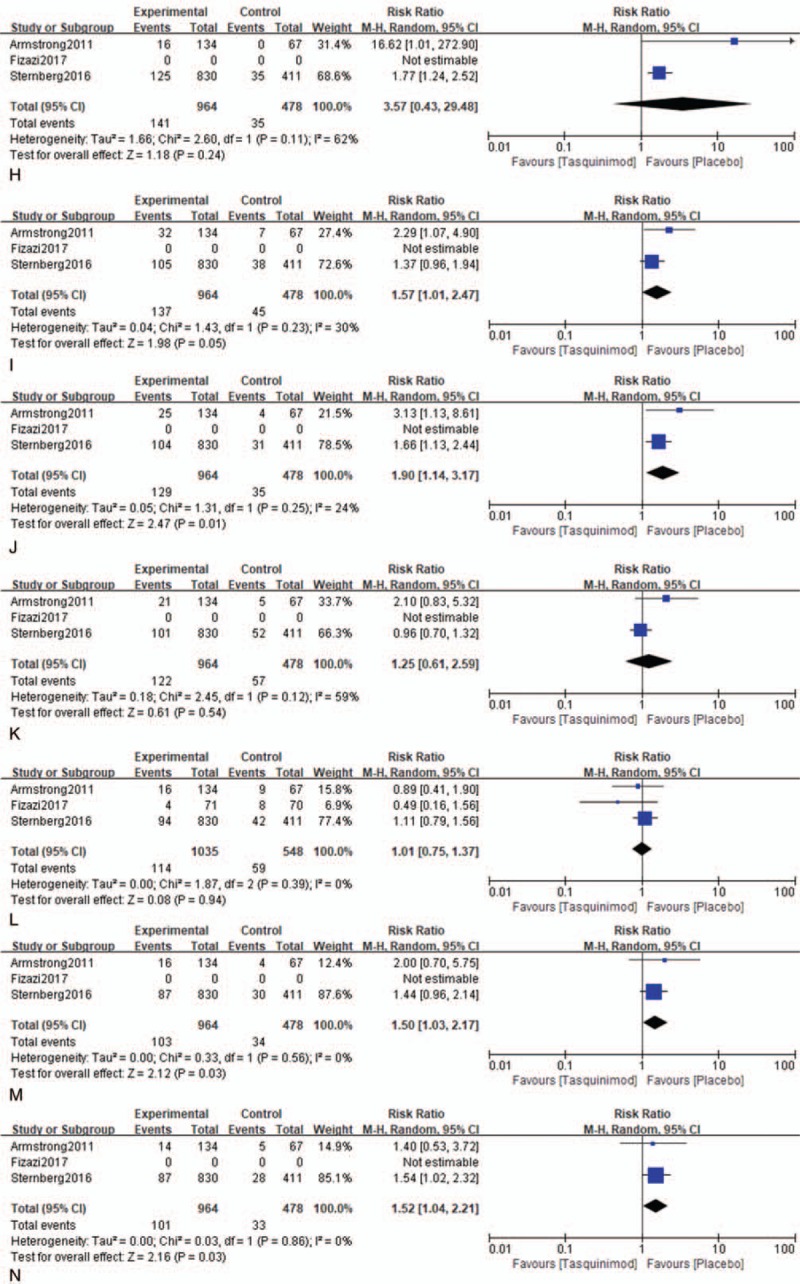
Forest plot of hazard ratio for the association of tasquinimod and AEs. A = All AEs, B = decreased appetite, C = nausea, D = fatigue, E = constipation, F = anemia, G = asthenia, H = decreased weight, I = back pain, J = pain in extremity, K = arthralgia, L = diarrhea, M = insomnia, N = vomiting, O = peripheral edema.
3.3. Publication bias
We used a funnel plot with pseudo 95% confidence limits to assess publication bias. As shown in Supplemental Figures 2 and 3, the shape of the funnel plot demonstrated cursory symmetry in terms of rPFS and all aspects of AEs.
4. Discussion
Tasquinimod is a novel small-molecule inhibitor and second-generation oral quinoline-3-carboxamide analog with antiangiogenic properties and tumor growth-inhibiting activity against human prostate cancer models.[27] The mechanism of inhibition of angiogenesis has been demonstrated in different in vitro assays including endothelial capillary tube formation, aortic ring, and chorioallantoic membrane assays.[15] In addition, tasquinimod binds to S100A9, which has a role in cancer via myeloid derived suppressor cells and displays antiangiogenic properties and antitumor activity in prostate cancer models.[16,18,19,28–31]
For patients with mCRPC, docetaxel is considered the reference systemic therapy, with investigational efforts through 2012 directed toward predocetaxel, docetaxel combinations, and postdocetaxel therapeutic domains. We observed that there is no clear benefit with endothelin-a inhibitors atrasentan or zibotentan among predocetaxel targeted therapy regimens for mCRPC. This evidence demonstrates that tasquinimod may be a superior treatment for mCRPC.
In our study, we pooled previous RCTs of tasquinimod to verify that it has better performance for extended rPFS in patients with mCRPC, however, the agent is controversial owing to AEs. Our meta-analysis revealed that significant AEs in patients using tasquinimod included anemia, back pain, pain in the extremities, insomnia, vomiting, and peripheral edema. These findings suggested that the toxicity of tasquinimod must be considered before it commercialized.
What's more, the following points are worth noting. First, there are few published results of RCTs about tasquinimod; therefore, the publication bias for rPFS and AEs may be a debatable point. Next, we could not explore treatment outcomes with subgroup analysis by different ethnicities owing to a lack of access to individual patient data. Last, we only included trials comparing tasquinimod with placebo and we excluded trials comparing other treatment strategies to reduce heterogeneity.
5. Conclusions
Our meta-analysis suggests that use of tasquinimod is associated with better rPFS in mCRPC. However, the toxicity of tasquinimod requires further investigation; therefore, it is not recommended for routine clinical use. Our findings should be interpreted with caution owing to the heterogeneities of the included studies and bias of the pooled analysis. Further, clinical studies should be carried out to determine the efficacy of tasquinimod, especially the related AEs. As always, patients should be encouraged to participate in clinical trials.
Author contributions
Conceptualization: Ping Gong.
Formal analysis: Xinyu Liu.
Methodology: Ping Gong, Xinyu Liu.
Project administration: Xiumin Zhang.
Software: Xinyu Liu.
Supervision: Xiumin Zhang.
Validation: Hongjian Liu.
Writing—original draft: Ping Gong, Ge Zhou, Meitian Liu, Xiaodi Yang, Wenjing Xiong, Qi Wang.
Writing—review & editing: Ping Gong, Hongjian Liu, Juan Ma, Zheng Ren, Minfu He, Xiumin Zhang.
Supplementary Material
Footnotes
Abbreviations: AEs = adverse effects, CI = confidence interval, CRPC = castration-resistant prostate cancer, HR = hazard ratio, mCRPC = metastatic castration-resistant prostate cancer, OS = overall survival, RCTs = randomized clinical trials, rPFS = radiologic progression-free survival, RR = risk ratio.
Funding: The authors received no funding for this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Shin H, Bray F, et al. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC cancer base no. 10. Int J Cancer 2012;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Ahmedin J, Freddie B, Center MM, et al. Global cancer statistics. CA Cancer J Clin 1900;61:69–90. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Center MM, Desantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907. [DOI] [PubMed] [Google Scholar]
- [4].GLOBOCAN2012. Available at: https://jingyan.baidu.com/article/fec4bce2752613f2618d8b29.html Accessed March 15, 2018. [Google Scholar]
- [5].Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011;65:1180–92. [DOI] [PubMed] [Google Scholar]
- [6].Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2007;25:1596–605. [DOI] [PubMed] [Google Scholar]
- [7].de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2011;59:1147–54. [DOI] [PubMed] [Google Scholar]
- [8].Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. Urol Clin North Am 2010;39:465–81. [DOI] [PubMed] [Google Scholar]
- [9].Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2014;191:213–23. [DOI] [PubMed] [Google Scholar]
- [10].Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- [12].Tannock IF, De WR, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. J Urol 2004;173:242–5. [DOI] [PubMed] [Google Scholar]
- [13].Dalrymple SL, Becker RE, Isaacs JT. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate 2007;67:790–7. [DOI] [PubMed] [Google Scholar]
- [14].Isaacs JT. The long and winding road for the development of tasquinimod as an oral second-generation quinoline-3-carboxamide antiangiogenic drug for the treatment of prostate cancer. Expert Opin Investig Drugs 2010;19:1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Isaacs JT, Pili R, Qian DZ, et al. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate 2010;66:1768–78. [DOI] [PubMed] [Google Scholar]
- [16].Källberg E, Vogl T, Liberg D, et al. S100A9 interaction with TLR4 promotes tumor growth. PLoS ONE 2012;7:e34207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bratt O, Haggman MG. Open-label, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br J Cancer 2009;101:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Björk P, Björk A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 2009;7:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Isaacs JT, Antony L, Dalrymple SL, et al. Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res 2013;73:1386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil 2006;87:875. [DOI] [PubMed] [Google Scholar]
- [21].Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007;335:914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [23].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sternberg C, Armstrong A, Pili R, et al. Randomized, double-blind, placebo-controlled phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J Clin Oncol 2017;34:2636–43. [DOI] [PubMed] [Google Scholar]
- [25].Pili R, Häggman M, Stadler WM, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 2011;29:4022–8. [DOI] [PubMed] [Google Scholar]
- [26].Fizazi K, Ulys A, Sengelov L, et al. A randomized, double-blind, placebo-controlled phase II study of maintenance therapy with tasquinimod in patients with metastatic castration-resistant prostate cancer responsive to or stabilized during first-line docetaxel chemotherapy. Ann Oncol 2017;28:2741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Osanto S, Van PH, Burggraaf J. Tasquinimod: a novel drug in advanced prostate cancer. Future Oncol 2013;9:1271–81. [DOI] [PubMed] [Google Scholar]
- [28].Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 2008;205:2235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol 2006;8:1321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sinha P, Okoro C, Foell D, et al. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 2008;181:4666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008;29:2035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


