Abstract
Wingless-Type MMTV Integration Site Family, Member 6 (WNT6) is a member of the Wnt family and its expression is abnormal in different human cancer cell lines. The purpose of this study was to investigate the clinical significance of WNT6 in osteosarcoma.
The levels of WNT6 mRNA and protein in tissue and serum were detected through quantitative real-time polymorperase chain reaction (qRT-PCR) and Enzyme Lined Immunosorbent Assay (ELISA), respectively. Chi-square test was performed to estimate the association of WNT6 expression with clinical parameters among osteosarcoma patients. Receiver operation characteristic (ROC) curve was plotted to determine diagnostic performance of serum WNT6 in osteosarcoma. Survival analysis was performed using Kaplan–Meier method. Cox regression analysis was adopted to evaluate prognostic significance of WNT6 expression among osteosarcoma patients.
Compared with the controls, WNT6 mRNA and protein levels were significantly elevated in patients with osteosarcoma (P > .05 for all). Furthermore, WNT6 upregulation showed positive correlation with patients’ age (P < .001), tumor grade (P < .001) and distant metastasis (P = .001). WNT6 might be a diagnostic marker for osteosarcoma with an AUC of 0.854 combining a specificity of 88.4% and a sensitivity of 77.8%. Survival analysis result indicated that high WNT6 expression predicted poor survival (log rank test, P = .001). WNT6 might be a potential prognostic biomarker for osteosarcoma (HR = 2.227, 95%CI = 1.061–10.842, P = .027).
WNT6 may be a diagnostic and prognostic marker in osteosarcoma.
Keywords: diagnosis, osteosarcoma, prognosis, WNT6
1. Introduction
Human osteosarcoma is one of the most common malignant bone tumors in children and young adult, with high mortality and disability.[1,2] The cancer is characterized by high propensity to metastasize and locally relapse.[3] Along with the development of comprehensive therapies, the 5-year survival rate among osteosarcoma patients is up to 60% to 70%, but over 50% osteosarcoma cases present metastasis when initially diagnosed, especially lung metastases, and the 5-year survival rate in these cases is <30%.[4,5] Until now, measures for osteosarcoma early diagnosis and prognosis are still unsatisfactory. Therefore, it is necessary to identify key components in osteosarcoma origination and development, thus contributing to the malignancy diagnosis, treatment, and prognosis.
Wingless-Type MMTV Integration Site Family, Member 6 (WNT6), located in chromosome 2q35, belongs to the WNT gene family showing diverse functions on cell fate, proliferation, migration, polarity, and death.[6] In previous studies, WNT6 was initially considered as a canonical WNT signaling molecule expressed strongly in epithelium.[7] With the advancement in molecular techniques, WNT6 dysregulation has already been observed in various pathological conditions, such as renal fibrosis, cleft palate, and recurrent miscarriage.[8–10] Recently, the effects of WNT6 on cancers have attracted increasing attentions. Growing evidences have suggested that WNT6 over-expression is associated with various human cancers, including colorectal cancer[11] and gastric cancer.[6] However, the role of WNT6 in osteosarcoma is still unclear.
In this study, we aimed to investigate the expression of WNT6 in osteosarcoma tissues and its relationship with clinicopathologic characteristics. Besides, clinical significance of WNT6 for early detection and prognosis evaluation in osteosarcoma were also estimated.
2. Materials and methods
2.1. Patients and samples
This study was conducted in Fudan University and approved by the Ethic Committee of the hospital. Around 88 osteosarcoma patients were collected who were diagnosed during 2011 to 2013 and had a median age of 20.89 ± 6.64. Besides, 32 patients with Ewing's sarcoma and 20 patients with osteomyelitis were recruited in the study as well. None of the patients had received any preoperative treatments, either chemotherapy or radiotherapy. In addition, the same number of healthy people who were matched with the cases in age (±5 years), sex and residential area were enrolled as controls. All participants signed informed consents in advance.
Osteosarcoma tissues and adjacent normal ones were collected from osteosarcoma patients. Besides, similar operations were also conducted for the healthy people, Ewing's sarcoma patients, and osteomyelitisthe cases. Then the tissues were immediately frozen in liquid nitrogen, and stored at −80°C for RNA extraction. Fasting blood samples were collected for all participants using blood collection tube with Ethylenediaminetetraacetic acid. Serum specimens were isolated from the blood samples through centrifugation, and then stored at −80°C for subsequent analysis. The levels of WNT6 mRNA and protein were detected via quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme lined immunosorbent assay (ELISA), respectively.
2.2. QRT-PCR assay
Total RNA was extracted from the tissue and serum samples applying Trizol reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was carried out to synthesize the first chain of cDNA using the Omniscript RT kit (Qiagen, Valencia, CA). RT-PCR reaction was conducted in Applied Biosystems 7500 real-time PCR system (Applied Biosystems, Foster City, CA). RNU6 was used as endogenous control for WNT6 expression. WNT6 expression relative to RNU6 in tissue and serum was calculated using the 2-△△CT method. All samples were examined in triplicate.
2.3. ELISA assay
Total protein was isolated on the basis the obtained tissue samples using the protein extraction kit (Bestbio, China) according to the manufacturer's instructions. The isolated protein samples were quantified via BCA assay (Thermo Scientific). ELISA analysis was performed using Human Protein Wnt-6 (WNT6) ELISA kit (CSB-EL026140HU, CUSABIO, China), and detailed protocols were as follows: the protein was loaded to each well of a 96-well immunoplate (Nunc, Denmark), and incubated overnight at 4°C; the plates were then washed with PBS and blocked with 5% BSA/PBS for 2 hours at room temperature; after washing with PBS again, biotin-conjugated antibody was added to the wells and incubated for 1 hour at room temperature; later, 50 μL HRP chromogenic substrate was put into each well of the plates to develop signals for 30 minutes at room temperature. The absorbance was read using an ELISA reader (Benchmark Microplate Reader; Bio-Rad, Hercules, CA).
2.4. Follow-up
A 5-years follow-up was performed among the osteosarcoma patients. The overall survival time was defined as the duration between the diagnosis day and the death day or the date at the end of the follow-up. Telephone inquiring or questionnaire was adopted to collect participants’ information during the follow-up. Clinicopathologic characteristics such as age, sex, tumor grade, tumor diameter, distant metastasis, tumor location, and recurrence were recorded in a database.
2.5. Statistical analysis
Statistical analyses were performed using SPSS 18.0 software. Differences in WNT6 expression between osteosarcoma patients and the controls were analyzed adopting Students’ t test. The relationship between WNT6 and clinicopathologic characteristics was evaluated through chi-square test. Receiver operating characteristic (ROC) curve was constructed to evaluate WNT6 diagnostic value. The association between WNT6 and overall survival was estimated utilizing Kaplan–Meier and cox regression analysis. Results would be statistically significant if P value was less than 0.05.
3. Results
3.1. Increased expression of WNT6 mRNA in osteosarcoma tissues and serum
The expression of WNT6 at mRNA level was detected through qRT-PCR. The result demonstrated that WNT6 expression was markedly upregulated in osteosarcoma tissues compared with the adjacent and healthy ones (Fig. 1, P < .05). Besides, the expression of serum WNT6 mRNA was also tested for osteosarcoma patients, Ewing's sarcoma patients, osteomyelitis patients, and healthy controls. The outcomes showed that serum WNT6 expression was higher in osteosarcoma patients than in other patients and healthy controls (Fig. 2, P < .05).
Figure 1.
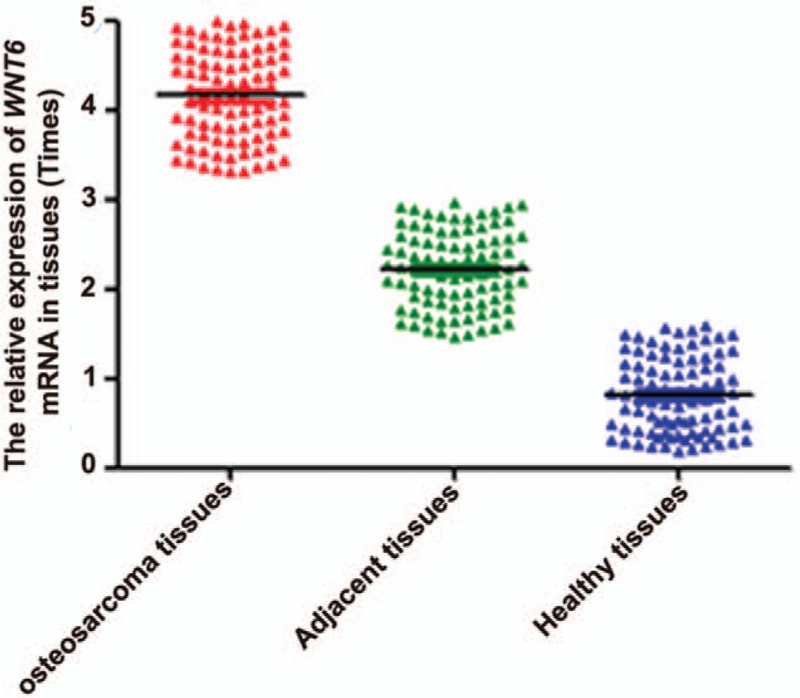
The expression of WNT6 mRNA in osteosarcoma, adjacent nontumor and healthy tissues. WNT6 expression was higher in osteosarcoma tissues than in adjacent and healthy ones (P < .05). WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
Figure 2.
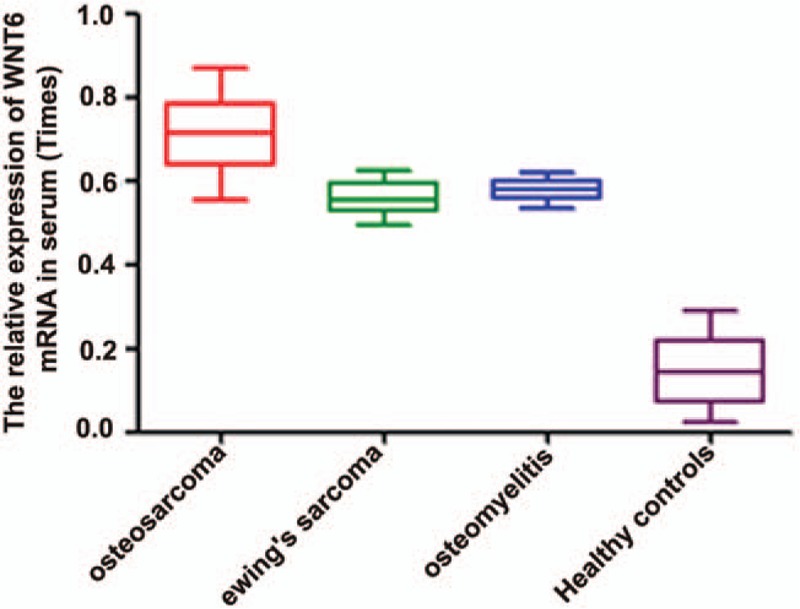
The expression of serum WNT6 mRNA in osteosarcoma patients, ewing's sarcoma individuals, osteomyelitis cases and healthy controls. Serum WNT6 expression was higher in osteosarcoma patients than in other cases and healthy controls (P < .05). WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
3.2. The expression of WNT6 at protein level
WNT6 protein expression was measured using ELISA. The results indicated that the expression of WNT6 protein was higher in osteosarcoma patients than in healthy controls (Fig. 3, P < .05), which was in accordance with the outcome from qRT-PCR.
Figure 3.
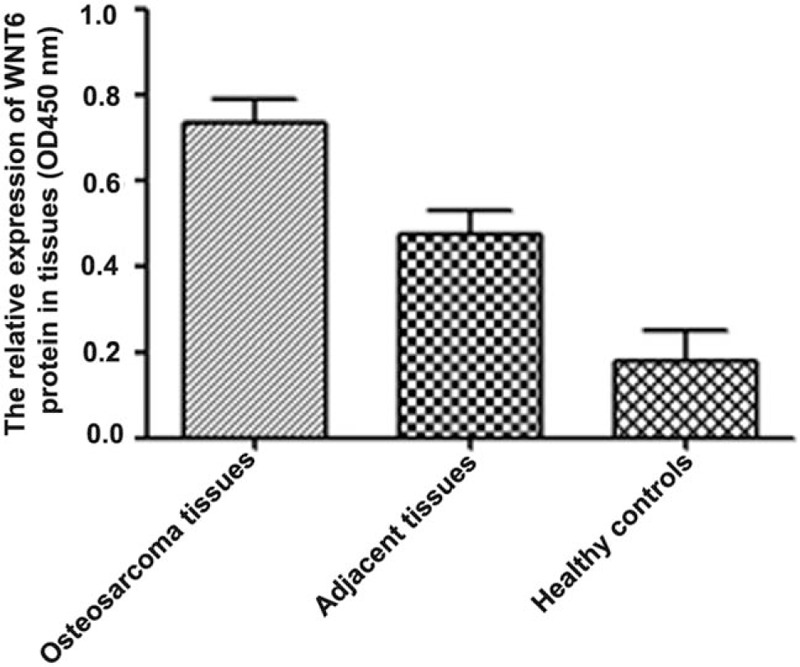
The expression of WNT6 protein in osteosarcoma, adjacent cancer-free and healthy tissues. WNT6 protein expression was higher in osteosarcoma tissues than in adjacent and healthy ones (P < .05). WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
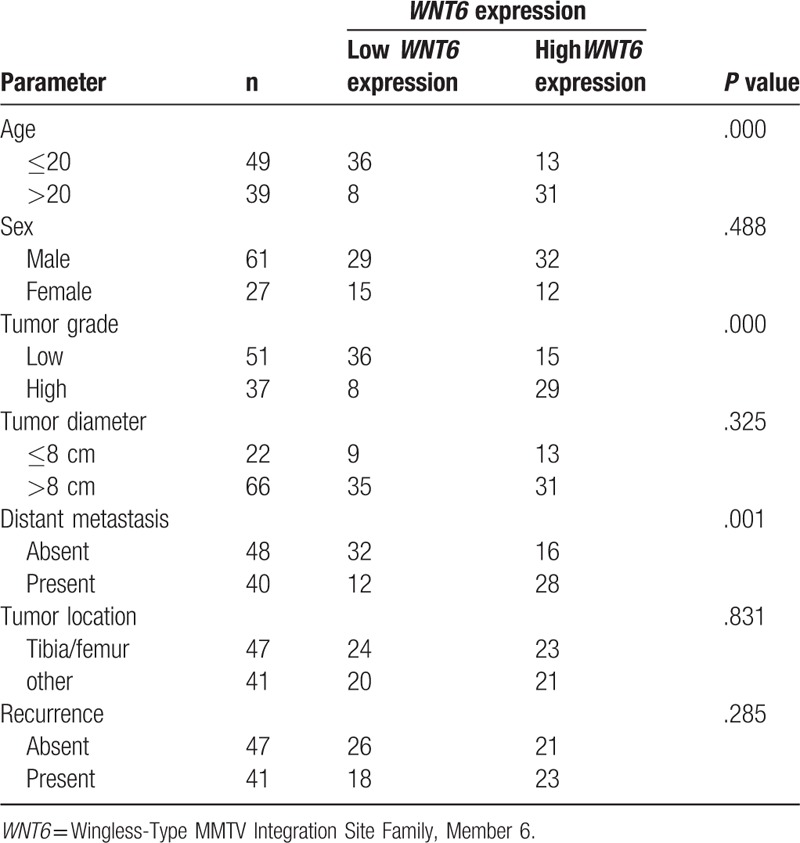
3.3. Association between WNT6 and clinicopathologic characteristics
In this study, we also analyzed the association between WNT6 and clinicopathologic characteristics among osteosarcoma patients. No significant correlation was found for WNT6 with sex, tumor diameter, tumor location, or recurrence (P > .05 for all). However, WNT6 expression showed close association with patients’ age (P < .001), tumor grade (P < .001), and distant metastasis (P = .001) (Table 1).
Table 1.
Association between WNT6 and clinicopathologic characteristics in osteosarcoma patients.
3.4. The diagnostic value of WNT6 in osteosarcoma
In the study, we used ROC curve to analyze the diagnostic value of serum WNT6 in osteosarcoma (Fig. 4). The AUC value was 0.854, with a specificity of 88.4% and a sensitivity of 77.8%, suggesting a splendid performance for WNT6 in osteosarcoma diagnosis. The cut off value was 4.255.
Figure 4.
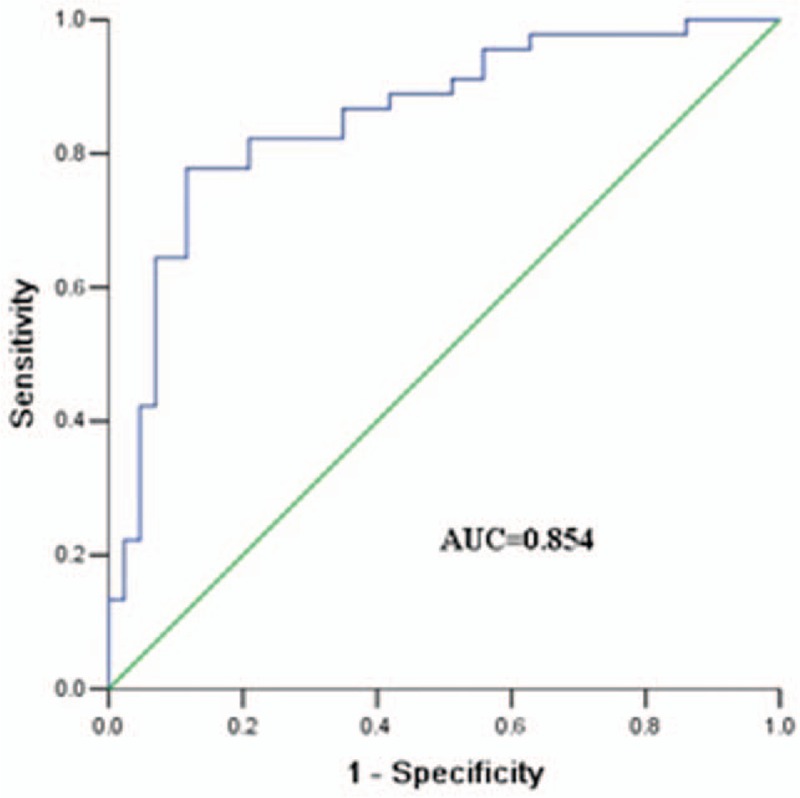
The ROC curve of WNT6. The AUC was 0.854 with a cut off value of 4.255, while the specificity and sensitivity were 88.4% and 77.8%, respectively. ROC = receiver operating characteristic, WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
3.5. The relationship between WNT6 and overall survival in patients with osteosarcoma
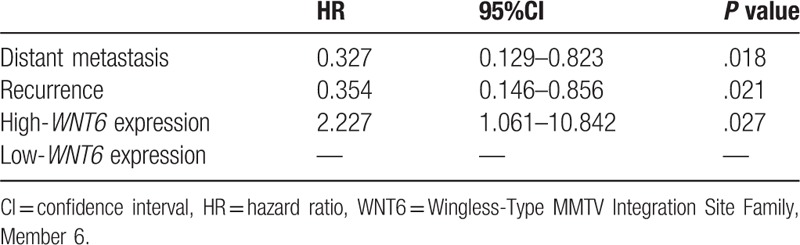
Kaplan–Meier analysis indicated that osteosarcoma patients with high WNT6 expression had shorter survival time than those with low expression (Fig. 5, log rank test P = .001). Cox regression analysis manifested that distant metastasis (HR = 0.327, 95%CI = 0.129–0.823, P = .018), recurrence (HR = 0.354, 95%CI = 0.146–0.856, P = .021) and WNT6 expression (HR = 2.227, 95%CI = 1.061–10.842, P = .027) were important factors affecting osteosarcoma prognosis (Table 2).
Figure 5.
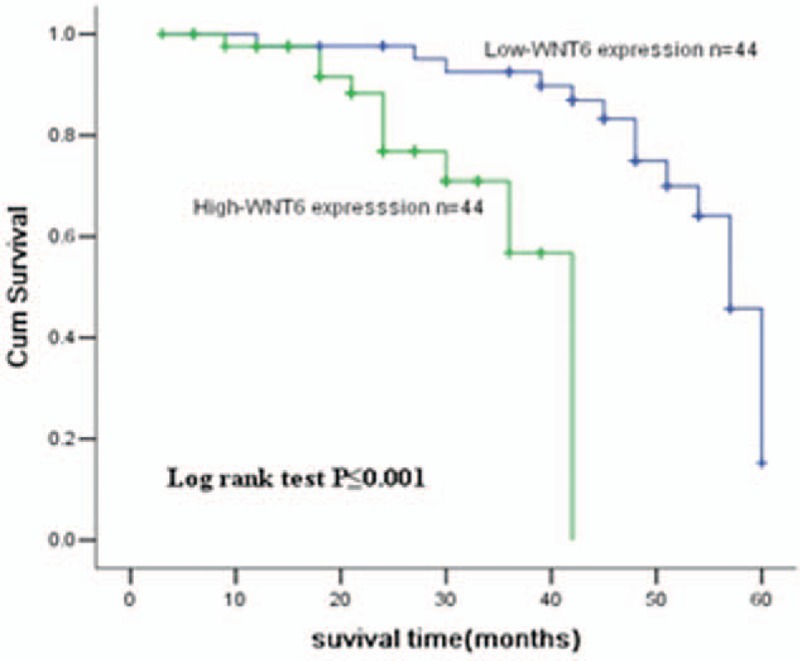
The Kaplan–Meier analysis on WNT6 in osteosarcoma patients. As shown in the curve, osteosarcoma patients with high WNT6 expression had shorter survival time than those with low levels (P < .001). WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
Table 2.
COX regression for the relationship between clinicopathological features and WNT6 expression.
4. Discussion
Osteosarcoma is caused by genetic changes that interrupt osteoblast differentiation from mesenchymal stem cells.[12,13] Due to the unclear molecular mechanism, it is difficult to identify key factors for osteosarcoma development.[14] In order to improve clinical outcomes of osteosarcoma patients, it is of great importance to explore molecular mechanism of this malignancy.
Wnt signal pathway has been confirmed to be related to the occurrence of osteosarcoma.[15] Disorder in the functions of Wnt signal transduction causes abnormalities in cell proliferation and in downstream genes’ expressions, thus leading to various malignant tumors, such as colorectal cancer, neuroblastomas, and thyroid cancer.[16–18]WNT6 gene is an important member of WNT gene family, and plays critical roles in various biological processes including embryogenesis, epithelial formation, and cell–cell communication.[8,19,20] The association of WNT6 with cancers has already been reported in some previously published studies. In the study by Galbraith et al,[21]WNT6 gene was upregulated in colorectal adenoma and associated with the cancer malignant degree. Yuan et al[6] found WNT6 upregulation could enhance epirubicin resistance in gastric cancer. WNT6 is expressed specifically by macrophage clusters within granulomatous lesions and possesses a distinctively immunomodulatory potential.[22]WNT6 has also been linked to the metastasis of melanoma and breast carcinoma.[23] Previous study has shown that WNT6 can induce satellite-cell quiescence as well as nuclear translocation of Act-beta-Cat appearing in acute and chronic skeletal muscle regeneration in rodents and humans.[24] While the study by Li et al[25] demonstrated that DNA methylation of WNT6 was low in children with osteosarcoma, which might contribute to malignant progression. However, whether the WNT6 gene is associated with osteosarcoma progression in adults is rarely reported.
In our study, the expression of WNT6 mRNA and protein was detected in osteosarcoma patients. The results showed that the mRNA and protein levels of WNT6 were markedly upregulated in osteosarcoma tissues when compared to the adjacent and healthy ones. Similarly, serum WNT6 expression in osteosarcoma patients was much higher than that in other patients and healthy controls. Then we further analyzed the relationship between WNT6 and clinicopathologic characteristics. WNT6 expression was uncovered to be significantly associated with age and distant metastasis. The results revealed that WTN6 might contribute to osteosarcoma development.
In addition, we evaluated the diagnostic and prognostic value of WNT6 in osteosarcoma. ROC curve was constituted to evaluate the diagnostic value for WNT6, and obtained an AUC value of 0.854, with a specificity of 88.4%, a sensitivity of 77.8% and a cut-off value of 4.255. All of these data suggested that WNT6 gene could be a diagnostic biomarker in osteosarcoma. Kaplan–Meier analysis showed that patients with relatively higher WNT6 expression survived significantly shorter than those with low levels. Meanwhile, cox regression analysis verified that WNT6 expression was a crucial indicator for osteosarcoma prognosis.
Several limitations in the current study should be stated here. Firstly, the sample size was relatively small, which might reduce the statistical power of our study. Secondly, molecular mechanisms underlying the functional roles of WNT6 in osteosarcoma development and progression remained unclear. Cell and animal experiments should be performed to address this issue. Additionally, all of the study subjects were collected from one region, and the conclusions obtained from our study might be less applicable for other populations. Therefore, further researches with larger sample sizes are required to verify and improve our conclusions.
In conclusion, WNT6 is upregulated in osteosarcoma and its expression is closely correlated with age and distant metastasis. WNT6 may be an independent diagnostic and prognostic biomarker for osteosarcoma.
Author contributions
Conceptualization: Kai Jiang.
Data curation: Kai Jiang.
Formal analysis: Kai Jiang.
Funding acquisition: Kai Jiang, Sha Li.
Investigation: Sha Li.
Methodology: Sha Li.
Project administration: Lu Li.
Resources: Lu Li.
Software: Lu Li, Xiaohua Wang.
Supervision: Xiaohua Wang.
Validation: Xiaohua Wang.
Visualization: Yuanjie Gu, Zhiqiang Jin.
Writing – original draft: Yuanjie Gu, Zhiqiang Jin.
Writing – review & editing: Yuanjie Gu, Zhiqiang Jin.
Footnotes
Abbreviations: AUC = area under curve, CI = confidence interval, ELISA = enzyme lined immunosorbent assay, HR = hazard ratio, QRT-PCR = quantitative real-time polymerase chain reaction, ROC = receiver operating characteristic, WNT6 = Wingless-Type MMTV Integration Site Family, Member 6.
The authors have no conflicts of interest to disclose.
References
- [1].Misaghi A, Goldin A, Awad M, et al. Osteosarcoma: a comprehensive review. SICOT J 2018;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr 2016;28:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xing D, Qasem SA, Owusu K, et al. Changing prognostic factors in osteosarcoma: analysis of 381 cases from two institutions. Hum Pathol 2014;45:1688–96. [DOI] [PubMed] [Google Scholar]
- [4].Raimondi L, De Luca A, Costa V, et al. Circulating biomarkers in osteosarcoma: new translational tools for diagnosis and treatment. Oncotarget 2017;8:100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma 2018;18:39–50. [DOI] [PubMed] [Google Scholar]
- [6].Yuan G, Regel I, Lian F, et al. WNT6 is a novel target gene of caveolin-1 promoting chemoresistance to epirubicin in human gastric cancer cells. Oncogene 2013;32:375–87. [DOI] [PubMed] [Google Scholar]
- [7].Li R, Wang C, Tong J, et al. WNT6 promotes the migration and differentiation of human dental pulp cells partly through c-Jun N-terminal kinase signaling pathway. J Endod 2014;40:943–8. [DOI] [PubMed] [Google Scholar]
- [8].Beaton H, Andrews D, Parsons M, et al. Wnt6 regulates epithelial cell differentiation and is dysregulated in renal fibrosis. Am J Physiol Renal Physiol 2016;311:F35–45. [DOI] [PubMed] [Google Scholar]
- [9].Jiang Z, Pan L, Chen X, et al. Wnt6 influences the viability of mouse embryonic palatal mesenchymal cells via the β-catenin pathway. Exp Ther Med 2017;14:5339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Li G, Fan Y, et al. Novel missense mutation in WNT6 in 100 couples with unexplained recurrent miscarriage. Hum Reprod 2015;30:994–9. [DOI] [PubMed] [Google Scholar]
- [11].Jumpertz S, Hennes T, Asare Y, et al. CSN5/JAB1 suppresses the WNT inhibitor DKK1 in colorectal cancer cells. Cell Signal 2017;34:38–46. [DOI] [PubMed] [Google Scholar]
- [12].Xie B, Li Y, Zhao R, et al. Identification of key genes and miRNAs in osteosarcoma patients with chemoresistance by bioinformatics analysis. Biomed Res Int 2018;2018:4761064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 2010;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pan Y, Lu L, Chen J, et al. Identification of potential crucial genes and construction of microRNA-mRNA negative regulatory networks in osteosarcoma. Hereditas 2018;155:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pridgeon MG, Grohar P, Steensma MR, et al. Wnt signaling in Ewing sarcoma, osteosarcoma, and malignant peripheral nerve sheath tumors. Curr Osteoporos Rep 2017;15:239–46. [DOI] [PubMed] [Google Scholar]
- [16].Novellasdemunt L, Antas P, Li VS. Targeting Wnt signaling in colorectal cancer. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am J Physiol Cell Physiol 2015;309:C511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Becker J, Wilting J. WNT signaling, the development of the sympathoadrenal-paraganglionic system and neuroblastoma. Cell Mol Life SCi 2018;75:1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ely KA, Bischoff LA, Weiss VL. Wnt signaling in thyroid homeostasis and carcinogenesis. Genes (Basel) 2018;9:E204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Adaimy L, Chouery E, Megarbane H, et al. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet 2007;81:821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Linker C, Lesbros C, Gros J, et al. Beta-Catenin-dependent Wnt signalling controls the epithelial organisation of somites through the activation of paraxis. Development 2005;132:3895–905. [DOI] [PubMed] [Google Scholar]
- [21].Galbraith RL, Poole EM, Duggan D, et al. Polymorphisms in WNT6 and WNT10A and colorectal adenoma risk. Nutr Cancer 2011;63:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schaale K, Brandenburg J, Kispert A, et al. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J Immunol 2013;191:5182–95. [DOI] [PubMed] [Google Scholar]
- [23].McCorkle JR, Leonard MK, Kraner SD, et al. The metastasis suppressor NME1 regulates expression of genes linked to metastasis and patient outcome in melanoma and breast carcinoma. Cancer Genomics Proteomics 2014;11:175–94. [PMC free article] [PubMed] [Google Scholar]
- [24].Otto A, Schmidt C, Luke G, et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 2008;121:2939–50. [DOI] [PubMed] [Google Scholar]
- [25].Li L, Xu C, Liu P, et al. Correlation study of DNA methylation of WNT6 gene with osteosarcoma in children. Oncol Lett 2017;14:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


