Abstract
The efficacy of stem cell (SC) transplantation in patients with type 1 diabetes mellitus (T1DM) has remained to be fully elucidated. In the present study, a systematic review and meta-analysis was performed to determine the clinical outcomes. Electronic databases, including PubMed, MEDLINE, WanFang and the Cochrane Library were screened for relevant studies published until January 13, 2018. The references of retrieved papers, systematic reviews and trial registries were manually screened for additional papers. Two authors were involved in screening the titles in order to select eligible studies, extract data and assess the risk of bias. Studies were pooled using a random-effects model as well as the Begg's funnel plot and subgroup analysis was performed using Stata 14.0 software. A total of 47 studies were retrieved for detailed evaluation, of which 22 met the inclusion criteria. No substantial publication bias was identified. The meta-analysis revealed that SC therapy increased C-peptide levels when compared with placebo treatment in randomized-controlled trials [RCT; standardized mean difference (SMD), 0.93; 95% confidence interval (CI) 0.23–1.63] and self-controlled trials (SMD, 0.66; 95% CI, −0.22 to 1.54). An analysis demonstrated that SC therapy was more efficient at reducing the glycated hemoglobin level compared with the control group in RCTs (SMD, 0.56; 95% CI; 0.06–1.06; and SMD, 1.63; 95% CI, 0.92–2.34, respectively). The graphs demonstrated that SC transplantation resulted in a reduction of insulin requirement. Furthermore, subgroup analyses revealed that patient age, medical history and the SC injection dose may be sources of the heterogeneity observed. The greatest benefit of SC transplantation was seen in patients aged ≥18 years or a medical history of <3 months. In addition, the SC injection dose of ≥107 IU/kg/day was more effective than <107 IU/kg/day when the cellular composition included mesenchymal SCs and hematopoietic SCs. In conclusion, SC therapy represents an efficient option for patients with T1DM. This systematic review was registered at the International prospective register of systematic reviews (no. 42018093930).
Keywords: cell therapy, meta-analysis, priority, stem cell, type 1 diabetes mellitus
Introduction
Diabetes mellitus (DM) has become a major public health problem worldwide (1). It is a major risk factor for ischemic heart disease and stroke, chronic kidney disease and blindness, and is responsible for high rates of morbidity and mortality among patients (2–4). Type 1 (T1) DM is characterized as a chronic autoimmune disease of the pancreas, causing progressive destruction of β-cells (5). Patients with T1DM eventually require exogenous insulin to control their blood glucose. Regarding the treatment of DM patients, various methods have been evaluated, including the use of cytostatic drugs (6,7), monoclonal antibodies (e.g. rituximab, teplizumab) (8,9) and vaccines for modifying the patient's abnormal immune system response (10). Transplantation of the entire human pancreas or isolated, cadaveric human islets, which has provided proof of concept for β-cell replacement therapies, has made T1DM patients insulin-independent (11,12). However, the transplantation mentioned above has remained to be fully developed and effectively implemented in clinical practice. The major limitations include surgery-associated complications, the requirement of donor cells (13) and immunosuppressants (14), and the risk of immune rejection (15) and recurrence (16).
Stem cell (SC) transplantation has emerged as a breakthrough treatment due to of the intrinsic regenerative capacity and immunomodulatory properties of SCs (17), which may achieve arrest of autoimmune β-cell destruction and generate functional β-cells (18). SC therapy may represent a novel paradigm for optimizing glycemic control in T1DM. Meta-analyses of studies on SC therapy for liver cirrhosis (19), chronic liver disease (20), inflammatory bowel disease (21) and ischemic heart disease (22) have indicated a certain potential for cell therapy-based strategies. In animal studies, it was demonstrated that SCs are able to relieve hyperglycemia by differentiating into insulin-producing cells, thereby promoting the transformation of α-cells to β-cells, improving pancreatic regeneration and ameliorating insulin resistance. In the present meta-analysis, the efficacy and safety of SC therapy for the treatment of patients with T1DM was assessed based on published and unpublished clinical trials. Representative outcome data were analyzed, including the mean values of C-peptide levels, glycated hemoglobin (HbA1c) levels, fasting plasma glucose (FPG) and daily insulin requirement every three months over 12 months.
C-peptide, a connecting peptide, is a short 31-amino-acid polypeptide that connects the A-chain of insulin to its B-chain in the pro-insulin molecule. In diabetes, measurement of C-peptide blood serum levels may be performed to distinguish between certain diseases with similar clinical features (23). HbA1c may be assessed to evaluate the average blood sugar levels over a period of weeks/months (24). For patients with diabetes, this is important, as higher HbA1c levels are linked with a greater risk of developing diabetes-associated complications. A fasting plasma glucose (FPG) test, a simple blood test taken after several h of fasting, also known as the fasting glucose test, may be used to diagnose diabetes or pre-diabetes (25). The insulin requirement reflects the degree of insulin dependence in diabetic patients. In the present study, two major subgroup analyses [data from randomized-controlled trials (RCTs) and self-controlled trials (SCTs)] were performed. Several subgroups were classified based on the origin and nature of SCs within each major group, including hematopoietic stem cells (HSC), mesenchymal stem cells (MSC) and umbilical cord blood (UCB). The RCTs reflected the efficacy of a population-based study, while that of individuals was demonstrated by the SCTs. In these two major groups, the present study aimed to explore the therapeutic efficacy of different SCs in patients with T1DM.
Materials and methods
Guidelines and data availability
This systematic review was registered at the International prospective register of systematic reviews (no. 42018093930). Study inclusion and exclusion was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta Analyses (26) and the Cochrane handbook for systematic reviews of interventions (27).
Literature search
A systematic search of studies published from inception until 13th January 2018 was performed in the databases PubMed (https://www.ncbi.nlm.nih.gov/pubmed), MEDLINE (https://www.medline.com/home.jsp), WanFang (http://g.wanfangdata.com.cn/index.html) and the Cochrane Library (including CENTRAL; http://www.cochranelibrary.com/). To accurately identify clinical trials of SC transplantation in T1DM patients, a search was performed using a combination of Me-SH terms and text words: (‘type 1 diabetes’ OR ‘diabetes mellitus type 1’ OR ‘hyperglycemia’) AND (‘stem cells’ OR ‘progenitor cells’ OR ‘mesenchymal stem cell’ OR ‘mononuclear stem cell’ OR ‘hematopoietic stem cell’ OR ‘progenitor cell’ OR ‘beta cell’) AND (‘cell therapy’ OR ‘treated’ OR ‘therapeutic’ OR ‘treatment’). No language restrictions were applied. Furthermore, potentially eligible studies were identified by screening the lists of references of the retrieved articles as well as in review articles and abstracts from relevant conferences. The most recent or complete studies were selected for analysis when the same or similar patient data were included. Furthermore, information of prospective and ongoing trials was retrieved from http://www.ClinicalTrials.gov, a database created to establish a registry of clinical trials involving investigational drugs as a result of the Food and Drug Administration Modernization Act of 1997 and a Final Rule issued by Department of Health and Human Services (28). All of the searches were performed by two investigators independently (GJD and WYJ).
Study selection
The following inclusion criteria were applied to select studies based on their titles and abstracts: i) The studies were clinical trials, but not pre-clinical trials, reviews, comments, case reports or basic scientific research; ii) the subjects included had been diagnosed with T1DM; iii) the participants received SC therapy (no restriction was applied regarding the source and route of administration of the stem cells). Studies were excluded if: i) They were animal studies; ii) the included participants did not undergo SC therapy; iii) they contained duplicate data; iv) the C-peptide levels, HbA1c levels and FPG or insulin requirement assessed every 3 months over a 12 month period were not included and could not be calculated from the data.
Furthermore, when the data overlapped or were duplicated between two or more studies by the same group, the most recent study, which contained more complete data were included. The articles were independently evaluated by two reviewers (GJD and WYJ) in duplicate and selected for inclusion if they met the abovementioned criteria. Discrepancies were resolved by consensus.
Data extraction and quality assessment
Primary items were independently extracted by two authors (GJD and WYJ), including the request for documentation and recalculation of the following variables, through a standardized data collection form. The form included: a) The first author's name, b) year of publication, c) country, d) size of study population, e) study design, f) mean age, g) gender of the participants, h) history of DM, i) type of SCs, j) mean dose of injected cells, k) path of injection, l) follow-up time, m) C-peptide levels, n) HbA1c levels, o) FPG and p) insulin requirement was assessed every 3 months over 12 months. The data for C-peptide levels, HbA1c levels and FPG were extracted to identify changes over time and evaluate the length of SC treatment. For continuous outcomes, the mean, standard deviation and total sample size were recorded. Furthermore, the corresponding authors of studies with insufficient data or studies that could not be located were contacted by email and asked whether they were willing to share their unpublished data from these trials for inclusion in the present analysis. If no reply was received, the study was excluded from the meta-analysis. Those data that were the outcomes of interest of the present study were pooled with the data from the primary trials.
Data from SCTs were analyzed to evaluate whether the SC therapy improved the symptoms of patients with T1DM. The data from RCTs were pooled to evaluate whether the SC therapy was superior to the standard or conventional therapy in improving the symptoms of T1DM patients.
Two reviewers (GJD and WYJ) independently assessed the methodological quality of the nine RCTs by rating them on the Jadad scale (29). The quality scale of Jadad ranges from 0 to 5 points, with a score of ≤2 indicating a low-quality study and a score of ≥3 indicating a high-quality study. Disagreements were resolved by discussion between the two reviewers.
Statistical analysis
Meta-analysis was performed with a random-effects model (DerSimonian-Laird method) by using Stata 14.0 (StataCorp LP, College Station, TX, USA). The standardized mean difference (SMD) was used to combine treatment effect data. A random-effects model was used to compute the pooled standardized mean deviation of C-peptide and HbA1c levels with 95% confidence intervals (CI), as this model takes into account any differences between studies even if there is no statistically significant heterogeneity (27). The heterogeneity among the studies was assessed using the inconsistency index (I2) statistics (30,31) [I2 describes the percentage of total variation across studies that are due to heterogeneity rather than to chance and I2>50% was considered to indicate substantial heterogeneity based on the suggestion in the Cochrane Handbook for Systemic Reviews of Interventions (27)] and the Chi-square test (the risk of random error is the risk of drawing a false conclusion based on sparse data). This risk is quantified as the P-value, and P<0.05 was considered to indicate significant statistical heterogeneity.
To determine the primary outcomes, Egger's test was employed to assess the publication bias (32). Clinical heterogeneity was assessed by subgroup analysis with patients stratified according to age, medical history and cell dose. Prespecified subgroup analyses were performed to explore potential heterogeneity between subgroups (P<0.05 was considered to indicate significant statistical heterogeneity). To evaluate whether the association between C-peptide levels or HbA1c levels and SC therapy was affected by clinical characteristics, the subjects were stratified into subgroups based on age (<18 or ≥18 years), medical history (<3 or ≥3 months) and cell dose (<107 or ≥107 IU/kg/day). Data were analyzed using Stata statistical software version 14.0 (StataCorp LP).
Results
Literature search and study selection
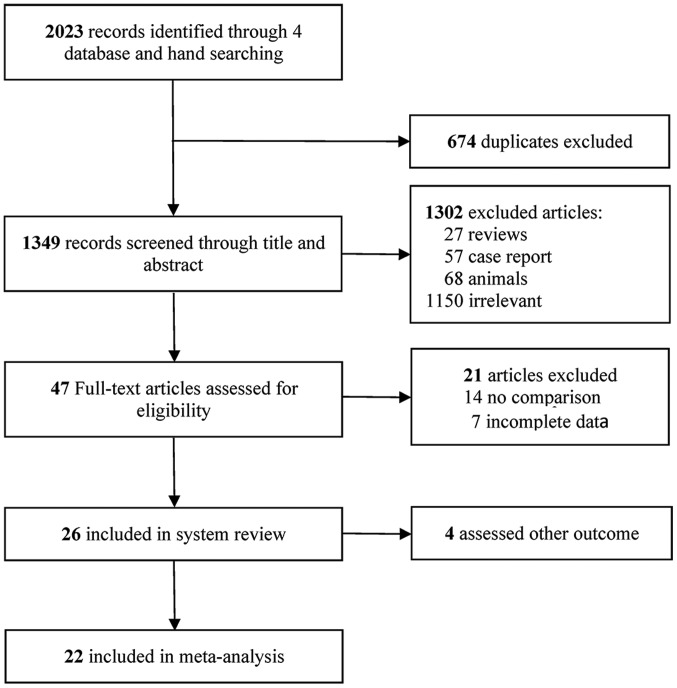
The literature search performed in the present study yielded 2,023 records of potentially eligible studies, of which 674 were excluded due to being duplicates. Following screening of titles and abstracts of the remaining 1,349 studies, a total of 1,302 studies were excluded based on the inclusion and exclusion criteria, among which 27 were reviews, 57 were case reports, 68 included animal studies and 1,150 were irrelevant. Finally, 47 full-text articles were considered for inclusion, among which 22 included original research papers (33–54). A flowchart illustrating the selection process is presented in Fig. 1.
Figure 1.
Flow chart of the selection of studies for the present systematic review and meta-analysis.
Study and patient characteristics
A total of 9 RCTs and 14 SCTs were included in the final meta-analysis. Their details are summarized in Table I. Of the studies, 2 were published prior to 2009 and 19 were published from 2010 onwards. The eligible studies represented an international population, as they were performed in a large range of countries, including China, India, Poland, Spain, USA, Sweden, Argentina, Germany and Brazil, with the age of participants ranging from 3 to 40 years. Individual patient data were obtained for 455 participants, including 189 from the RCTs and 266 from SCTs. The length of follow-up period ranged between 1 and 12 months. The most common injection route of the SCs in the studies included was intravenous. Thakkar et al (34) and Vanikar et al (50) used intra-pancreatic injection. Furthermore, Cai et al (37) used a pancreatic artery cannulation method.
Table I.
Characteristics of the trials included.
| First author (year) | Country | Cases/Total | Design | Mean age (years) | Gender (F/M) | BMI (kg/m2) | History of DM | Intervention | Dose | Injection path | Time to follow-up (months) | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ye (2017) | China | 10/18 | RCT | 18.9±1.5 | 6/4 | 19.2 ± 1.1 | <6 months | HSC | 2.00×105 | IV | 12 | (33) |
| 8/18 | 20.2±4.0 | 5/3 | 18.2±1.3 | <6 months | Insulin | |||||||
| Thakkar (2015) | India | 10/20 | RCT | 20.2 | 1/9 | NA | 8.1 years | MSC+HSC | 2.07×104 | IP | 3,6,9,12,24 | (34) |
| 10/20 | 19.7 | 4/6 | NA | 8.1 years | Insulin | |||||||
| Snarski (2016) | Poland | 20 | SCT | 24.3±4.5 | 5/15 | 22.2±2.3 | NA | HSC | 3.0×106 | IV | 3,6,12,24 | (35) |
| Delgado (2015) | Spain | 6/15 | SCT | 33.8±10.9 | 2/4 | 6.5 years | UCB | 1.0×106 | IV | 12 | (36) | |
| Cai (2016) | USA | 21/42 | RCT | 18.3 | 12/9 | 22.0 | 9.2 years | MSC+HSC | 106.8×106 | IP | 3,6,9,12 | (37) |
| 21/42 | 20.4 | 10/11 | 22.1 | 7 years | Insulin | |||||||
| Giannopoulou | Germany | 7/17 | RCT | 3.0 | 2/5 | NA | <3 months | UCB | 1.27×106 | IV | 3,6,9,12 | (38) |
| (2014) | 10/17 | 6.6 | 6/4 | NA | <4 months | Insulin | ||||||
| D'Addio (2014) | USA | 65 | SCT | 20.4±5.5 | 24/41 | 18.1±3.1 | <3 months | HSC | 5.8×106 | IV | 12,24 | (39) |
| Carlsson (2015) | Sweden | 9/18 | RCT | 24±2 | 1/8 | 23.3±1.1 | <3 weeks | MSC | 2.75×106 | IV | 12 | (40) |
| 9/18 | 27±2 | 4/5 | 22.5±0.9 | <3 weeks | Insulin | |||||||
| Hu (2013) | China | 15/29 | RCT | 17.6±8.7 | 6/9 | 20.9±3.7 | New onset | MSC | 2.6×107 | IV | 3,6,9,12,24 | (41) |
| 14/29 | 18.2±7.9 | 6/8 | 21.3±4.2 | New onset | Insulin | |||||||
| Haller (2013) | USA | 10/15 | RCT | 7.2 | NA | NA | 3.9 months | UCB | 1.1×107 | IV | 6,12 | (42) |
| 5/15 | 6.6 | NA | NA | 3.9 months | Insulin | |||||||
| Zhao (2012) | USA | 6/9 | RCT | 30±9 | 4/2 | NA | 6 years | UCB-SC | 1.0×106 | IV | 12 | (43) |
| 3/9 | 33±9 | 0/3 | NA | 6 years | Insulin | |||||||
| Zhang (2012) | China | 9 | SCT | 17.5±3.5 | 4/5 | 18.5±1.5 | 2 years | HSC | 10.49×106 | IV | 6,12 | (44) |
| Li (2012) | China | 13 | SCT | 14.1±4.0 | 3/10 | 18.1±2.3 | New onset | HSC | 4×106 | IV | 12 | (45) |
| Gu (2012) | China | 28 | SCT | 18.4±4.1 | 9/8 | 19.2±1.6 | 3 months | HSC | 1.0×106 | IV | 12 | (46) |
| Yu (2011) | China | 6/12 | RCT | 19.6±2.5 | 3/3 | 21.5±1.6 | <3 months | MSC | 1.0×107 | IV | 12 | (47) |
| 6/12 | 14.8±8.1 | 2/4 | 20.0±3.4 | <3 months | Insulin | |||||||
| Haller (2011) | USA | 24 | SCT | 5.1 | NA | NA | 4 months | UCB | 1.88×107 | IV | 3,6,9,12,24 | (48) |
| Feng (2011) | China | 17 | SCT | 13.0±3.8 | 9/8 | NA | 4 months | HSC | 3×106 | IV | 12 | (49) |
| Vanikar (2010) | India | 11 | SCT | 21.1 | 4/7 | NA | 8.2 years | MSC+HSC | 3.15×106 | IP | 12 | (50) |
| Snarski (2011) | Poland | 8 | SCT | 25.8 | 4/4 | NA | 2 years | HSC | 4.14×106 | IV | 3,6 | (51) |
| Gu (2010) | China | 18 | SCT | 18.8±4.4 | 12/6 | NA | <6 months | HSC | NA | IV | 3,6,12 | (52) |
| Haller (2009) | USA | 15 | SCT | 5.5 | 7/8 | NA | 4.1 months | UCB | 1.5×107 | IV | 12 | (53) |
| Couri (2009) | Brazil | 23 | SCT | 18.4±4.6 | 6/17 | 19.7±2.2 | <3 months | HSC | 10.52×106 | IV | 12 | (54) |
RCT, randomized controlled trial; SCT, self-controlled trial; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; UCB, umbilical cord blood; UCB-SC, umbilical cord blood-stem cells; IV, intra-venous; IP, intra-pancreatic; NA, not available; M, male; F, female; BMI, body mass index; DM, diabetes mellitus.
Quality assessment
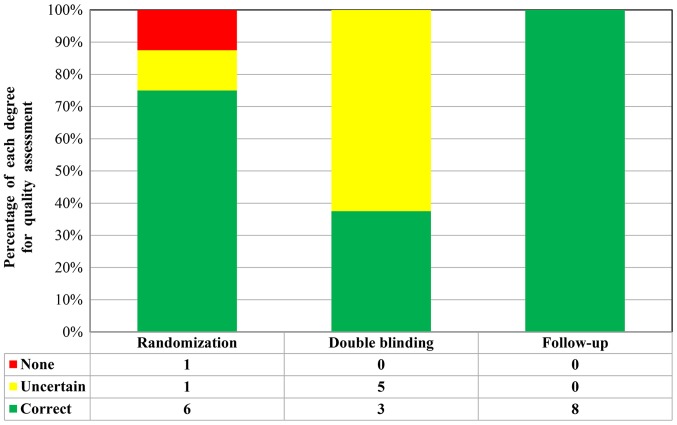
A total of 9 RCTs included a control group (placebo or hypoglycemic agent) in a double-blinded design. Through Jadad score tools, 5 studies [Cai et al (37), Carlsson et al (40), Hu et al (41), Haller et al (42) and Yu et al (46)] received 5 points, one study [Zhao et al (43)] received 4 points and one study [Ye et al (33)] received 3 points. These eight studies were considered to be of high quality, whereas another study [Giannopoulou et al (38)] received 2 points and was considered a low-quality study (Fig. 2). In addition, 13 studies were designed as SCTs, for which no approved assessment tools were available to evaluate the SCTs. However, these studies were also repeatedly reviewed and quality assessments were performed. Each of these SCTs included detailed selection procedures, strict descriptions of the procedures and an adequate follow-up period.
Figure 2.
Methodological quality of trials included in the meta-analysis.
Publication bias
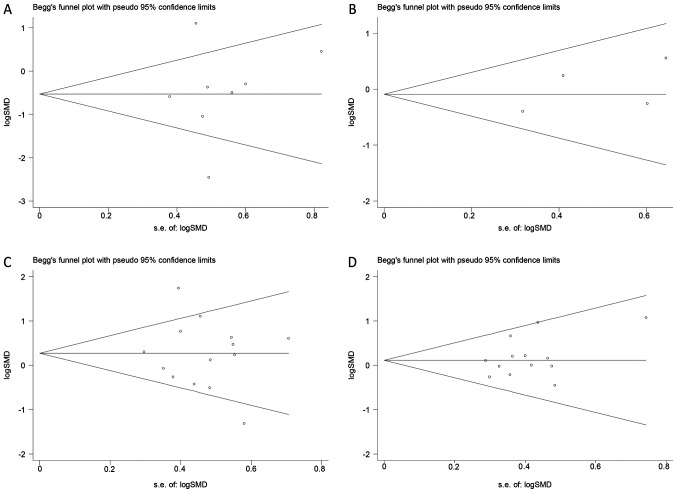
Begg's funnel plot revealed no evidence of publication bias for the outcomes examined (P>0.05; Fig. 3). This indicated that the present results may slightly overestimate the true effect size if non-significant studies are not identified. The potential publication bias of the effects of SC therapy on C-peptide and HbA1c estimated in RCTs (Fig. 3A and B). The potential publication bias of the effects of SC therapy on C-peptide and HbA1c estimated in SCTs (Fig. 3C and D).
Figure 3.
Begg's funnel plot for potential publication bias analysis. Each circle represents an eligible study. The potential publication bias of the effects of SC therapy on (A) C-peptide and (B) HbA1c estimated in RCTs. The potential publication bias of the effects of SC therapy on (C) C-peptide and (D) HbA1c estimated in SCTs. SMD, standardized mean difference.
Efficacy of SC treatment in RCTs
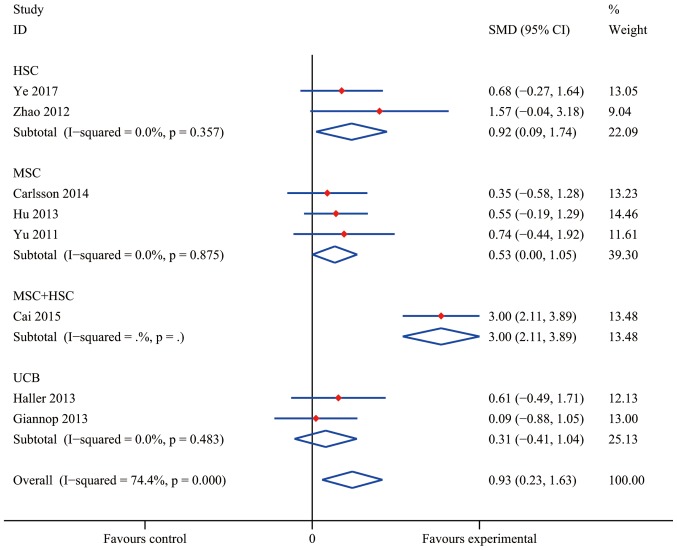
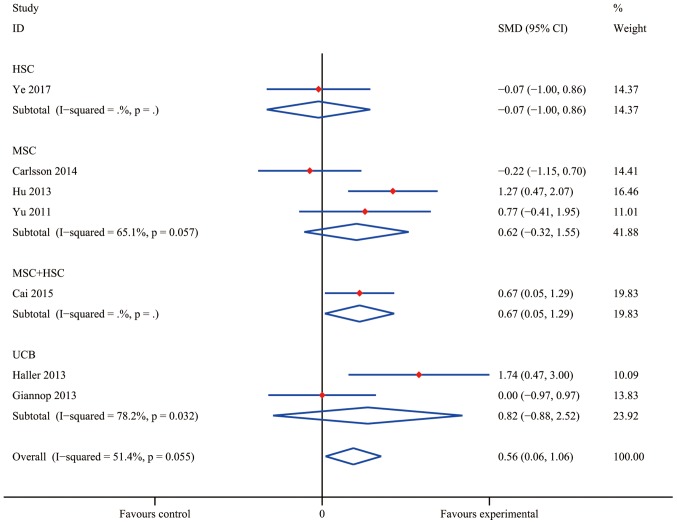
Compared with conventional insulin therapy, SC treatment resulted in a statistically significant difference in the increase of C-peptide levels in T1DM patients with an overall SMD of 0.93 (95% CI, 0.23–1.63; I2=74.4%; P=0.009). As presented in Fig. 4, a statistically significant pooled effect was determined in the HSC group (SMD, 0.92; 95% CI, 0.09–1.74; I2=0.0%; P=0.029) and in the MSC group (SMD, 0.53; 95% CI, 0.00–1.05; I2=0.0%; P=0.048), whereas in the UCB group, no statistically significant effect was observed (SMD, 0.31; 95% CI, −0.41 to 1.04; I2=0.0%; P=0.397). The data obtained by Cai et al (37) indicated a significant efficacy of MSC+HSC (SMD, 3.00; 95% CI, 2.11–3.89). At the same time, the pooled effect of HbA1c reduction was significantly different from that in the insulin treatment group (the control group) with an overall SMD of 0.56 (95% CI, 0.06–1.06; I2=51.4%; P=0.028; Fig. 5). Furthermore, MSC+HSC exerted a decent therapeutic effect on T1DM patients in terms of HbA1c with an SMD of 0.67 (95% CI, 0.05–1.29; P=0.034). However, the effect in the groups treated with HSC (SMD, −0.07; 95% CI, −1.00 to 0.86; P=0.089), UCB (SMD, 0.82; 95% CI, −0.88 to 2.52; I2=78.2%; P=0.345) and MSC (0.62; 95% CI, −0.32 to 1.55; I2=65.1%; P=0.197) overlapped with the no effect lines, indicating that the differences in these groups were not statistically significant.
Figure 4.
Effects of stem cell therapy on C-peptide estimated after 12 months in randomized-controlled trials. CI, confidence interval; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; SMD, standardized mean difference; UCB, umbilical cord blood.
Figure 5.
Effects of stem cell therapy on glycated hemoglobin estimated after 12 months in randomized-controlled trials. CI, confidence interval; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; SMD, standardized mean difference; UCB, umbilical cord blood.
Efficacy of SC therapy in SCTs
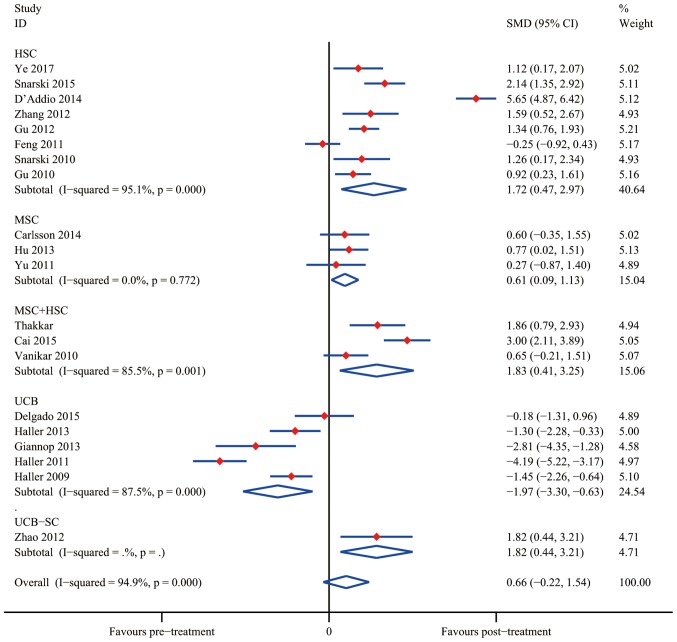
The overall meta-analyses of data from SCTs are presented in Figs. 6 and 7. The results indicated that SC treatment significantly increased C-peptide levels after 12 months in the HSC group (SMD, 1.72; 95% CI, 0.47–2.97; I2=95.1%; P=0.007), the MSC group (SMD, 0.61; 95% CI, 0.09–1.13; I2=0.0%; P=0.021) and the MSC+HSC group (SMD, 1.83; 95% CI, 0.41–3.25; I2=85.5%; P=0.011). On the contrary, the results obtained using UCB suggested that SC therapy reduced C-peptide levels (SMD, −1.97; 95% CI, −3.30 to −0.63; I2=87.5%; P=0.004); however, Zhao et al (43) reported a decent curative effect with UCB-SC therapy (SMD, 1.82; 95% CI, 0.44–3.21; P=0.010). It was therefore apparent that UCB-SC therapy had a different effect when compared with that of treatment with UCB.
Figure 6.
Effects of stem cell therapy on C-peptide estimated after 12 months in self-controlled trials. CI, confidence interval; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; SMD, standardized mean difference; UCB, umbilical cord blood.
Figure 7.
Effects of stem cell therapy on glycated hemoglobin estimated after 12 months in self-controlled trials. CI, confidence interval; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; SMD, standardized mean difference; UCB, umbilical cord blood.
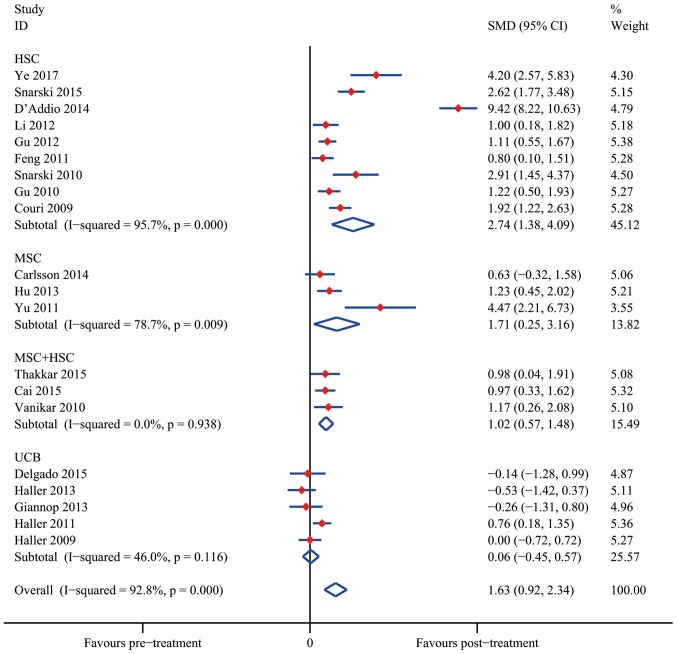
The analysis of changes in HAb1c levels demonstrated that SC therapy resulted in a statistically significant difference from the control group after 12 months in the groups treated with HSC (SMD, 2.74; 95% CI, 1.38–4.09; I2=95.7%; P<0.001), MSC (SMD, 1.71; 95% CI, 0.25–3.16; I2=78.7%; P=0.022) and MSC+HSC (SMD, 1.02; 95% CI, 0.57–1.48; I2=0.0%; P<0.001). Similar to the result regarding the C-peptide levels, UCB treatment did not significantly reduce the HbA1c levels (SMD, 0.06; 95% CI, −0.45 to 0.57; I2=46.0%; P=0,831).
FPG and insulin requirement
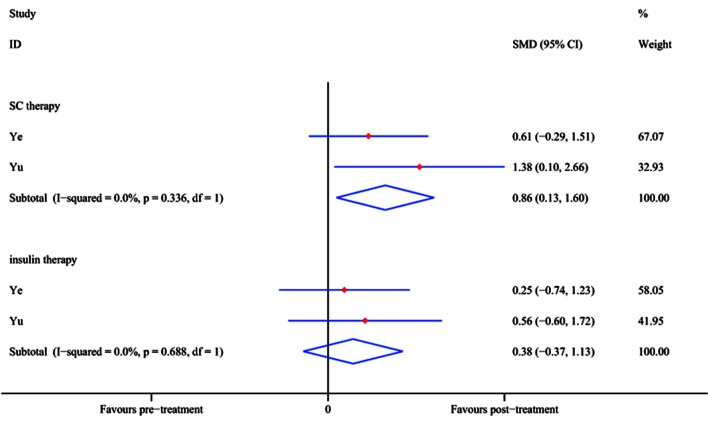
In two RCTs [Ye et al (33) and Yu et al (47)], the FPG level (mmol/l) was reported at the 12-month follow-up time-point. The pooled effect of FPG reduction at 12 months after SC treatment achieved an SMD of 0.86 (95% CI, 0.13–1.60; P=0.021), whereas in the control group, no significant difference was identified with insulin treatment (SMD, 0.38; 95% CI, −0.37 to 1.13; P=0.323; Fig. 8) compared with baseline. The results demonstrated that SC therapy is an efficient option while insulin may not.
Figure 8.
Effects of stem cell and insulin therapy estimated after 12 months in self-controlled trials. CI, confidence interval; SC, stem cell; SMD, standardized mean difference.
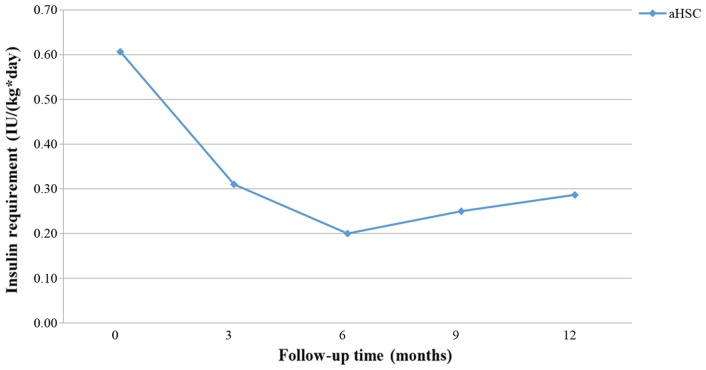
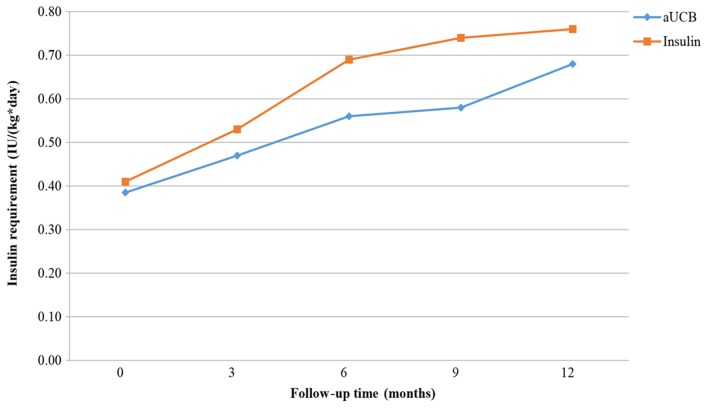
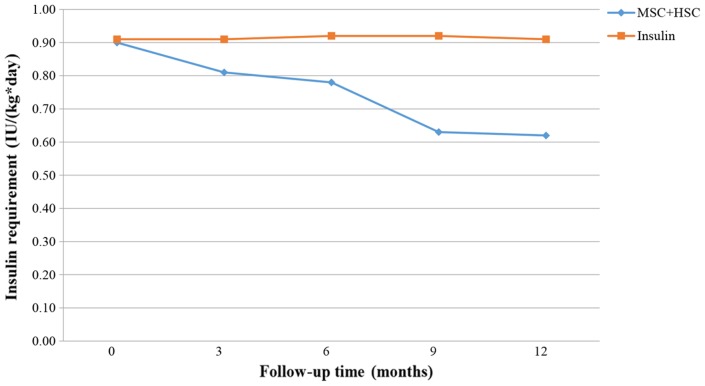
Figs. 9–11 present the trend of insulin requirement after treatment. The insulin requirement vs. time curves exhibited a downward trend, demonstrating that SC transplantation resulted in a reduction in exogenous insulin requirement in patients with T1DM.
Figure 9.
Fold line diagram of insulin requirement during 12 months in patients treated by intravenous administration of HSC. aHSC, autologous hematopoietic stem cells.
Figure 11.
Fold line diagram of insulin requirement during 12 months in patients treated by intravenous administration of UCB. aUCB, autologous umbilical cord blood.
Subgroup analysis
A subgroup analysis was performed to assess the influence of clinical characteristics and the type of SCs on the therapeutic effect compared with the insulin-treated control group. As presented in Table IIA, an age of ≥18 years and a disease history of <3 months were associated with a greater increase in C-peptide levels in patients transplanted with HSC (P<0.001), whereas in patients treated with MSC+HSC, an age of <18 years and a cell dose of ≥107 IU/kg/day was associated with higher C-peptide levels. The subgroup analysis also indicated that patient age, disease history and the dose of SCs used led to different results in patients treated with UCB. However, UCB therapy was not recommended since it had a worse outcome compared with the other treatments. No significant differences were identified in patients transplanted with MSC.
Table II.
Subgroup analysis of C-peptide and glycated hemoglobin changes at 12-month follow-up.
| A, C-peptide | |||||
|---|---|---|---|---|---|
| Variable | N | SMD | 95% CI | P-value | |
| HSC | 211 | ||||
| Age (years) | |||||
| <18 | 118 | 0.87 | 0.54, 1.20 | <0.001 | <0.001 |
| ≥18 | 93 | 3.37 | 2.88, 3.87 | <0.001 | |
| Disease history (months) | |||||
| <3 | 159 | 1.74 | 1.43, 2.04 | <0.001 | <0.001 |
| ≥3 | 52 | 1.31 | 0.71, 1.90 | <0.001 | |
| MSC | 30 | ||||
| Age (years) | |||||
| <18 | 15 | 0.77 | 0.02, 1.51 | 0.043 | 0.254 |
| ≥18 | 15 | 0.46 | −0.26, 1.19 | 0.212 | |
| Dose (IU/kg/day) | |||||
| <107 | 9 | 0.60 | −0.35, 1.55 | 0.214 | 0.946 |
| ≥107 | 21 | 0.62 | −0.01, 1.24 | 0.052 | |
| MSC+HSC | 42 | ||||
| Age (years) | |||||
| <18 | 21 | 3.00 | 2.11, 3.89 | <0.001 | <0.001 |
| ≥18 | 21 | 1.13 | 0.06, 1.80 | 0.001 | |
| Dose (IU/kg/day) | |||||
| <107 | 21 | 1.13 | 0.06, 1.80 | 0.001 | <0.001 |
| ≥107 | 21 | 3.00 | 2.11, 3.89 | <0.001 | |
| UCB | 62 | ||||
| Age (years) | |||||
| <18 | 56 | −2.21 | −2.72, −1.71 | <0.001 | <0.001 |
| ≥18 | 6 | −0.18 | −1.31, 0.96 | 0.757 | |
| Dose (IU/kg/day) | |||||
| <107 | 13 | −1.11 | −2.02, −0.19 | 0.017 | <0.001 |
| ≥107 | 49 | −2.14 | −2.67, −1.61 | <0.001 | |
| Disease history (months) | |||||
| <3 | 6 | −2.81 | −4.35, −1.28 | <0.001 | <0.001 |
| ≥3 | 56 | −1.79 | −2.27, −1.31 | <0.001 | |
| B, Glycated hemoglobin | |||||
| Variable | N | SMD | 95% CI | P-value | |
| HSC | 211 | ||||
| Age (years) | |||||
| <18 | 118 | 1.31 | 1.01, 1.61 | <0.001 | <0.001 |
| ≥18 | 93 | 4.53 | 3.90, 5.16 | <0.001 | |
| Dose (IU/kg/day) | |||||
| <107 | 188 | 1.91 | 1.62, 2.20 | <0.001 | 0.899 |
| ≥107 | 23 | 1.92 | 1.22, 2.63 | <0.001 | |
| Disease history (months) | |||||
| <3 | 159 | 2.26 | 1.93, 2.59 | <0.001 | <0.001 |
| ≥3 | 52 | 1.21 | 0.73, 1.68 | <0.001 | |
| MSC | 30 | ||||
| Age (years) | |||||
| <18 | 15 | 1.23 | 0.45, 2.02 | 0.007 | 0.947 |
| ≥18 | 15 | 1.21 | 0.33, 2.08 | 0.002 | |
| Dose (IU/kg/day) | |||||
| <107 | 9 | 0.63 | −0.32, 1.58 | 0.192 | 0.006 |
| ≥107 | 21 | 1.58 | 0.84, 2.32 | <0.001 | |
| MSC+HSC | 42 | ||||
| Age (years) | |||||
| <18 | 21 | 0.97 | 0.33, 1.62 | 0.001 | 0.616 |
| ≥18 | 21 | 1.07 | 0.42, 1.73 | 0.003 | |
| Dose (IU/kg/day) | |||||
| <107 | 21 | 1.07 | 0.42, 1.73 | 0.003 | 0.616 |
| ≥107 | 21 | 0.97 | 0.33–1.62 | 0.001 | |
| UCB | 62 | ||||
| Age (years) | |||||
| <18 | 56 | −0.14 | −1.28, 0.99 | 0.327 | 0.485 |
| ≥18 | 6 | 0.19 | −0.19, 0.57 | 0.804 | |
| Dose (IU/kg/day) | |||||
| <107 | 13 | −0.20 | −0.98, 0.57 | 0.603 | 0.005 |
| ≥107 | 49 | 0.25 | −0.15, 0.66 | 0.217 | |
| Disease history (months) | |||||
| <3 | 6 | 0.19 | −0.19, 0.57 | 0.804 | 0.483 |
| ≥3 | 56 | −0.14 | −1.28, 0.99 | 0.327 | |
SMD refers to comparisons between the pre- and post-SC therapy levels. CI, confidence interval; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; SMD, standardized mean difference; UCB, umbilical cord blood.
As presented in Table IIB, regarding the change in HbA1c levels at the 12-month follow-up, statistically significant differences in the effect of HSC treatment were identified in patients of different age and disease history subgroups (an age of ≥18 years and a disease history of <3 months were associated with lower HbA1c levels). Furthermore, an SC dose of ≥107 IU/kg/day was associated with lower HbA1c levels in patients with MSC therapy, which demonstrated SC therapy at the aforementioned dose was more efficient. Regarding UCB therapy, a significant difference between dose groups was identified. Regarding UCB therapy, a significant difference between the two dose groups was identified, but the treatment did not significantly improve the overall outcome. No statistically significant differences were identified for any of the other comparisons.
Discussion
In recent years, significant progress has been made regarding the treatment of diabetes. With the development of genomics, genetics, cell biology, and other major advances in science and technology, a major breakthrough has emerged in the treatment of diabetes (55,56). The present systematic review and meta-analysis suggested that SC therapy has a potential therapeutic effect in T1DM patients.
As presented by the pooled effect of RCTs, HSC, MSC and MSC+HSC therapy resulted in a higher C-peptide level increase when compared with that achieved by conventional insulin treatment, whereas UCB did not. The higher C-peptide levels significantly proved the regeneration of β-cell function after SC therapy. As for the reduction of HbA1c levels, MSC+HSC had a significantly better efficacy when compared to insulin and MSC treatment resulted in a similar trend. However, the results did not support that HSC and UCB had a better effect on the regulation of HbA1c levels when compared to insulin, with the reduction in HbA1c indirectly representing the degree of glycemic control.
As indicated by the pooled effect of SCTs, HSC, MSC and MSC+HSC all had a significant effect on the C-peptide increase after 12 months, compared to the baseline level. These results indicated that these three methods all improved β-cell function. In addition, UCB treatment had an opposite effect to that of and UCB-SC: UCB-SC was effective, whereas UCB was not or even resulted in a worse outcome. This result demonstrated that the dose of SCs may affect the effect of SC therapy, which may result from the variation of SCs volume between UCB and UCB-SC. Similar results were obtained regarding the reduction of HbA1c levels. HSC, MSC and MSC+HSC all reduced the HbA1c levels over a period of 12 months after SC transplantation, whereas the UCB was not effective.
Given that the present analysis was obtained from measurements every three months the results may have some deviation, since the measurement of HbA1c is slightly delayed in the diagnosis of hyperglycemia (57). In addition, C-peptide levels have a moderate capacity to reflect the regeneration of β-cell function.
Based on the results of the subgroup analysis, it may be concluded that the group aged ≥18 years and patients with a medical history of <3 months benefited significantly more from HSC transplantation. An SC injection dose of ≥107 IU/kg/day may be more suitable when MSC+HSC therapy is employed in patients aged ≥18 years with a recent (<3 months) diagnosis of DM.
At present, methods for SC therapy are limited by certain bottlenecks. To implement novel cell therapy-based strategies in the clinic, numerous issues require addressing. For instance, the mechanism for the differentiation of SCs into islet β-cells remains to be fully elucidated, reproducible induction methods require to be established and there is no consensus regarding the definitive functional criteria for induced cells. Therefore, it is challenging to determine whether the obtained cells are suitable for replacing the islet β-cells. Furthermore, the number of implanted SCs, the site of transplantation, the host's immune response after transplantation and carcinogenicity are all issues that require addressing by future studies. In a previous study, only few of the pediatric T1DM patients treated by autologous umbilical cord blood transfusion no longer required insulin (58). Thus, the major function of β-cells, namely robust glucose-induced insulin secretion, may not be easily realized by SCs. The combination of multiple approaches and methods may be the safest and most effective type of immunomodulatory therapy.
One issue that must be considered is the safety of SC therapy. Besides the immune reaction mentioned above, Gu et al (46) demonstrated that patients experienced different degrees of gastrointestinal reactions, hair loss, fever, bone marrow suppression and other adverse reactions during the process of transplantation. Fortunately, no significant organ, heart, liver or kidney dysfunction was observed and adverse reactions gradually disappeared at 2–4 weeks after SC reinfusion.
Furthermore, a major safety issue exists regarding the numerous rounds of replication that these SCs undergo prior to transplantation into the patient, which may result in the accumulation of chromosomal abnormalities and the cells may eventually become malignant.
The analysis of the present study has several strengths. Firstly, the robust methodology is a major strength. It was attempted to consider all available published studies via searching four major databases, and the data quality was assessed and to obtain suitable data. Furthermore, no language restrictions were imposed on the articles included. In addition, the present study compared data between SC therapy and conventional insulin treatment groups, and also included data from prior to and after SC therapy, which not only indicated the individuality of the treatments, but also reflected their applicability. In the present analysis, a random-effects model was employed, which was able to correlate individual observations to fit non-independent observation data. In addition, subgroup analyses were performed to confirm the consistency of conclusions among the different subgroup populations.
Several limitations of the present analysis reduce the value of the information obtained. First, in several trials included, the baseline C-peptide levels, HbA1c levels and FPG concentrations of participants were not assessed. Therefore, results of the subgroup analyses may have been different if all of the indexes had been assessed. Furthermore, several of the studies included [Ye et al (33), Giannopoulou et al (38) and Zhao (43)] were of poor quality, e.g. due to using unclear allocation concealment. In addition, there was a lack of original trials and the sample size of the studies included was limited. Finally, one inevitable limitation of the present study was the heterogeneity between the studies, which was based on differences between the institutions from various parts of the world.
In conclusion, according to the present meta-analysis, the use of SCs, including HSC, MSC or MSC+HSC, may be considered an effective approach for the therapy of T1DM. These results supported the routine application of these therapies in patients with T1DM. The use of USB is not recommended; however, a USB-SC component may be considered. Furthermore, patients aged ≥18 years or with a duration of T1DM of <3 months demonstrated a greater benefit from HSC transplantation. A SC injection dose of ≥107 IU/kg/day is more suitable in patients undergoing MSC+HS transplantation. Taken together, the present results may guide the further development of SC therapy and its implementation in the clinic, and indicate that SC therapy holds great promise for treating T1DM. In the future, additional trials are required to further elucidate the subject.
Figure 10.
Fold line diagram of insulin requirement during 12 months in patients treated by intravenous administration of MSC+HSC. HSC, hematopoietic stem cells; MSC, mesenchymal stem cells.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (grant nos. 81160307 and 81560395), the Jiangxi Science & Technology Pillar Program and the Science Foundation for Young Scholars of Jiangxi Province (grant no. 2007GQY1167) and the Voyage Project of Jiangxi Province Science and Technology Association.
Availability of data and materials
The analyzed data sets generated during the study are available in the published article.
Authors' contributions
JG performed the meta-analysis, YW was responsible for the statistical analysis and XZ prepared the manuscript. All authors have read and approved the final version of the manuscript prior to submission.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 3.Garofalo C, Iazzetta N, Camocardi A, Pacilio M, Iodice C, Minutolo R, De Nicola L, Conte G. Anti-diabetics and chronic kidney disease. G Ital Nefrol. 2015;32:pii. [PubMed] [Google Scholar]
- 4.Gholamhossein Y, Behrouz H, Asghar Z. Diabetic retinopathy risk factors: Plasma erythropoietin as a risk factor for proliferative diabetic retinopathy. Korean J Ophthalmol. 2014;28:373–378. doi: 10.3341/kjo.2014.28.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JJ, Hudson I, Harrison LC, Dean B, Colman PG, Werther GA, Warne GL, Court JM. Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes. 1989;38:779–783. doi: 10.2337/diabetes.38.6.779. [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson J, Carlsson A, Deli A, Forsander G, Ivarsson SA, Kockum I, Lindblad B, Marcus C, Lernmark Å, Samuelsson U. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013;100:203–209. doi: 10.1016/j.diabres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, et al. Rituximab, B-lymphocyte depletion and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagopian W, Ferry RJ, Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, et al. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: Two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: Where are we at and where should we be going. Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant. 2010;10:1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 13.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong JH, Yook S, Hwang JW, Jung MJ, Moon HT, Lee DY, Byun Y. Synergistic effect of surface modification with Poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat. Transplant Proc. 2013;45:585–590. doi: 10.1016/j.transproceed.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Evgenov NV, Medarova Z, Pratt J, Pantazopoulos P, Leyting S, Bonner-Weir S, Moore A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55:2419–2428. doi: 10.2337/db06-0484. [DOI] [PubMed] [Google Scholar]
- 16.Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996;61:1272–1274. doi: 10.1097/00007890-199604270-00027. [DOI] [PubMed] [Google Scholar]
- 17.Newman RG, Ross DB, Barreras H, Herretes S, Podack ER, Komanduri KV, Perez VL, Levy RB. The allure and peril of hematopoietic stem cell transplantation: Overcoming immune challenges to improve success. Immunol Res. 2013;57:125–139. doi: 10.1007/s12026-013-8450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: From hype to hope. Stem Cells Transl Med. 2013;2:328–336. doi: 10.5966/sctm.2012-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi X, Guo X, Su C. Clinical outcomes of the transplantation of stem cells from various human tissue sources in the management of liver cirrhosis: A systematic review and meta-analysis. Curr Stem Cell Res Ther. 2015;10:166–180. doi: 10.2174/1574888X09666141112114011. [DOI] [PubMed] [Google Scholar]
- 20.Kim G, Eom YW, Baik SK, Shin Y, Lim YL, Kim MY, Kwon SO, Chang SJ. Therapeutic effects of mesenchymal stem cells for patients with chronic liver diseases: Systematic review and Meta-analysis. J Korean Med Sci. 2015;30:1405–1415. doi: 10.3346/jkms.2015.30.10.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA., Jr Mesenchymal stem cell therapy for inflammatory bowel disease: A systematic review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2696–2707. doi: 10.1097/MIB.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong H, Yim HW, Park HJ, Cho Y, Hong H, Kim NJ, Oh IH. Mesenchymal stem cell therapy for ischemic heart disease: Systematic review and Meta-analysis. Int J Stem Cells. 2018;11:1–12. doi: 10.15283/ijsc17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoekstra JB, van Rijn HJ, Erkelens DW, Thijssen JH. C-peptide. Diabetes Care. 1982;5:438–446. doi: 10.2337/diacare.5.4.438. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Perez FJ, Aguilar-Salinas CA, Almeda-Valdes P, Cuevas-Ramos D, Lerman Garber I, Rull JA. HbA1c for the diagnosis of diabetes mellitus in a developing country. A position article. Arch Med Res. 2010;41:302–308. doi: 10.1016/j.arcmed.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Preedy VR, Watson RR. Vol. 25. Springer; New York: 2010. Fasting Plasma Glucose. [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group, corp-author. Preferred reporting items for systematic reviews and Meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] naunyn-schmiedebergs archiv für experimentelle pathologie und pharmakologie. 2014;5(Suppl):S38. [Google Scholar]
- 28.Anderson ML, Peterson ED. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372:2370–2371. doi: 10.1056/NEJMsa1409364. [DOI] [PubMed] [Google Scholar]
- 29.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Deeks JJ, Altman DG, Bradburn MJ. Systematic Reviews in Health Care Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd. Wiley-Blackwell; Chichester: 2008. Statistical methods for examining heterogeneity and combining results from several studies in Meta-analysis. [Google Scholar]
- 32.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 33.Ye L, Li L, Wan B, Yang M, Hong J, Gu W, Wang W, Ning G. Immune response after autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus. Stem Cell Res Ther. 2017;8:90. doi: 10.1186/s13287-017-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakkar UG, Trivedi HL, Vanikar AV, Dave SD. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015;17:940–947. doi: 10.1016/j.jcyt.2015.03.608. [DOI] [PubMed] [Google Scholar]
- 35.Snarski E, Milczarczyk A, Hałaburda K, Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K, Franek E, Wiktor-Jedrzejczak W. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: Long-term observations. Bone Marrow Transplant. 2016;51:398–402. doi: 10.1038/bmt.2015.294. [DOI] [PubMed] [Google Scholar]
- 36.Delgado E, Perez-Basterrechea M, Suarez-Alvarez B, Zhou H, Revuelta EM, Garcia-Gala JM, Perez S, Alvarez-Viejo M, Menendez E, Lopez-Larrea C, et al. Modulation of autoimmune T-cell memory by stem cell educator therapy: Phase 1/2 clinical trial. EBiomedicine. 2015;2:2024–2036. doi: 10.1016/j.ebiom.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: A pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39:149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 38.Giannopoulou EZ, Puff R, Beyerlein A, von Luettichau I, Boerschmann H, Schatz D, Atkinson M, Haller MJ, Egger D, Burdach S, Ziegler AG. Effect of a single autologous cord blood infusion on beta-cell and immune function in children with new onset type 1 diabetes: A non-randomized, controlled trial. Pediatr Diabetes. 2014;15:100–109. doi: 10.1111/pedi.12072. [DOI] [PubMed] [Google Scholar]
- 39.D'Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: A multicenter analysis. Diabetes. 2014;63:3041–3046. doi: 10.2337/db14-0295. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 41.Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, Chen Y, Zhao W, Jia Z, Yan S, Wang Y. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endoc J. 2013;60:347–357. doi: 10.1507/endocrj.EJ12-0343. [DOI] [PubMed] [Google Scholar]
- 42.Haller MJ, Wasserfall CH, Hulme MA, Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster JJ, Ferguson RJ, et al. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with type 1 diabetes. Biol Blood Marrow Transplant. 2013;19:1126–1129. doi: 10.1016/j.bbmt.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, Li H, Zhang Y, Diao Y, Li Y, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:3. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Ye L, Hu J, Tang W, Liu R, Yang M, Hong J, Wang W, Ning G, Gu W. Acute response of peripheral blood cell to autologous hematopoietic stem cell transplantation in type 1 diabetic patient. PLoS One. 2012;7:e31887. doi: 10.1371/journal.pone.0031887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W, Zhang N, Zhuge YZ, Chen B, Xu J, Zhu D. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab. 2012;97:1729–1736. doi: 10.1210/jc.2011-2188. [DOI] [PubMed] [Google Scholar]
- 46.Gu W, Hu J, Wang W, Li L, Tang W, Sun S, Cui W, Ye L, Zhang Y, Hong J, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care. 2012;35:1413–1419. doi: 10.2337/dc11-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Gao H, Yu X, Wang L, Yan S, Wang Y. Umbilical cord mesenchymal stem cells transplantation for newly-onset type 1 diabetes. J Clin Rehabil Tissue Engineering Res. 2011;15:4363–4366. [Google Scholar]
- 48.Haller MJ, Wasserfall CH, Hulme MA, Cintron M, Brusko TM, McGrail KM, Sumrall TM, Wingard JR, Theriaque DW, Shuster JJ, et al. Autologous umbilical cord blood transfusion in young children with type 1 diabetes fails to preserve C-peptide. Diabetes Care. 2011;34:2567–2569. doi: 10.2337/dc11-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng K, Xu YW, Ye FG, Xiao L, Ma XH, Gao Y, Zhang X, Yao SZ, Shi BY. Autologous peripheral blood hematopoietic stem cell transplantation in the treatment of type 1 diabetic mellitus: A report of 16 cases. Zhonghua Yi Xue Za Zhi. 2011;91:1966–1969. (In Chinese) [PubMed] [Google Scholar]
- 50.Vanikar AV, Dave SD, Thakkar UG, Trivedi HL. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010;2010:582382. doi: 10.4061/2010/582382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snarski E, Milczarczyk A, Torosian T, Paluszewska M, Urbanowska E, Król M, Boguradzki P, Jedynasty K, Franek E, Wiktor-Jedrzejczak W. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant. 2011;46:562–566. doi: 10.1038/bmt.2010.147. [DOI] [PubMed] [Google Scholar]
- 52.Gu WQ, Sun SY, Hu J, Tang W, Wei JS, Zhu LP, Hong J, Tang ZY, Liu JM, Li XY, Wang WQ, et al. Efficacy and safety of autologous hematopoietic stem cell transplantation in treating type 1 diabetes mellitus. Chin J Endocrinol Metabol. 2010;53:1023–1026. (In Chinese) [Google Scholar]
- 53.Haller MJ, Wasserfall CH, Mcgrail KM, Cintron M, Brusko TM, Wingard JR, Kelly SS, Shuster JJ, Atkinson MA, Schatz DA. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care. 2009;32:2041–2046. doi: 10.2337/dc09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simões BP, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19:538–546. doi: 10.1016/j.bbmt.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Yanai G, Hayashi T, Zhi Q, Yang KC, Shirouzu Y, Shimabukuro T, Hiura A, Inoue K, Sumi S. Electrofusion of mesenchymal stem cells and islet cells for diabetes therapy: A rat model. PLoS One. 2013;8:e64499. doi: 10.1371/journal.pone.0064499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glycosylated hemoglobin assays in the management and diagnosis of diabetes mellitus. Health and Public Policy Committee, American College of Physicians. Ann Intern Med. 1984;101:710–713. doi: 10.7326/0003-4819-101-5-710. [DOI] [PubMed] [Google Scholar]
- 58.Haller MJ, Viener H, Brusko T, Wasserfall C, Mcgrail K, Staba S, Cogle C, Atkinson M, Schatz DA. Insulin requirements, HbA1c and stimulated C-peptide following autologous umbilical cord blood transfusion in children with T1D. Diabetes. 2007;56:A82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available in the published article.