Abstract
Inadequate ablation lesion formation may be responsible for post-ablation ventricular tachycardia (VT) recurrences.
We aimed to evaluate whether visualisation of radiofrequency (RF) lesion size by cardiac magnetic resonance imaging (CMR) has any role in predicting adequacy of lesion and in estimating outcome.
Retrospective pilot study
Nine consecutive patients (8 male, age 60 ± 13 years) underwent ablation for sustained VT because of ischemic scar were evaluated for pre- and post-procedure scar tissue by CMR to characterize ablation lesions. Microvascular obstruction (MVO) surrounded by late gadolinium enhancement was defined as irreversible RF lesion. All patients were followed for at least 6 months for recurrences.
Five of the patients had previous inferior myocardial infarction (MI), whereas remaining 4 had anterior MI. Acute procedural success, as defined by termination of the arrhythmia without recurrence in 30 minutes, was attained in all patients. Contrast enhancement and wall motion abnormality in presumed infarction area were confirmed by pre-ablation CMR images. MVO was detected at the reported ablation site in 6/9 patients, all arrhythmia- and symptom-free at median 24 months (range 8–38 months) follow-up. In remaining 3 patients who had VT recurrence (clinical VT in 2, sustain VT with a new morphology in 1), MVO was not detected despite achievement of acute procedural success. There was no correlation with pre-ablation scar size and clinical arrhythmia recurrence.
CMR is a useful imaging modality to guide ablation procedures by detecting scar tissue. Additionally MVO seen by post-procedural imaging may be related to adequacy of RF ablation lesions and may correlate with clinical outcome.
Keywords: cardiac magnetic resonance, radiofrequency catheter ablation, recurrence, ventricular tachycardia
1. Introduction
Catheter ablation of ventricular tachycardia (VT) has been proven to be an effective strategy in patients with structural heart disease.[1,2] In patients with ischemic cardiomyopathy, activation mapping during stable VT or substrate-based mapping strategies during sinus rhythm have become the mainstay of VT ablation.[3,4] Although prophylactic role of radiofrequency (RF) ablation before defibrillator implantation was previously studied in detail, data obtained from large series of patients reveal that prevention of VT recurrence is not achieved after endocardial ablation in a significant proportion of patients.[4–8] The following variables have been implicated as possible factors in the failure of ablation: hemodynamic instability; complex arrhythmia substrate; multiple VTs, non-inducibility; and uncertainty over the best ablation method to undertake.[4,9–11] Although RF ablation using an irrigated-tip ablation catheter in combination with an electroanatomical mapping system is most commonly attempted method, the success of procedure is dependent some confusing factors such as the choice of mapping technique (activation-mapping, pace-mapping, or substrate-mapping), the choice of ablation area (endocardial, epicardial, or endo-epicardial ablation), and ablation technique (focal, linear, or substrate isolation).[12] All these variables are not only VT dependent but also are operator dependent, and currently, the best approach has yet to be defined. Endocardial ablation attempts may fail if the location of critical components of VT circuit is extending deeper to the endocardium.[13] Epicardial ablation has become an essential alternative strategy in these patients. However, in some cases, scar architecture and inadequate ablation lesion may be the only cause of endocardial ablation failure. Acute assessment of adequate ablation lesion creation is currently performed via indirect measures such as termination of arrhythmia, changes in the intracardiac electrograms, measurement of impedance, delivered power, thermal changes on catheter tip.[14] Imaging of myocardial scar tissue by cardiac magnetic resonance imaging (CMR) was previously studied to identify patients at risk of VT recurrence.[15,16] As an indicator of acute RF lesion size, contrast-enhanced CMR was used in patients underwent atrial fibrillation ablation.[17] In a recently published study, Grant et al [18] evaluated acute post-procedure ablation lesions by MRI and correlated these findings with clinical outcomes in pediatric patients with ventricular arrhythmia. However, role of post-ablation contrast-enhanced CMR in patients with scar-related VT has not been investigated yet. This pilot study, therefore, aimed to evaluate whether visualization of RF lesion size by CMR has any role in predicting adequacy of lesion and in guiding further ablation.
2. Materials and methods
2.1. Study population
Consecutive patients undergoing ablation for ischemic scar-related sustained monomorphic VT were included in the study. Patients were included if they had ischemic scar-related ventricular tachycardia (ISRVT) and their ablation was performed with an open-irrigation ablation catheter. Patients were excluded if they had non-ischemic scar-related VT and idiopathic VT or if their ablation was performed with a non-irrigated ablation catheter. All patients signed informed consent, and the study protocol was approved by the Local Ethics Committee (Istanbul University, Istanbul Faculty of Medicine). The study design was a single arm retrospective collection of the data recorded in the electrophysiology procedural notes and the electronic medical records. All included patients were followed in a prospective fashion.
The diagnosis of ischemic heart disease was established by prior myocardial infarction with Q waves, focal wall motion abnormality, or fixed perfusion defect correlated with >70% coronary stenosis. Exclusion criteria included VT that presented with non-sustain episodes; age <18 years; severe renal insufficiency (glomerular filtration rate <15 mL/min/1.73 m2), thrombus formation on the left ventricle, unstable angina or acute myocardial infarction, severe aortic stenosis, end-stage heart failure with limited life expectancy, and prior failed VT ablation and without preprocedural CMR.
2.2. Electrophysiological study and ablation procedure
All antiarrhythmic drugs except amiodarone were stopped for more than 5 half-lives unless incessant VT was present. With the patients under conscious sedation, transvenous multipolar catheters were placed into the coronary sinus, the right ventricle. Left ventricular mapping was performed via the retrograde aortic or transseptal approach according to operator preference. An activated clotting time goal of 300 seconds was maintained throughout the procedure with intravenous heparin. 3D electroanatomic activation maps (CARTO, Biosense Webster, Diamond Bar, California) were obtained during hemodynamically stable VT using a 3.5-mm open irrigated tip catheter (NaviStar, Thermocool, Biosense Webster) with the fill threshold setting at 15 mm. Intracardiac signals were filtered at 30 to 400 Hz. All induced monomorphic VTs except for cycle length <250 ms were targeted.
Preablation CMR was reviewed by the operators before and during the procedures and was correlated anatomically to the electroanatomic map to guide more meticulous mapping of the areas corresponding to the scar. Whether If the scar was endocardial or not, an endocardial procedure was performed. Entrainment and activation mapping were performed in all cases. Pace mapping along scar borders or sites with late potentials within scar were performed. Those sites with late potentials and demonstrating accurate pace maps (≥11/12 lead match between QRS-paced and QRS-VT, stimulus-paced QRS interval >40 ms) were targeted (Fig. 1). A site was defined as critical in the presence of concealed entrainment and VT termination during RF energy delivery. Ablation was performed at individual sites for ≥60 seconds at ≤50 W with a temperature limit of 40°C with cool rate of 30 cm3/min. All patients underwent bipolar substrate mapping with standard scar settings defined as normal tissue >1.5 mV and dense scar <0.5 mV. Maps included higher density points around areas of scar, focusing on the scar border and electrograms within the scar. Normal tissue was less densely mapped. The details of substrate mapping strategy were explained in our previous studies.[15,19] All ablation lesions were put in areas less than 1.5 mV.
Figure 1.
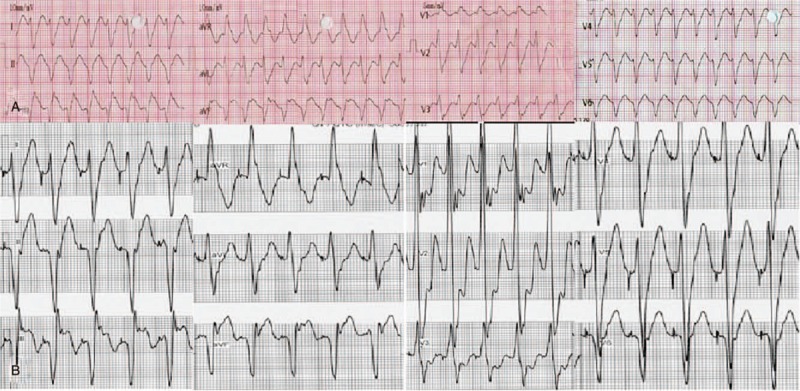
Twelve lead ECG during clinical ventricular tachycardia (A) and pace mapping (B) in case 8. ECG = electrocardiography.
After ablation, programmed right ventricular stimulation was repeated with up to 4 extrastimuli from 2 right ventricular sites unless the patient was hemodynamically unstable. Acute procedural outcome was classified as successful when no sustained monomorphic VTs were inducible by programmed stimulation at the end of the procedure, in contrast to partial success (nonclinical VTs inducible), or failure (clinical VT inducible).
2.3. CMR
All study patients underwent both pre- and post-ablation (within 24 h) CMR imaging. Patients were placed supine in a 1.5-T whole-body magnetic resonance imaging (MRI) scanner (Philips Achieva Intera, Philips, Best, the Netherlands), fiberoptic electrocardiography (ECG) leads were placed for scanner gating, and a phased-array receiver coil was placed on the chest for imaging. All images were acquired using 10- to 15-second breath holds. Short-axis slices were acquired from the base to apex, making sure to include the entire left ventricle using methods previously described. A gadolinium-based contrast agent (0.1–0.2 mmol/kg, Gadobutrol, Gadovist; Schering, Berlin, Germany) was administered intravenously, and images were obtained as described previously. [9] CMR images were analyzed by a radiologist expert on cardiovascular imaging (SS), who was blinded to clinical data. Microvascular obstruction (MVO) was defined as black areas surrounded by peripheral white regions in late gadolinium-enhanced images. MVO was considered as irreversible RF ablation lesions. Myocardial edema seen on T2 weighted images were ignored, due to reversible nature of myocardial edema seen on CMR.
2.4. Follow-up
All patients were followed for at least 6 months for clinical recurrences. An implantable cardiac defibrillator (ICD) was inserted for secondary prevention in patients with left ventricular ejection fraction lower than 35% or if VT recurred after ablation. Recurrent VT was defined as any VT documented electrocardiographically or by ICD electrograms, regardless of whether ICD therapies were delivered.
2.5. Statistical analysis
SPSS software for Windows (release 17.0.0, SPSS, Chicago, IL) was used for statistical analysis. Continuous variables were reported as mean ± standard deviation or median (minimum-maximum). Categorical variables were reported as the number (percentage) of participants.
3. Results
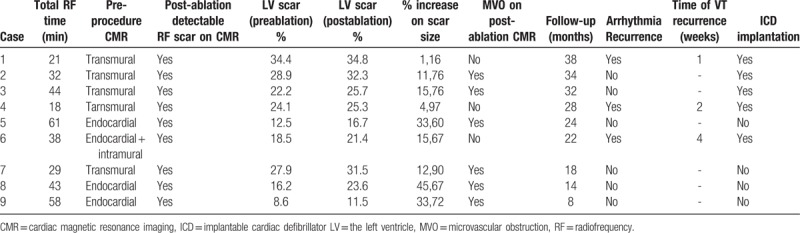
Between 8/2012 and 3/2015, 9 patients (8 male, age 59,7 ± 13 years) with ISRVT underwent ablation of VT. Patient's characteristics were summarized in Table 1. The left ventricular ejection fraction was preserved in only 1 patient, although segmental wall-motion abnormality was detected in inferior and posterior walls due to inferior myocardial infarction in the case. Mean left ventricular ejection fraction was 39.2 ± 8%. Clinical sustained VT could be induced in all patients (mean tachycardia cycle length was 368 ± 64 ms). Acute procedural success was attained in all 9 patients. There was no procedure-related complication.
Table 1.
Baseline characteristics of patients.
All 9 patients with diagnostic pre-procedure CMR studies had ≥1 myocardial segment involved with late gadolinium enhancement and 5 patients had late gadolinium enhancement in ≥3 segments. Late gadolinium enhancement was transmural in 5 patients, endocardial in 3, and both endocardial and intramural in 1.
All patients were in sinus rhythm without pharmacologic rate or rhythm control at the time of post-ablation CMR. Post-ablation CMR images were obtained within 24 hours of procedure and delayed enhancement images showed ablation lesions as MVO (MVO) surrounding contrast enhancement. As demonstrated in Figure 2, a new region of MVO was identified on CMR late gadolinium enhancement imaging in the areas of ventricular scar in which late gadolinium enhancement was detected on pre-ablation study in 6/9 patients. There were no MVO in remote healthy myocardium after RF ablation. In 3 of the 9 patients, there was no ventricular MVO area despite successful termination of intra-procedural arrhythmia with RF ablation (Fig. 3). In 1 of 3 patients with no MVO, CMR was showed a suspicious myocardial edema image.
Figure 2.
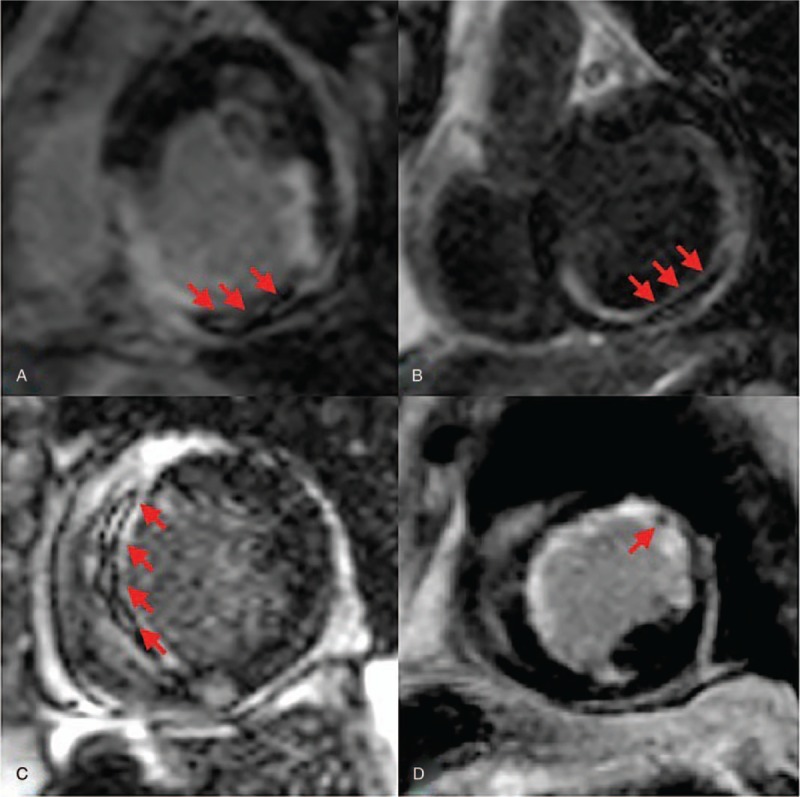
Delayed enhanced images obtained after radiofrequency ablation. Arrows demonstrate microvascular obstruction areas which indicate complete scar formation due to radiofrequency ablation in patients with no recurrence. A. Case number 2, B. Case number 8, C. Case number 7, and 4. Case number 9.
Figure 3.
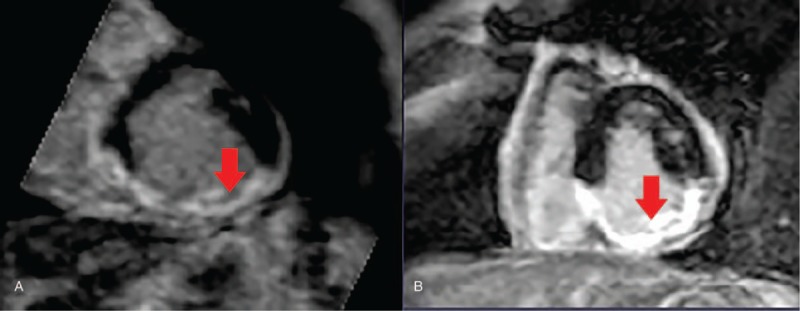
Delayed enhanced images obtained before (A) and after (B) radiofrequency ablation in case 4. A. Pre-ablation image demonstrates late gadolinium enhancement on scar site (red arrow). B. There is no microvascular obstruction in radiofrequency ablation site which may indicate incomplete ablation.
During follow-up, 6 patients with identifiable MVO on CMR were arrhythmia- and symptom-free at median 24 month follow-up period (range 8–38 months). Three patients in whom a MVO could not be identified by CMR had early VT recurrence which was detected on intracardiac defibrillator recordings. The VT recurrences were clinical in 2 patients and originated from a new site in 1 (Table 2 ).
Table 2.
Procedural and follow-up characteristics of patients.
4. Discussion
Main findings of this pilot study are;
-
(1)
acute ventricular RF ablation lesions can be visualized by CMR with late gadolinium enhancement imaging in patients underwent ablation for sustained VT because of ischemic scar with irrigated tip RF ablation catheters;
-
(2)
VT recurred despite achievement of acute procedural success in 1/3 of patients,
-
(3)
MVO on CMR with late gadolinium enhancement demonstrated RF lesions in corresponding scar regions seen on pre-ablation images in 2/3 of the patients and
-
(4)
there may be a correlation between lesion visibility with CMR, and arrhythmia recurrence risk because there was no identifiable MVO on CMR in patients demonstrating recurrence during follow-up.
Post-procedural recurrence rate of VT is related to several factors. In our study, recurrence was detected in 3 patients (33%) at median 24-month follow-up period. In Termocool study cohort, 69% of post-myocardial infarction patients recurred at sixth month after substrate-based endocardial ablation. Patients who had VT recurrence had multiple myocardial infarction locations, more inducible VTs, partial success, and necessity of more RF application.[5] It may be explained by importance of complex arrhythmic substrate and potential inadequate lesion formation in this population. In our previously published study, we evaluated whether endocardial unipolar low voltage area surrounding bipolar low voltage area predicts endocardial ablation recurrence in patients with structural heart disease undergoing substrate modification.[19]Although bipolar and unipolar low voltage areas were similar in patients with and without VT recurrence, peripheral unipolar low voltage areas surrounding bipolar scar predicted recurrence of VT, highlighting the importance of deeper VT circuits.
Yokokawa et al [20] studied the reasons for VT recurrence based on comparisons of ICD recordings in patients with post-infarction VT. Comparing our data in which 6 of 9 cases (67%) demonstrated only 1 documented VT, in 37 of 98 patients (38%), there was only 1 documented VT. Multiple VTs were documented in the remaining patients. The main reason of this discrepancy might be that some slow VTs may not be detected due to asymptomatic nature of arrhythmia in our study whereas ICD could probably detect all VTs in the other study. Secondly, VT recurrence rate and ablation success was found comparable between our series and Yokokawa's study (VT recurrence rate was 33%, and 34%, respectively and ablation success was 100%).[20]As a main difference, 79% of the recurrent VTs had new morphology which was only 33% in the present study. The majority of VTs that recurred were different from the initially induced and targeted VTs at a distance of 6 ± 3 mm to the prior ablation lesions. One of the reason for this difference might be related to higher detection of pre-procedural multiple VTs morphologies. Also, they postulated that ablation lesions from prior procedures formed the borders for some of the recurring new VTs. It is well known that creation of complete linear lesions in left ventricle is difficult. And, any gap in ablation line may change the exit site of the VT and produce a new morphology.
In the present study, we imaged to the left ventricle by CMR to demonstrate transmural nature of ablation lesions. There was no identifiable ablation lesion on CMR imaging in 3 patients who had early recurrence of arrhythmia. This data suggests the possible role of inadequate lesion formation in VT recurrence pathogenesis and a possible correlation between lesion visibility with CMR and arrhythmia recurrence risk. However, it cannot be explained by the present study why there was no MVO in some cases despite same ablation strategy and achievement of same ablation endpoints.
As the most important part of the present study, there is no larger sized study to investigating correlation of arrhythmia recurrence and post-ablation scar size in patients with ISRVT. This correlation was previously studied for atrial fibrillation ablation. Different groups demonstrated that poor scar formation on CMR after atrial fibrillation ablation correlates with risk of arrhythmia recurrence.[21–23]Our preliminary report confirmed these results in patients with ISRVT underwent ablation.
CMR can be used with different techniques in cardiovascular imaging. [24–27] Feasibility of CMR imaging after ventricular ablation was only studied in paediatric cohort.[18] Ten pediatric patients who underwent VT ablation were immediately evaluated by CMR after ablation to assess immediate and mid-term arrhythmia recurrences. Similar to our study, acute procedural success was achieved in all cases. Late gadolinium enhancement was identified at the reported ablation site in 9/10 patients, all arrhythmia-free at median 7 months follow-up. Late gadolinium enhancement was not visible in 1 patient who had recurrence within 2 hours.
CMR has potential advantages to show ablation targets in patients with scar-related tachycardias. Furthermore, delayed enhanced CMR based signal intensity mapping can be used to determine conducting channels, as well as targets located in epicardium.[28,29] Imaging of the scar tissue prior to ablation may help to focus on an area of interest intra-procedurally and may provide valuable informations for outcomes.[30] At this time, with the advent of real-time MRI, technology has gone a step further. Currently, real-time CMR-guided atrial arrhythmia ablation is being trialed.[31–33] Despite promising developments in both guiding pre-procedural ablation plan, intra-procedural catheter manipulation under CMR guidance and the use of gadolinium-based contrast agents for the evaluation of acute RF lesions have significant disadvantages. The differences in the temporal kinetics of contrast-agent passage in various tissue states resulted in clear depiction of lesions, but with a tendency to overestimate their extent.[34] Furthermore, the number of times delayed enhancement can be used in a single session is limited by clinical restrictions on contrast dosage, as well as effects on imaging from accumulated contrast agent. Guttman et al [35] studied a novel non-contrast-enhanced T1weighted CMR technique for visualization of RFA lesions which can be repeated as needed and may be useful during ablation procedure.
4.1. Limitations
Our study should be considered as a pilot study hypothesis generating a proof of concept since the sample size is small and the follow-up is relatively short. In addition, it was a retrospective and non-randomized study. Another potential limitation is that the physicians adjudicating recurrences were not blinded to the study hypothesis. There is no clear data about whether MVO is a proper method to assess the acute effect of ablation or not in the relevant literature because MVO may be not related to RF lesions. To overcome this dilemma, comparison between CMR and CARTO map should be performed in the future studies. To prove that comparison of pre and post-ablation CMR imaging was performed in the same layers is not possible for present study although there is no missing data on the scar size. Also, even with knowledge of the likely ablation lesion location, the selected CMR orthogonal planes may not capture the lesion in all planes due to size discrepancy between CMR slice thickness (usually about 10 mm) and ablation lesion (usually about 4–6 mm). In our study patients substrate-based ablation was preferred rather than focal approach, which may increase the possibility of lesion visualization. The lack of MVO in substantial number of patients despite lengthy application (21–61 min) time may suggest poor electrode contact. Using contact force catheters with novel Ablation Index algorithm would provide more definite conclusion. But, with financial reasons, contact force catheter could not be used in our country in those times. Although we speculated that ablation lesions were located in the areas of ventricular scar detected pre-ablation study in all patients, it cannot be proven unless CARTO-Merge (Biosense Webster, Diamond Bar, California) module used which combines both pre-procedural CMR based signal map and electroanatomical map by using special software. During the imaging protocol, CMR lesion identification was usually guided by knowledge of the ablation site, allowing for specific site imaging in orthogonal planes. When there is any discrepancy between reported ablation site and actual ablation site, it should be kept in mind that lesions may be difficult to identify by CMR in this technique. Lastly, the left ventricular ejection fraction was 50% in case 7. Thus, we use ISRVT definition rather than ischemic cardiomyopathy in the present article.
5. Conclusion
Acute ventricular RF ablation lesions can be visualized by CMR in patients with ISRVT by late gadolinium enhancement imaging techniques. Appearance of lesion on CMR may correlate with clinical outcomes and therefore may be useful to predict arrhythmia recurrence risk. Further studies are necessary to verify clinical correlation of acute ablation lesion appearance on CMR before considering applying post-ablation CMR to routine clinical practice. If the findings are confirmed through larger studies, acute post-ablation CMR may be used to ensure adequate ablation lesion creation by guiding targeted ablation of lesion gaps or extension of lesion depth.
Author contributions
Concept/design: TEG, KY, EG, Data analysis/interpretation: KY, SS, Critical revision of article: KY, AKB, KY, TA, Data collection: AKB, KA, TA
Conceptualization: Tümer Erdem Guler.
Data curation: Tümer Erdem Guler, Kivanç Yalin.
Formal analysis: Kivanç Yalin, Ebru Golcuk.
Investigation: Kivanç Yalin.
Methodology: Tümer Erdem Guler, Kivanç Yalin, Ebru Golcuk.
Project administration: Tolga Aksu.
Supervision: Tümer Erdem Guler, Tolga Aksu.
Writing – original draft: Tolga Aksu, Sukru Sanli, Ahmet Kaya Bilge, Kamil Adalet.
Writing – review & editing: Kivanç Yalin, Tolga Aksu, Sukru Sanli, Ahmet Kaya Bilge, Kamil Adalet.
Footnotes
Abbreviations: CMR = cardiac magnetic resonance imaging, ICD = implantable cardiac defibrillator, ISRVT = ischemic scar-related ventricular tachycardia, MVO = microvascular obstruction, RF = radiofrequency, VT = ventricular tachycardia.
TEG and KY contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Maskoun W, Saad M, Abualsuod A, et al. Outcome of catheter ablation for ventricular tachycardia in patients with ischemiccardiomyopathy: A systematic review and meta-analysis of randomized clinical trials. Int J Cardiol 2018;267:107–13. [DOI] [PubMed] [Google Scholar]
- [2].Piers SR, Leong DP, van Huls, et al. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy: the impact of noninducibility. Circ Arrhythm Electrophysiol 2013;6:513–21. [DOI] [PubMed] [Google Scholar]
- [3].Vergara P, Trevisi N, Ricco A, et al. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol 2012;23:621–7. [DOI] [PubMed] [Google Scholar]
- [4].Kottkamp H, Wetzel U, Schirdewahn P, et al. Catheter ablation of ventricular tachycardia in remote myocardial infarction: substrate description guiding placement of individual linear lesions targeting noninducibility. J Cardiovasc Electrophysiol 2003;14:675–81. [DOI] [PubMed] [Google Scholar]
- [5].Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;118:2773–82. [DOI] [PubMed] [Google Scholar]
- [6].Sacher F, Tedrow UB, Field ME, et al. Ventricular tachycardia ablation: evolution of patients over 8 years. Circ Arrhythm Electrophysiol 2008;1:153–61. [DOI] [PubMed] [Google Scholar]
- [7].Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. New Engl J Med 2007;357:2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- [9].Della Bella P, Riva S, Fassini G, et al. Incidence and significance of pleomorphism in patients with postmyocardial infarction ventricular tachycardia. Acute and long-term outcome of radiofrequency catheter ablation. Eur Heart J 2004;25:1127–38. [DOI] [PubMed] [Google Scholar]
- [10].Soejima K, Stevenson WG, Maisel WH, et al. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation 2002;106:1678–83. [DOI] [PubMed] [Google Scholar]
- [11].Bansch D, Oyang F, Antz M, et al. Successful catheter ablation of electrical storm after myocardial infarction. Circulation 2003;108:3011–6. [DOI] [PubMed] [Google Scholar]
- [12].Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 2014;11:e166–96. [DOI] [PubMed] [Google Scholar]
- [13].Baldinger SH, Stevenson WG, John RM. Ablation of ischemic ventricular tachycardia: evidence, techniques, results, and future directions. Curr Opin Cardiol 2016;31:29–36. [DOI] [PubMed] [Google Scholar]
- [14].Kumar S, Barbhaiya CR, Balindger S, et al. Better lesion creation and assessment during catheter ablation. J Atr Fibrillation 2015;8:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yalin K, Golcuk E, Buyukbayrak H, et al. Infarct characteristics by CMR identifies substrate for monomorphic VT in post-MI patients with relatively preserved systolic function and ns-VT. Pacing Clin Electrophysiol 2014;37:447–53. [DOI] [PubMed] [Google Scholar]
- [16].Siontis KC, Kim HM, Sharaf Dabbagh G, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm 2017;14:1487–93. [DOI] [PubMed] [Google Scholar]
- [17].Dickfield T, Kato R, Zviman M, et al. Characterization of radiofrequency ablation lesions with gadolinium enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2006;47:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grant EK, Berul CI, Cross RR, et al. Acute cardiac MRI assessment of radiofrequency ablation lesions for pediatric ventricular arrhythmia: feasibility and clinical correlation. J Cardiovasc Electrophysiol 2017;28:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yalin K, Golcuk E, Bilge AK, et al. Combined analysis of unipolar and bipolar voltage mapping identifies recurrences after unmappable scar-related ventricular tachycardia ablation. Europace 2015;17:1580–6. [DOI] [PubMed] [Google Scholar]
- [20].Yokokawa M, Desjardins B, Crawford T, et al. Reasons for recurrent ventricular tachycardia after catheter ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol 2013;61:66–73. [DOI] [PubMed] [Google Scholar]
- [21].Arujuna A, Karim R, Caulfield D, et al. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation. Circ Arrhythm Electrophysiol 2012;5:691–700. [DOI] [PubMed] [Google Scholar]
- [22].Parmar B, Jarrett TR, Kholmovski EG, et al. Poor scar formation after ablation is associated with atrial fibrillation recurrence. J Interv Card Electrophysiol 2015;44:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peters DC, Wylie JV, Hauser TH, et al. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging 2009;2:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen J, Zhang H, Zhang W, et al. Correlated regression feature learning for automated right ventricle segmentation. IEEE J Transl Eng Health Med 2018;6:1800610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang H, Gao Z, Xu L, et al. A Meshfree representation for cardiac medical image computing. IEEE J Transl Eng Health Med 2018;6:1800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao Z, Li Y, Sun Y, et al. Motion tracking of the carotid artery wall from ultrasound image sequences: a nonlinear state-space approach. IEEE Trans Med Imaging 2018;37:273–83. [DOI] [PubMed] [Google Scholar]
- [27].Du X, Zhang W, Zhang H, et al. Deep regression segmentation for cardiac bi-ventricle MR images. IEEE Access 2018;6:3828–38. [Google Scholar]
- [28].Perez-David E, Arenal A, Rubio-Guivernau JL, et al. Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction comparison of signal intensity scar mapping and endocardial voltage mapping. J Am Coll Cardiol 2011;57:184–94. [DOI] [PubMed] [Google Scholar]
- [29].Arenal A, Perez-David E, Avilla P, et al. Noninvasive identification of epicardial ventricular tachycardia substrate by magnetic resonance-based signal intensity mapping. Heart Rhythm 2014;11:1456–64. [DOI] [PubMed] [Google Scholar]
- [30].Njeim M, Yokokawa M, Frank L, et al. Value of cardiac magnetic resonance imaging in patients with failed ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol 2016;27:183–9. [DOI] [PubMed] [Google Scholar]
- [31].Grothoff M, Piorkowski C, Eitel C, et al. MR imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: initial results. Radiology 2014;271:695–702. [DOI] [PubMed] [Google Scholar]
- [32].Nordbeck P, Beer M, Kostler H, et al. Cardiac catheter ablation under real-time magnetic resonance guidance. European Heart Journal 2012;33:1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hilbert S, Sommer P, Gutberlet M, et al. M Real- time magnetic resonance-guided ablation of typical right atrial flutter using a combination of active catheter tracking and passive catheter visualization in man: initial results from a consecutive patient series Europace 2016;18:572–7. [DOI] [PubMed] [Google Scholar]
- [34].Oshinski JN, Yang Z, Jones JR, et al. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation 2001;104:2838–42. [DOI] [PubMed] [Google Scholar]
- [35].Guttman MA, Tao S, Fink S, et al. Non-contrast-enhanced T1 -weighted MRI of myocardial radiofrequency ablation lesions. Magn Reson Med 2018;79:879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


