Abstract
Rationale:
Periurethral abscess is a life-threatening disease, and the occurrence of a urethral defect with periurethral abscess is a rare finding. In this case, the patient had a lengthy urethral defect from the bulbous urethra to the membranous urethra accompanied by periurethral abscess that developed within a short time. Herein, we report a case of a pedicle-sparing tunica vaginalis flap utilized in urethral reconstruction which degenerated due to fibrotic changes and soft tissue defects in the urethral bed.
Patient concerns:
The patient was a 36-year-old man with fever and lower urinary tract symptoms who had been treated with antibiotics and anti-inflammatory drugs for urinary tract infections 3 days before admission. Purulent necrosis was formed by the urethral abscess, and a long-length urethral defect was formed in the bulbous urethra.
Diagnosis:
Based on the initial computed tomography and laboratory findings, empirical antibiotics were administered to treat a lower urinary tract infection. On the 7th day of hospitalization, ultrasonography was performed due to the sudden swelling of the scrotum, and the patient was diagnosed with a periurethral abscess that was 10 × 3 cm in size.
Intervention:
Initial urinary diversion, wide debridement, and a large amount of abscess drainage were performed. Necrosis of the urethral ventral part caused a urethral defect that was 5 cm in size. After treatment with antibiotics, long-term disinfection and intermittent debridement were conducted and urethral reconstruction was performed using a tunica vaginalis flap with preserved vascular structure.
Outcomes:
No complications occurred until 6 months after urethral reconstruction.
Lessons:
Urethral reconstruction using a tunica vaginalis flap is a good method for selected patients. Pedicle-sparing tunica vaginalis is an advantageous material for resolving urethral defects, especially when the surrounding circulation conditions are poor.
Keywords: periurethral abscess, tunica vaginalis flap, urethral reconstruction
1. Introduction
Periurethral abscess is a rare, infectious, life-threatening disease that occurs around the periurethral and urethral tissues in men. The initial infection may begin with a small focal lesion localized within the Buck fascia, but crossing this border can lead to extensive tissue necrosis, including the fascia and subcutaneous tissue. Early diagnosis and treatment can reduce the morbidity and mortality of patients and reduce post-treatment complications.[1]
The occurrence of urethral defect with periurethral abscess is a rare finding and has been reported only in some cases in the literature.[2] We noted a long urethral defect from the bulbous urethra to the membranous urethra, accompanied by purulent necrosis in a middle-aged man, which developed within a short time. Herein, we report a case of pedicle-sparing tunica vaginalis flap utilized in urethral reconstruction with inflammatory fibrotic changes and soft tissue defects in the urethral bed after initial urinary diversion, wide debridement, and antibiotic treatment.
2. Case report
2.1. Patient information
This study was performed with the approval and supervision of the Institutional Review Board of the Gangneung Asan Hospital, Gangneung, Republic of Korea. We conducted this study after obtaining informed consent from the patient. The overall course of the patient is summarized in Figure 1. A 36-year-old man was admitted to the emergency room with fever, gross hematuria, and dysuria of sudden onset 4 days ago. He visited a local clinic and was suspected to have urinary tract infection. He took ciprofloxacin 500 mg twice a day and nonsteroidal anti-inflammatory drugs (NSAIDs) for 3 days. He had no underlying disease diagnosed without taking medication regularly. He had social alcohol consumption, and he is a current 20-pack-year smoker.
Figure 1.
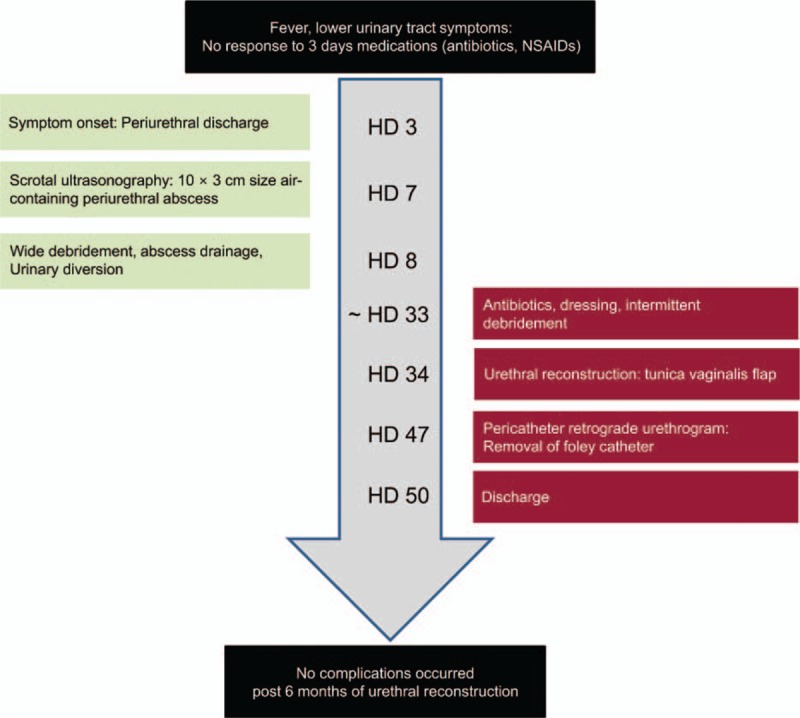
CARE-compliant flow diagram of the patient.
2.2. Physical examination and diagnostic tests
Initial vital signs at the emergency room were body temperature of 39.6°C which indicated high grade fever, blood pressure of 105/50 mm Hg, a heart rate of 121 beats/min, and respiratory rate of 22 breaths/min. Chills were observed as a symptom. No specific voiding symptoms other than dysuria were observed, and there were no suspicions of sexually transmitted disease and lymph node enlargement in the inguinal area. A history of urethral discharge was not observed, and digital rectal examination showed a tenderness pattern with a burning sensation in the prostate. No costovertebral angle tenderness was observed.
Initial laboratory test revealed the following: white blood cell (WBC) count of 3,900 (neutrophil 88.2%), elevated C-reactive protein of 18.5 mg/dL, hemoglobin of 13.5 g/dL, and decreased platelet count of 80,000/mm3. The following values were also recorded: serum calcium, 8.5 mg/dL; blood urea nitrogen, 26.5 mg/dL; creatinine, 2.05 mg/dL; aspartate aminotransferase/alanine aminotransferase ratio, 75/107 u/L; Na/K/Cl, 138/3.2/107; glucose, 255 mg/dL; and prostate-specific antigen, 0.23 ng/mL. The stick urine test revealed the following values: pH 5.0, albumin 4+, slight cloudy brown color, WBC 2+, and negative nitrite test. The urine polymerase chain reaction test for urethritis was negative. Nonenhanced computed tomography (CT) was performed along with an initial imaging test to find the cause of fever. No evidence of inflammation in the urogenital tract as well as lymph node enlargement was observed.
2.3. Initial diagnosis and treatment
The initial diagnosis was low urinary tract infection, and a 14-Fr Foley catheter was inserted due to difficult and painful urination. Intravenous ciprofloxacin 400 mg twice a day, hydration, and NSAIDs were used to control pain. On hospital day (HD) 3, symptoms of periurethral discharge occurred, and on HD 5, blood culture revealed the presence of methicillin-susceptible Viridans streptococcus, and antibiotics were changed from ciprofloxacin (400 mg bid, BAYER) to ceftriaxone (2 g q, Roche).
2.4. Therapeutic intervention and exclusion diagnosis
Scrotal swelling started on HD 7. Scrotal ultrasonography was performed because of scrotal swelling, urethral discharge, and continuous urethral pain. Scrotal ultrasonography revealed a 10 × 3 cm air-containing periurethral abscess with severe soft tissue swelling (Fig. 2). We changed the antibiotics to a combined regimen of meropenem (500 mg bid, YUHAN) and metronidazole (500 mg bid, Alvogen). On HD 8, scrotal exploration with wide debridement and suprapubic urinary diversion was performed (Fig. 3).
Figure 2.
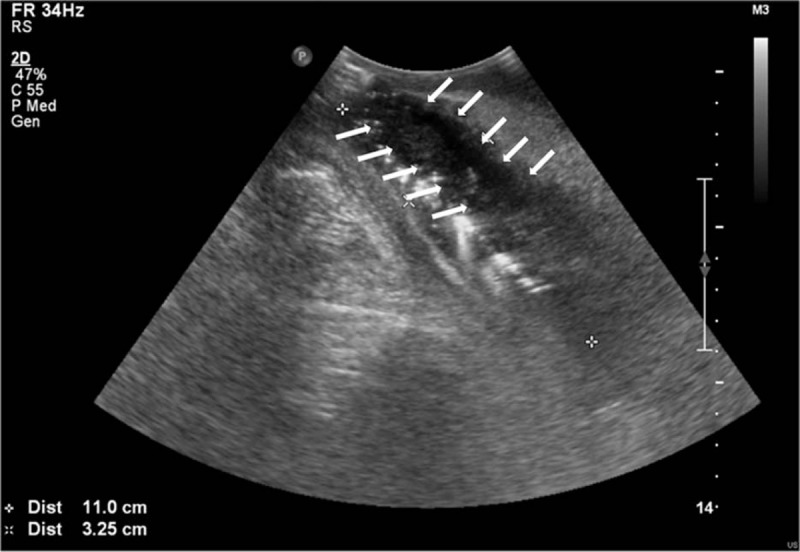
Scrotal ultrasonography reported a 10 × 3 cm air-containing periurethral abscess with severe soft tissue swelling. The arrow indicates the abscess.
Figure 3.
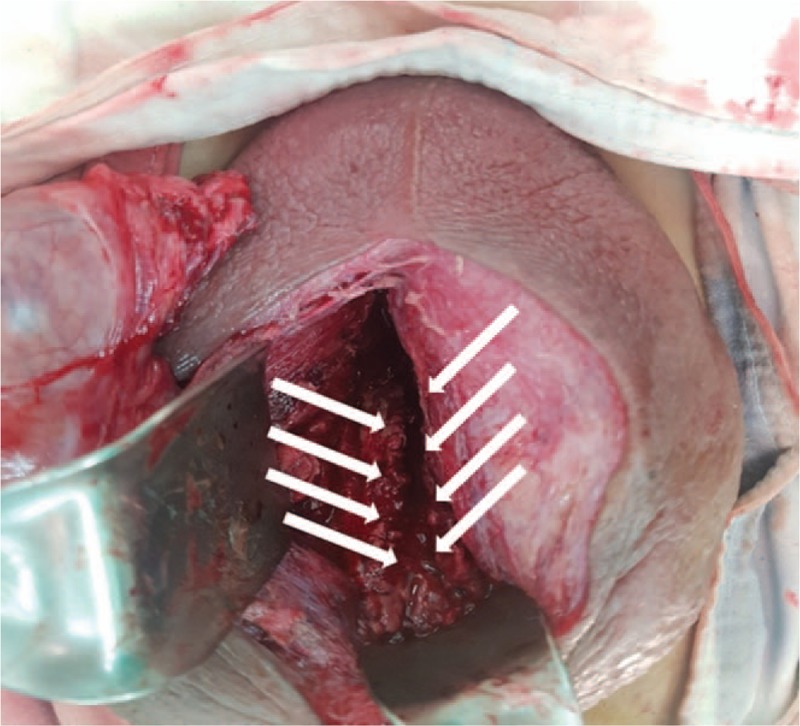
Scrotal exploration with wide debridement and suprapubic urinary diversion were performed. The arrow indicates the urethral defect.
During the operation, inflammation originating from the rectum was excluded with the help of a general surgeon. Magnetic resonance imaging detected an 8- to 9-cm infiltration inferior to the penile root extension from the penoscrotal junction inferior to the membranous urethra without rectal involvement.
2.5. Reconstructive surgical technique (tunica vaginalis flap)
On HD 34, urethral reconstruction was performed using a tunica vaginal flap by the ventral onlay technique. Fibrous tissue near the urethral reconstructed sites (Fig. 4) was debrided. The tunica vaginalis surrounding the right testis was appropriately marked with the length and width of the urethral defect size (Fig. 5). The tunica vaginalis was preserved in the proximal direction of the pedicle vascular structure and was dissected into a U-shape (Fig. 6). The harvested tunica vaginalis was trimmed and rotated to fit the urethral defect, and it was sutured to the urethral dorsal part using the running method and a 5-0 Vicryl (ETHICON, USA) absorbable suture (Fig. 7).
Figure 4.
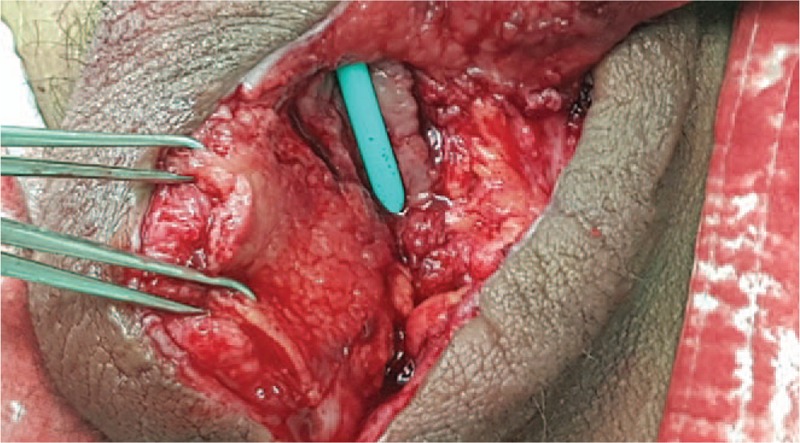
Debridement of the fibrous tissue near the urethral reconstructed site.
Figure 5.
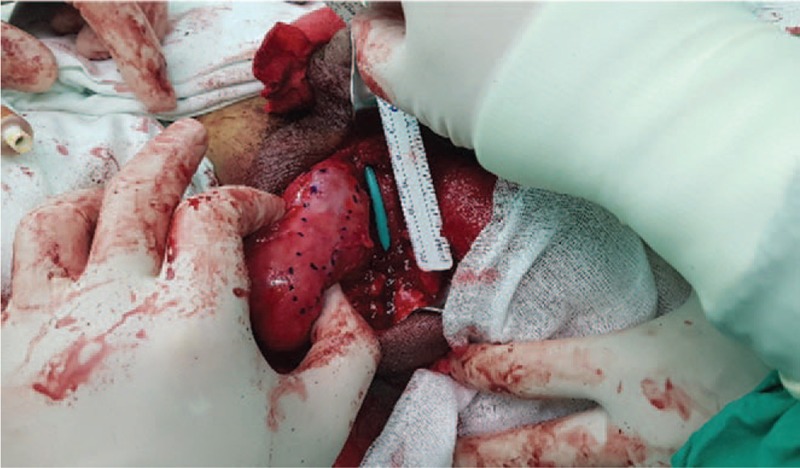
The tunica vaginalis surrounding the right testis was appropriately marked with the length and width of the urethral defect size.
Figure 6.
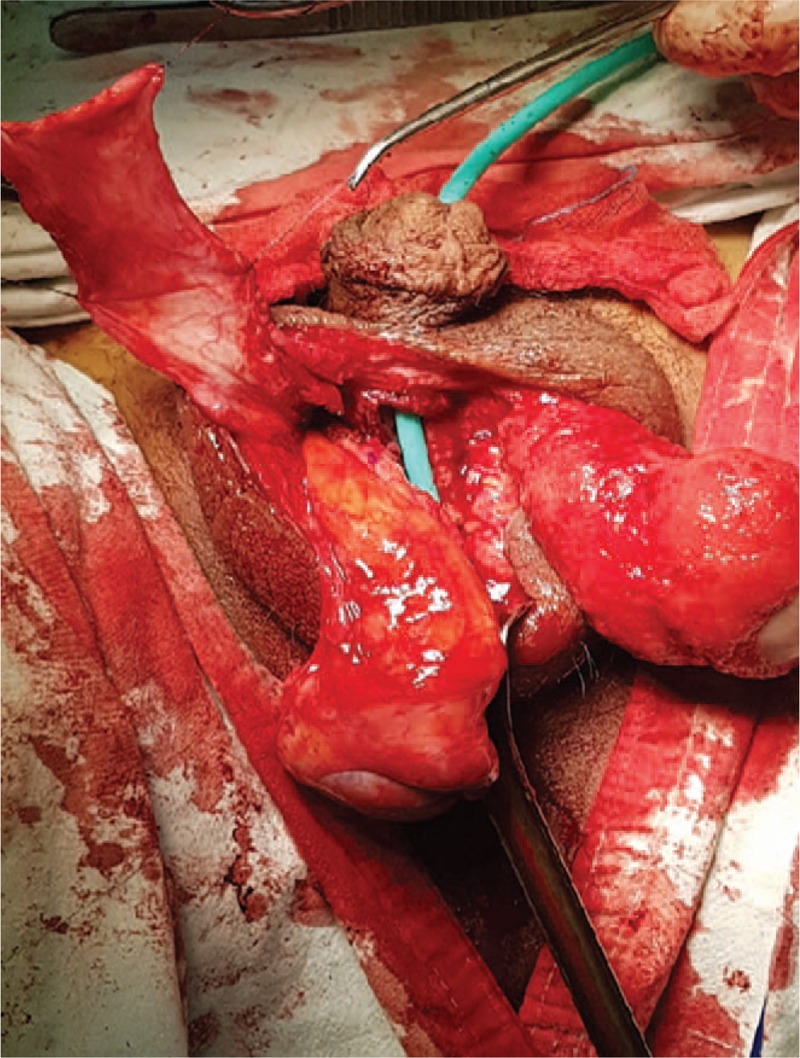
The tunica vaginalis was preserved in the proximal direction of the pedicle vascular structure and was dissected into a U shape.
Figure 7.
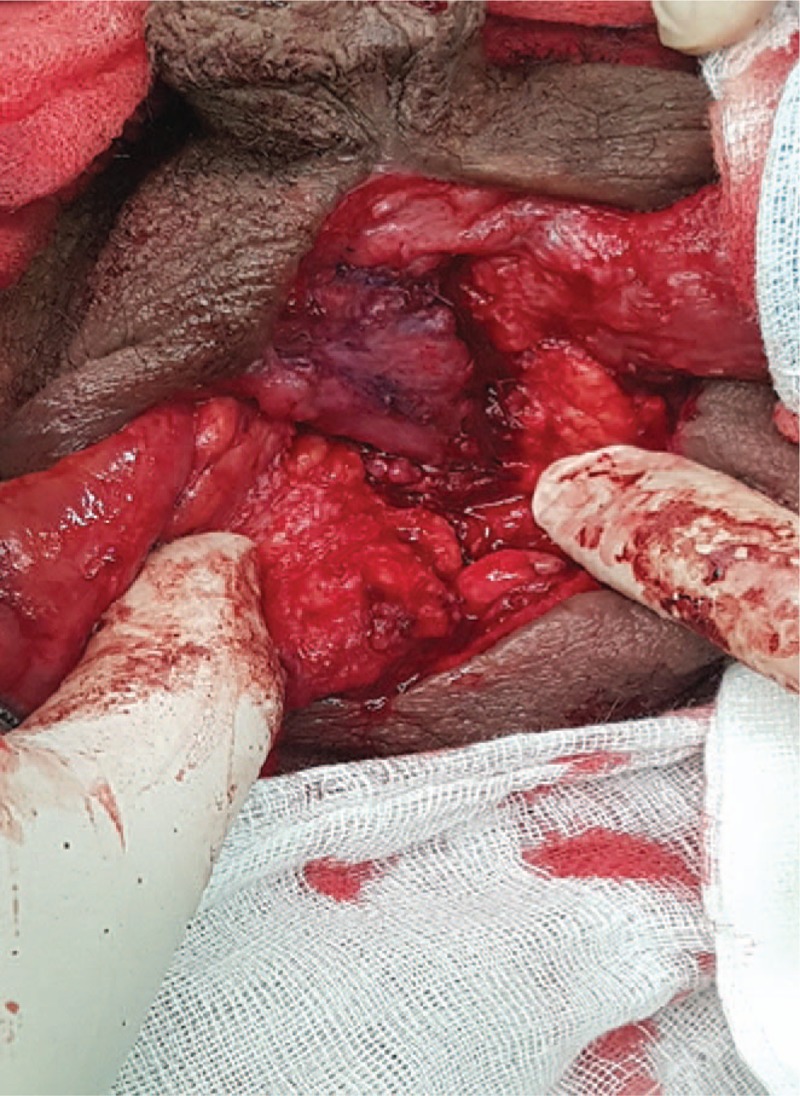
The harvested tunica vaginalis was trimmed and rotated to fit the urethral defect, and the urethral dorsal part was sutured using the running method and 5-0 Vicryl.
2.6. Follow-up and outcomes
On HD 47, pericatheter retrograde urethrography was performed to check for urethral leakage. Favorable results were revealed when his urination was evaluated by uroflowmetry test (voided volume: 101 mL, maximum flow rate: 22.4 mL/s, average flow rate: 7.9 mL/s, residual urine: 11 mL) without any discomfort, and the operation site was clean during the 6-month follow-up period.
3. Discussion
The treatment of periurethral abscess is mainly based on antibiotic therapy including cephalosporin and aminoglycoside and immediate urinary diversion and wide debridement. Long-term suprapubic diversion or perineal urethrostomy is known to be helpful in the course of treatment. However, there are no prospective studies on this; thus, clinical experience should follow empirical treatment, and after inflammatory treatment, delayed reconstruction depending on the extent and location of the urethral injury should be performed.[1]
Buccal mucosal grafts are most commonly used for urinary reconstruction. The beneficial aspects of the urethral graft material are the viable nature of the moist environment, resistance to the formation of urethral stone because of the absence of hair bulbs, ease of management, and a high graft success rate.[3–5] In addition, the stomach, small intestines, and vesical mucosa have been studied as urethral reconstruction materials, and these are associated with disadvantages as invasive surgery required donor organ tissue, with a success rate of 0% to 66%.[6,7]
Urethral reconstructive procedures are selected considering the overall environment, including the extent of the urethral defect size, viability of tissues around the urethra, and general circulation status of the patient. The urethral defect of the patient was 5 cm long, and the anatomical cheek was very short. If grafting of the buccal mucosa was performed, the buccal mucosa should be grafted from both cheeks and the graft connected. In local conditions, for graft transplantation, the urethral bed is denaturalized to inflammatory fibrotic tissues; however, in this case, there are soft tissue deficits due to previous wide debridement. The patient had unfavorable systemic conditions, in that he had a persistent high blood sugar level and was a 20-pack-year current smoker.
The maintenance of early viability for successful graft is known to go through an imbibition period, where nutrients and oxygen diffuse through the basal cells of the epidermis and the lamina propria. After this period, the lamina propria of the buccal mucosal graft is very thin, which is known to be beneficial to the neovascularization in the recipient site. However, buccal mucosa is a limited material, and availability depends on the cheek area. Moreover, it may not be suitable for treating large-sized, complicated urethral deficits.[7,8] In patients with postinflammatory fibrotic changes and soft tissue defects in the urethral bed, as in this case, tunica vaginalis flaps with preserved vascularity may be more advantageous than grafts that require vitality through diffusion because of the lack of imbibition. Pedicle-sparing tunica vaginalis has many advantages in addition to being well vascularized. It can reduce operation time because of its short distance from the donor site to the recipient site. It is easy to handle, versatile, rich in tissue, is waterproof, and has a high reported success rate of 86.6%–100%.[9,10] In studies on histologic feasibility, the mesothelium in the tunica vaginalis is found to change, similar to the urothelial lining of the naive urethra, by gradually replacing the stratified epithelial lining.[11] This patient showed favorable outcomes during the 6-month follow-up period, but long-term results will be needed to understand the histologic suitability of the urinary tract epithelium discussed above.
4. Conclusion
In this case, urethral reconstruction with tunica vaginalis flap is a good method for long-length urethral defect in urethral beds with inflammatory changes. Pedicle-sparing tunica vaginalis is an advantageous material for resolving urethral defects, especially when the circulation is poor. Further studies are needed with long-term follow-up periods.
Author contributions
Conceptualization: Sung Jin Kim, Sang Hoon Song, Kun Suk Kim, Han Gwun Kim.
Data curation: Jongpill Lee, Chang-Hoo Park.
Formal analysis: Sung Jin Kim, Jong Yeon Park.
Investigation: Sung Jin Kim, Jongpill Lee.
Methodology: Sung Jin Kim, Han Gwun Kim.
Project administration: Sung Jin Kim, Jong Yeon Park, Han Gwun Kim.
Resources: Sung Jin Kim, Jong Yeon Park, Han Gwun Kim.
Software: Sung Jin Kim, Jong Yeon Park, Han Gwun Kim.
Supervision: Sang Hoon Song, Kun Suk Kim, Han Gwun Kim.
Validation: Sung Jin Kim, Sang Hoon Song, Kun Suk Kim.
Visualization: Sung Jin Kim.
Writing – original draft: Sung Jin Kim, Jongpill Lee.
Writing – review & editing: Sung Jin Kim, Han Gwun Kim.
Footnotes
Abbreviations: CT = computed tomography, HD = hospital day, NSAIDs = nonsteroidal anti-inflammatory drugs, WBC = white blood cell.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Walther MM, Mann BB, Finnerty DP. Periurethral abscess. J Urol 1987;138:1167–70. [DOI] [PubMed] [Google Scholar]
- [2].Kanno T, Shibasaki N, Ito M, et al. Infection-induced urethral defect treated by urethral reconstruction with a radial forearm flap. Int J Urol 2005;12:228–30. [DOI] [PubMed] [Google Scholar]
- [3].Dubey D, Kumar A, Mandhani A, et al. Buccal mucosal urethroplasty: a versatile technique for all urethral segments. BJU Int 2005;95:625–9. [DOI] [PubMed] [Google Scholar]
- [4].Barbagli G, Vallasciani S, Romano G, et al. Morbidity of oral mucosa graft harvesting from a single cheek. Eur Urol 2010;58:33–41. [DOI] [PubMed] [Google Scholar]
- [5].Kovell RC, Terlecki RP. Ventral inlay buccal mucosal graft urethroplasty: a novel surgical technique for the management of urethral stricture disease. Korean J Urol 2015;56:164–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meeks JJ, Erickson BA, Fetchev P, et al. Urethroplasty with abdominal skin grafts for long segment urethral strictures. J Urol 2010;183:1880–4. [DOI] [PubMed] [Google Scholar]
- [7].Xu YM, Qiao Y, Sa YL, et al. Substitution urethroplasty of complex and long-segment urethral strictures: a rationale for procedure selection. Eur Urol 2007;51:1093–8. discussion 98–9. [DOI] [PubMed] [Google Scholar]
- [8].Chen ML, Odom BD, Johnson LJ, et al. Combining ventral buccal mucosal graft onlay and dorsal full thickness skin graft inlay decreases failure rates in long bulbar strictures (>/ = 6 cm). Urology 2013;81:899–902. [DOI] [PubMed] [Google Scholar]
- [9].Foinquinos RC, Calado AA, Janio R, et al. The tunica vaginalis dorsal graft urethroplasty: initial experience. Int Braz J Urol 2007;33:523–9. discussion 29-31. [DOI] [PubMed] [Google Scholar]
- [10].Yaqubi AA, Yuan WW, Mei SH, et al. Tunica vaginalis pedicle flap for reconstruction of anterior urethral stricture. Low Urin Tract Symptoms 2014;6:15–9. [DOI] [PubMed] [Google Scholar]
- [11].Rosito TE, Pires JA, Delcelo R, et al. Macroscopic and histological evaluation of tunica vaginalis dorsal grafting in the first stage of Bracka's urethroplasty: an experimental study in rabbits. BJU Int 2011;108:E17–22. [DOI] [PubMed] [Google Scholar]


