Abstract
Background:
Dipeptidyl peptidase-4 (DPP-4) inhibitor and pioglitazone combination therapy have been widely used for patients with inadequate glycemic control on monotherapy. This meta-analysis assessed the efficacy and safety of this combination therapy in patients with type 2 diabetes mellitus (T2DM).
Methods:
We searched the MEDLINE, Embase, and Cochrane databases. Studies were eligible if they were randomized controlled trials (RCTs) on DPP-4 inhibitor and pioglitazone combination therapy in patients with T2DM through the end of February 2016, using the keywords “alogliptin,” “dutogliptin, ” “linagliptin,” “saxagliptin,” “sitagliptin,” “vildagliptin,” “gliptins,” “DPP-4 inhibitor,” and “pioglitazone.” RCTs were selected if they compared DPP-4 inhibitors and pioglitazone as combination therapy; treatment duration was ≥12 weeks; and the reported data included hemoglobin A1c (HbA1c) or fasting plasma glucose (FPG) change, total or any other system Adverse Events (AEs). We estimated effect size with random-effects or fixed-effects meta-analysis, I2 statistic was used to estimate heterogeneity of results.
Results:
Seven RCTs were included. Compared with pioglitazone monotherapy, combination DPP-4 inhibitor and pioglitazone therapy were associated with increased reduction in HbA1c ([MD]-0.64%;−0.73 to −0.55) and FPG ([MD] −0.94; −1.12 to −0.76) levels, more patients in the combination therapy groups versus pioglitazone monotherapy groups had an A1c of < 7% ([OR]2.52; 2.18, 3.17) at the end of the studies, but was not associated with further reduction in higher risk of hypoglycaemia, edema, or any other system AEs. We also noticed that DPP-4 inhibitor and pioglitazone combination therapy were associated with better improvement of pancreatic β-cell function.
Conclusions:
DPP-4 inhibitor and pioglitazone combination therapy provided better glycemic control, both according to HbA1c and FPG levels, than pioglitazone monotherapy. Safety analysis showed well tolerance of combination therapy, even in hypoglycemic and edema AEs. However, additional large-scale, high quality, long-term follow-up clinical trials are necessary to confirm its long-term effectiveness.
Keywords: dipeptidyl peptidase-4 inhibitors, efficacy and safety, meta-analysis, pioglitazone
1. Introduction
Diabetes is a worldwide disease, and type 2 diabetes mellitus (T2DM) accounts for more than 90% diabetes cases. It is estimated that approximately 415 million people in the world have diabetes in 2015, and this figure is projected to increase to 642 million by 2040.[1]
The pathogenesis of T2DM includes a progressive decline in pancreatic β-cell function and insulin resistance. Due to the progressive nature of this disease, many patients, especially those with moderate-to-severe hyperglycemia, are unlikely to attain hemoglobin A1c (HbA1c) targets <7% with monotherapy.[2,3] Combination therapy with oral anti-hyperglycemic agents is considered a valid treatment option for these cases.
A classic hypoglycemic agent, pioglitazone, is an insulin sensitizer that can activate peroxisome proliferator-activated receptors in order to increase peripheral glucose uptake and improve insulin resistance. The beneficial effects of pioglitazone on β-cell function have been shown in rodent and cell culture studies. Dipeptidyl peptidase (DPP)-4 inhibitors, also known as gliptins, can prolong the plasma half-life of active glucagon-like peptide-1, an incretin hormone released from the gut in response to food intake, which improves glycemic control by stimulating insulin secretion, inhibiting glucagon secretion, and slowing gastric emptying. Similarly, several DPP-4 inhibitors, such as sitagliptin, have been shown to improve pancreatic β-cell function,[4–6] with antiapoptotic and pro-proliferative effects on β-cells reported in rodent and cell culture studies. Given these complementary mechanisms, the combination of DPP-4 inhibitors with pioglitazone has been proposed to be a valid treatment for T2DM.[7–9] Indeed, glycemic control was attained by improving 2 core pathophysiologic defects associated with the disease, diminished β-cell function with reduced insulin release, resulting in increased insulin resistance and increased hepatic glucose output.[10]
The present study assessed the efficacy and safety of initial combination therapy with DPP-4 inhibitors and pioglitazone in patients with T2DM. The main objective of this meta-analysis was to assess the efficacy and safety of this combination therapy compared to pioglitazone monotherapy for the management of patients with T2DM. The outcome measures included glycemic control, lipid profile, β-cell function, insulin resistance, and adverse events AEs. Homeostatic model assessment (HOMA) of β-cell function and insulin resistance was first described in 1985. The technique is a method for assessing β-cell function and insulin resistance from basal glucose and insulin or C-peptide concentrations. The HOMA model has proved as a robust clinical and epidemiological tool in evaluation of the pathophysiology of diabetes.[11]
We followed the methods specified in the Cochrane Handbook for Reviews on Interventions. To the best of our knowledge, this is the first meta-analysis to comparison the effects of dipeptidyl peptidase-4 inhibitors and pioglitazone combination therapy versus pioglitazone monotherapy in type 2 diabetes based on RCTs.
2. Methods
2.1. Search strategy and selection criteria
The meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Guidelines. No official protocol was published or registered.
Eligible studies, which were limited to those written in the English language, were identified through electronic searched of the PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) databases through May 2016. The main search term was a combination of MESH terms and text words for “DPP-4 inhibitors” and “pioglitazone,” in addition to restricting the study type to “Randomized Controlled Trial,” “RCT,” or “random.” These terms were adjusted according to the requirements for each database. Two independent reviewers (WB and SY) perform the search. The details of the search are presented in Figure 1.
Figure 1.
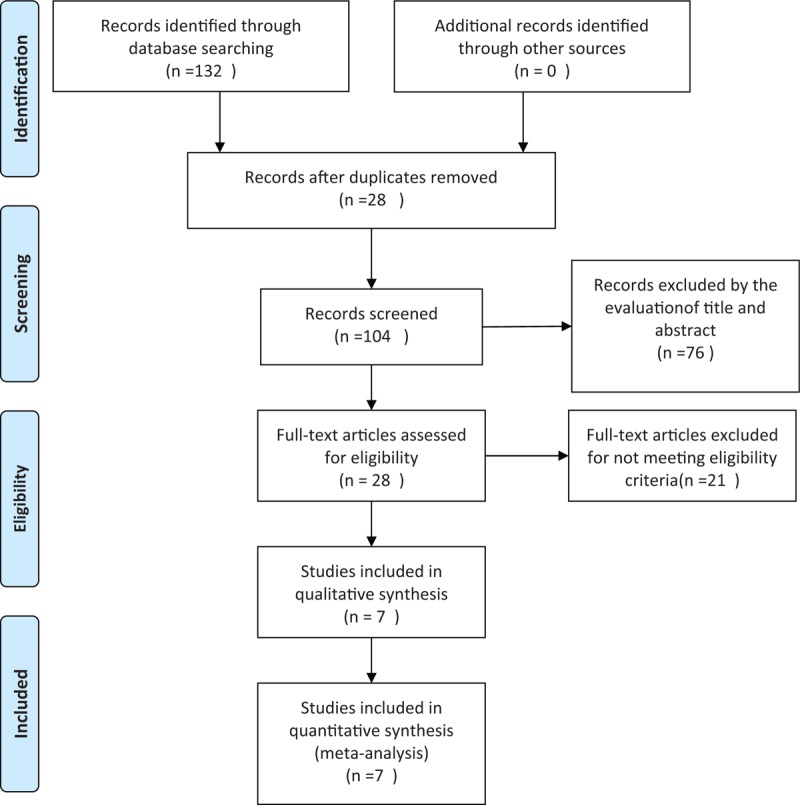
Article selection diagram for meta-analysis.
After deleting the duplicate results, 2 reviewers (SY and SYQ) independently screened all titles and abstracts and investigated full texts for eligible studies. The inclusion criteria were as follows: double-blind RCTs with duration ≥12 weeks, which enrolled patients with T2DM with HbA1c levels >7%, and analyzed the efficacy of pioglitazone and DPP-4 inhibitor therapy or placebo by comparing changes in HbA1c levels or during the intervention.
We performed a systematic review and meta-analysis of randomized clinical trials (RCTs) in order to compare combination DPP-4 inhibitors and pioglitazone therapy versus pioglitazone monotherapy for the management of T2DM. The primary outcome measures included changes in HbA1c and fasting plasma glucose (FPG) changes from baseline as well as AEs. A random effects model was used to perform the meta-analyses, and the results were expressed as weighted mean differences for continuous outcomes and relative risks for dichotomous outcomes, both with 95% confidence intervals, and with I2 and P values as markers of heterogeneity.
2.2. Study selection
The results of the electronic literature search were imported into reference management software (Endnote X 7.4). Potential studies were identified by screening article titles and abstracts, and target articles were assessed by reading the full text. Discrepancies were resolved by consensus. Two independent reviewers (WB and SY) extracted the data. Eligible studies were identified through independent screening of titles and abstracts or full texts by 2 reviewers as per the aforementioned inclusion criteria.
2.3. Quality assessment
Quality assessment was performed using Review Manager (Revman Version 5.3, Copenhagen, Denmark). The risk of bias was evaluated using the Cochrane Collaboration's risk of bias tool.[12] The overall risk of bias was assessed as low, high, or unclear based on study randomization methods, blinding, completeness of outcome data, reporting of data and other biases.
2.4. Data extraction
Data were extracted independently by 2 reviewers (WB and SY). The full text of the included studies were assessed, and the data were grouped according to study characteristics (title, publication date, authors, region, study design), participants (age, duration of T2DM, gender, and ethnicity), intervention (dosage of each group, duration), efficacy (HbA1c, lipid profiles, FPG, HOMA assessments of β-cell function and insulin resistance changes from baseline, and the proportion of participants that achieved the HbA1c goal of <7%), and safety (incidence of hypoglycaemia, edema, respiratory AEs, digestive tract AEs, nervous systemic AEs, total AEs,). Combination therapy was defined as pioglitazone (30 mg) and the maximum licensed dose of DPP-4 inhibitors. We choose the recommended starting dose; for example, 30 mg vildagliptin, and 3 of the included studies included drug-naïve participants.
2.5. Assessment of risk of bias
Two reviewers (WB and SYQ) independently assessed the risk of bias as recommended by the Cochrane Hand book for Systematic Reviews of Interventions. The following methodological domains were considered: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential threats to validity. We explicitly judged each of the domains as having high risk, low risk or unclear risk of bias
2.6. Data analysis
Meta-analysis was conducted with the Review Manager. Heterogeneity among studies was assessed using the I2 statistic. Random or fixed effects model was used to perform the meta-analysis depending on the heterogeneity. The weighted mean difference (MD) was applied for continuous variables, whereas the risk ratio was used for dichotomous outcomes, both with 95% confidence intervals (CI).
We performed subgroup analyses to examine different interventions according to drugs naive or not. The mean difference or relative risk was further evaluated by classifying each study into one of these categories.
2.7. Ethics
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
3. Results
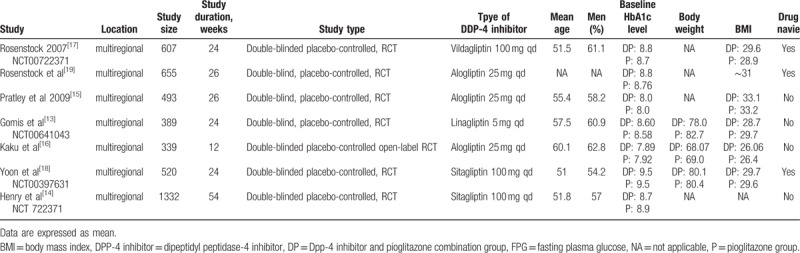
A total of 132 potentially relevant articles published until May 2016 were identified in the electronic search of the PubMed, EMBASE, and Cochrane CENTRAL databases. After screening the abstracts, 104 articles were excluded because they did not meet the inclusion criteria. Further detailed evaluation was performed for 28 articles, and 21 excluded for not being a real RCT. Finally, 7 studies[13–19] were included in the meta-analysis (Fig. 1). All 7 were published as full paper articles between 2007 and 2014 and were designed as multicentral studies lasting for 12 to 54 weeks. The DPP-4 inhibitors included sitagliptin,[14,18] alogliptin,[15,16,19] vildagliptin,[17] and linagliptin.[13] The baseline data for each trial were similar. The mean HbA1c values at baseline ranged from 7.89% to 9.5% and 7.92% to 9.5% for the combination groups and pioglitazone monotherapy groups, respectively. The mean body mass indexes ranged from 26.07 to 33.1 kg/m2 in the combination groups and 26.4 to 33.2 kg/m2 in the pioglitazone groups. Three RCTs were performed on drug-naive patients.[17–19] Four types of gliptins were included, and the recommended dosage for each medicine (pioglitazone, 30 mg; sitagliptin, 100 mg; vildagliptin, 100 mg; alogliptin, 25 mg; and linagliptin, 5 mg) was chosen to avoid the risk of clinical heterogeneity (Table 1).
Table 1.
Characteristics of RCTs included in the meta-analysis.
All 7 trials reported their randomization methods. None of the trials found differences in the baseline characteristics of participants between the combination therapy groups and pioglitazone groups. All 7 trials described random sequence generation and allocation concealment, reported clinically relevant outcome measures, and undertook sample size calculations. Three trials provided a clear description of losses to follow-up and accounted for patients with missing data in the analyses.[11,12,15] None of the included trials were terminated prematurely (Fig. 2).
Figure 2.
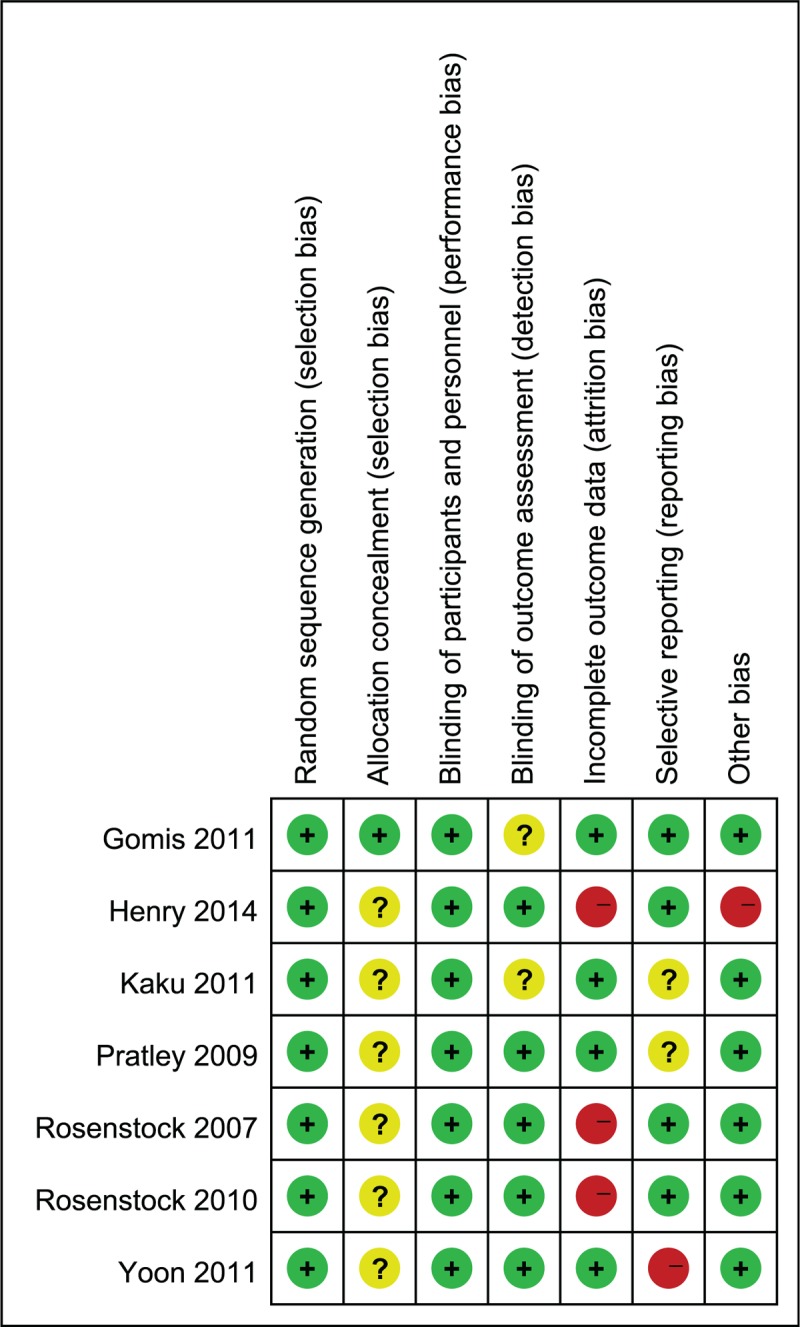
Risk of bias summary.+, Low risk of bias; 2 high risk of bias; ?, unknown risk of bias. Risk of bias assessment for random sequence generation and allocation concealment is performed at the study level. Risk of bias assessment for blinding of participants and personnel, incomplete outcome data, selective reporting, and overall risk of bias are for the primary outcome (change in HbA1c). HbA1C = hemoglobin A1c.
Six studies reported FPG levels. Analysis of these data revealed that the combination therapy of DPP-4 inhibitor and pioglitazone led to a greater reduction in FPG level (mean difference [MD] −0.94; −1.12 to −0.76) without significant heterogeneity (I2 = 0%, P = .52) (Fig. 3).
Figure 3.
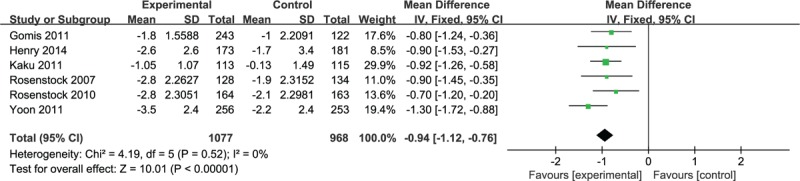
Meta-analysis of change in fasting plasma glucose (mmol/L).
All 7 studies assessed change in HbA1c levels at baseline and the end of the study. Six of them, including a total of 1081 patients had valid data. A greater reduction in HbA1c levels was observed in combination therapy groups, with heterogeneity (MD -0.64;-0.73,-0.55, I2 = 59%, P = .03; Fig. 4A). The percentage of patients achieving target HbA1c levels (<7%) was also calculated in all 7 studies. Patients with combination treatment manifested a higher propensity in achieving this goal (Odds Ratio [OR] 2.59; 2.18, 3.07, I2 = 61%, P = .02; Fig. 4B). Further subgroup analysis in drug-naïve patient also showed a higher propensity in achieving target HbA1c levels in combination groups with less heterogeneity (OR 3.30; 2.58, 4.22, I2 = 11%, P = .33; Fig. 4C).
Figure 4.
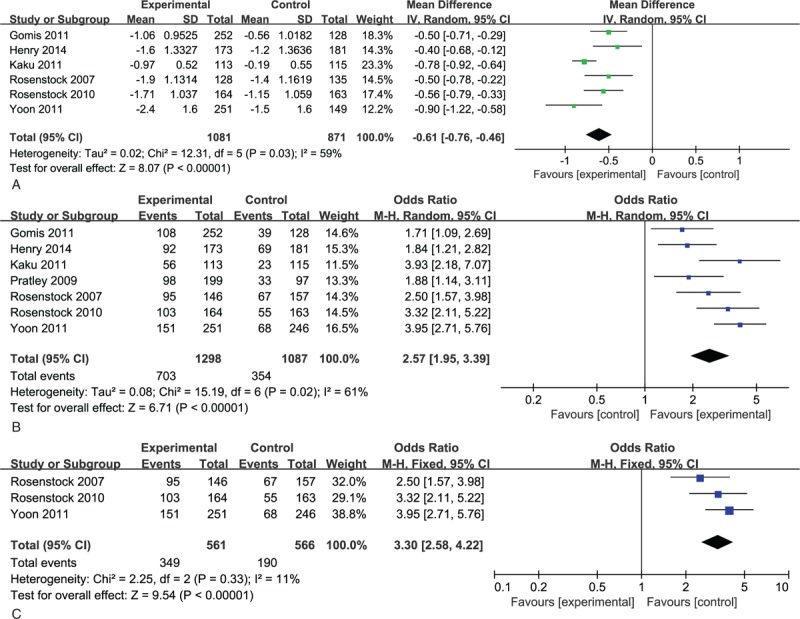
(A) Meta-analysis of change in HbA1c (%). (B) Percentage of patients achieving HbA1c< 7.0%. (C) Percentage of patients achieving HbA1c< 7.0% in subgroup analysis. HbA1C = hemoglobin A1c.
Three studies assessed changes in lipid profiles.[16–18] Two of them presented data as percentage change,[17,18] while one as absolute change. Statistically significant reduction in total cholesterol levels between different interventions was documented in 2 studies. Tendency for reduced triglyceride and low-density lipoprotein was reported in all the studies.
Meta-analysis of the 3 RCTs that assessed changes in HOMA-β revealed a higher increase in β-cell function in combination groups (MD 6.48; 3.91, 9.05, Fig. 5), as well as a tendency for reduced HOMA-IR for the combination treatment, which did not reach statistical significance.
Figure 5.
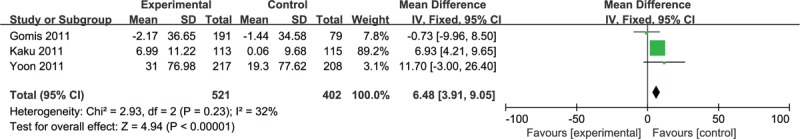
Meta-analysis of change in HOMA-β. HOMA = homeostatic model assessment.
Combination therapy with DPP-4 inhibitors and pioglitazone were generally well tolerated over the 12 to 54 week treatment period. There were no differences in AEs between combination therapy with DPP-4 inhibitors and pioglitazone and pioglitazone in any of the 7 RCTs (Fig. 6A). There were also no differences in reported hypoglycaemia between these studies (relative risk [RR] 0.80, 95% CI 0.43–1.50, Fig. 6B). The risk of digestive system adverse events with combination therapy was not significantly different from that of pioglitazone monotherapy (RR 0.85, 95% CI 0.54–1.34, P = .98, Fig. 6C).No difference in the incidence of edema, upper respiratory tract infection, nasopharyngitis, and headache were observed between combination and monotherapy groups (Fig. 6D–F).Overall, combination therapy was better tolerated, with lower absolute rates of AEs.
Figure 6.
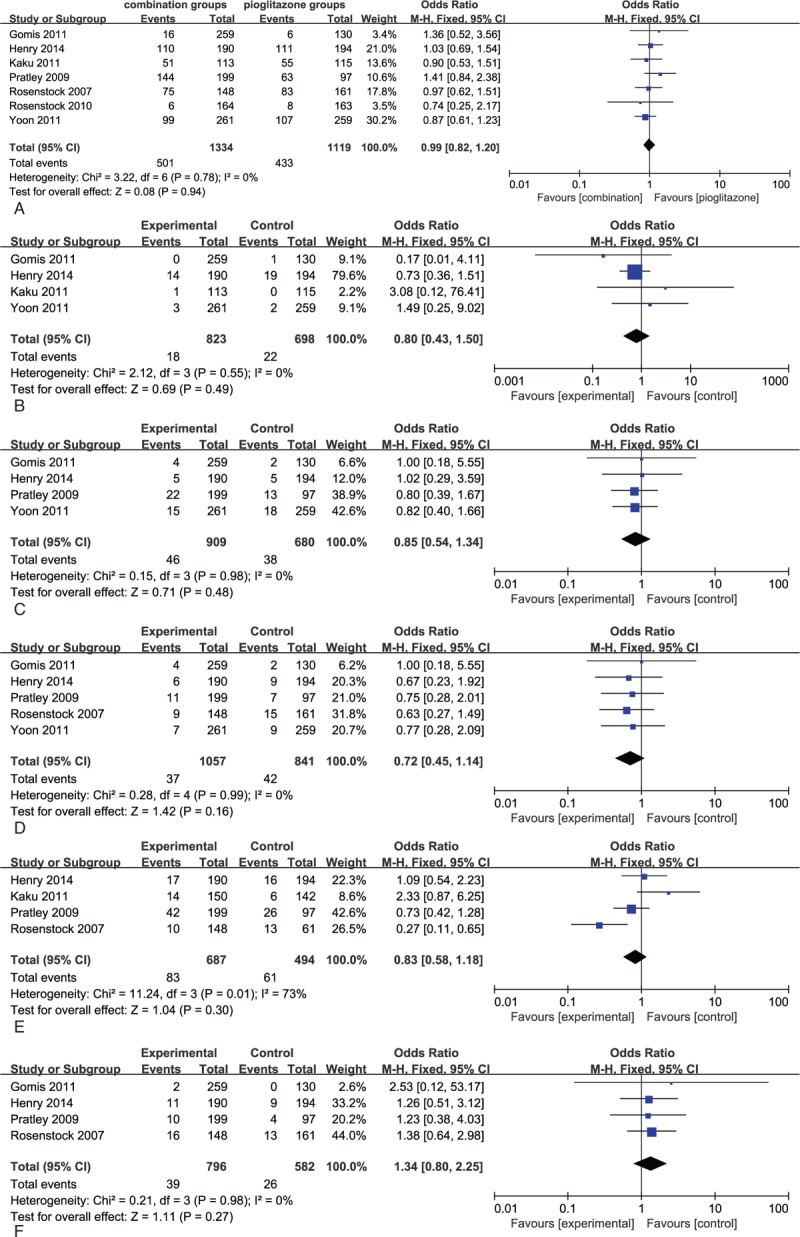
(A) Meta-analyses of total adverse events in patients with combination therapy versus pioglitazone. (B) Meta-analyses of hypoglycemia events in patients with combination therapy versus pioglitazone. (C) Meta-analyses of digestive system adverse events in patients with combination therapy versus pioglitazone. (D) Meta-analyses of edema events in patients with combination therapy versus pioglitazone. (E) Meta-analyses of respiratory system adverse events in patients with combination therapy vs pioglitazone. (F) Meta-analyses of nervous system events in patients with combination therapy versus pioglitazone.
4. Discussion
DPP-4 inhibitors combined with pioglitazone, administered once daily, produced significant, clinically meaningful, and sustained improvement in glycemic control from baseline compared with pioglitazone monotherapy. Overall, combination therapy was associated with a 0.61% reduction in HbA1c, as well as a higher propensity ([OR] 2.59; 2.18, 3.07) in achieving target HbA1c levels. Subgroup analysis in drug naïve patients revealed same results with less heterogeneity. Additional reduction in FPG level ([MD]-0.94; −1.12 to −0.76) was also documented.
Compared with pioglitazone monotherapy, combination therapy did not increase the incidence of AEs. DPP4-inhibtors are generally well tolerated, with the frequency of AEs being similar to placebo and a low frequency of hypoglycemia. In our meta-analysis, when added to pioglitazone therapy, similar hypoglycemia risk occurred.[13,14,16,18] In addition, the use of thiazolidinediones in the management of type 2 diabetes mellitus has been associated with an increased risk of fluid retention and edema.[20] However, in our meta-analysis, combination treatment did not seem to increase the risk of edema. The cardiovascular effects of DPP-4 inhibitors remain controversial.[21] One original RCT of our meta-analysis which focused on alogliptin and pioglitazone combination therapy, reported this AE.[15] None significant difference was revealed between groups. However, it is needed for more RCTs from multicenter for more reliable conclusions.
Pancreatic β-cell dysfunction and insulin resistance is the pathological basis of T2DM. HOMA is a “ease-of-use” approach to estimate the degree of β-cell deficiency and the target-tissue sensitivity to insulin.[22] Significant improvements in HOMA-β were consistently observed in the combination groups, indicating better protective effect for pancreatic β-cell function. Although lacking of significant between-group difference, trend of reduced HOMA-IR was noticed in all the 3 studies. The lack of a statistically significant difference in our meta-analysis may reflect the biological variation and the limited sample size. Further analysis needs to perform when new RCTs updated.
Combination therapy also shows potential benefits on lipid profiles, especially on total cholesterol levels, statistically significant reduction was reported in all 3 studies. More RCTs need to be performed to confirm the effect on triglyceride and low density lipoprotein.
The study by Henry et al[14] did not blind the participants or account for the intention to treat population in their results and analyses. The study duration ranged between 12 and 54 weeks, and the participants included both those who were drug naïve as well as those under treatment. These aspects weakened the internal validity of our findings. Because the treatments included in our meta-analysis were restricted to clinically relevant doses, the results can be extrapolated to clinical practice.
The strengths of this meta-analysis are related to the incorporation of direct evidence from recently published high-quality RCTs, sufficient outcomes were assessed. Considering treatment duration may influence primary outcome, subgroup meta-analysis in drug naïve studies was performed.
However, some limitations should also be recognized. Firstly, we assessed DPP-4 inhibitors as a whole, certain heterogeneity was hard to avoid. Furthermore, intervention effects of each gliptins were not examined in subgroup analysis due to the paucity of available data. Secondly, intervention duration is the major influence factor for HbA1c, however, different intervention durations was noticed among studies, Kaku et al[16] with 12 weeks duration and Henry et al[14] with 54 weeks duration, which may bring further heterogeneity. Most trials lasted <24 weeks, limited the observation of long-time outcomes among some comparisons. Thirdly, the included trials did not provide enough data to perform meta-analysis of lipid profiles, cardiovascular AEs, weight gain. Further analysis for each gliptins and other risks was necessary to be performed once new RCTs were published.
Based on currently available data from a limited number of RCTs, combination therapy was effective and well tolerated, thus offering a valuable option for T2DM patients with inadequate glycemic control on monotherapy. These results were consistent with original RCTs. In addition, DPP-4 inhibitors and pioglitazone combination therapy might be considered as a more favorable option for T2DM patients with dislipdemia and insulin resistance.
5. Conclusion
DPP-4 inhibitor and pioglitazone combination therapy provided better glycemic control, both according to HbA1c and FPG levels, than pioglitazone monotherapy. Safety analysis showed well tolerance of combination therapy, even in hypoglycemic and edema AEs. However, additional large-scale, high quality, long-term follow-up clinical trials are necessary to confirm its long-term effectiveness.
Author contributions
Jun Liang, Yan Sun, and Ben Wang did the study design. Yan Sun and YQS did the scientific search. Ben Wang and Yan Sun extracted data. Ben Wang and Kui Xue Liu did the analysis. Jun Liang, Ben Wang and Yan Sun wrote the first draft of the report. All authors contributed to interpretation and edited the draft report.
Conceptualization: Ben Wang, Yan Sun, Jun Liang.
Data curation: Ben Wang, Kui Xue Liu.
Formal analysis: Kui Xue Liu.
Methodology: Ben Wang, Quan Yi Sang, Kui Xue Liu.
Project administration: Jun Liang.
Resources: Yan Sun.
Software: Yan Sun, Quan Yi Sang.
Supervision: Jun Liang.
Writing – original draft: Ben Wang, Yan Sun.
Writing – review & editing: Jun Liang.
Footnotes
Abbreviations: DPP-4 = inhibitor dipeptidyl peptidase-4 inhibitor, T2DM = type 2 diabetes mellitus, RCT = randomized controlled trials, HbA1C = hemoglobin A1c, FPG = fasting plasma glucose, AEs = adverse events, HOMA = homeostatic model assessment, MD = weighted mean difference, CI = confidence intervals, OR = odds ratio, BMI = body mass index.
BW and YS contributed equally to this work.
Declaration of interests: We declare no competing interests.
This work was sponsored by Xuzhou Science and Technology Grant (KC14SX013, KC14SH).
The authors have no conflicts of interest to disclose.
References
- [1].Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- [2].Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015;21:438–47. [DOI] [PubMed] [Google Scholar]
- [3].Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach—update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2015;38:140–9. [DOI] [PubMed] [Google Scholar]
- [4].Ahrén B1, Foley JE2. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia 2016;59:907–17. [DOI] [PubMed] [Google Scholar]
- [5].Esposito K, Chiodini P, Maiorino MI, et al. Glycaemic durability with dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and meta analysis of long-term randomised controlled trials. BMJ Open 2014;4:e005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Esposito K, Chiodini P, Maiorino MI, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24163 patients. BMJ Open 2015;5:e005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Priscilla H, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 2009;94:4810–9. [DOI] [PubMed] [Google Scholar]
- [8].Derosaa G, Maffiolia P, Sibilla SA, et al. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metab Clin Exp 2010;59:887–95. [DOI] [PubMed] [Google Scholar]
- [9].Al-Majed A, Bakheit AH, Abdel Aziz HA, et al. Pioglitazone. Profiles Drug Subst Excip Relat Methodol 2016;41:379–438. [DOI] [PubMed] [Google Scholar]
- [10].Mikhail N. Combination therapy with DPP-4 inhibitors and pioglitazone in type 2 diabetes: theoretical consideration and therapeutic potential. Vasc Health Risk Manag 2008;4:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Green S. (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 Available at: http://handbook.cochrane.org Accessed April 15, 2014. [Google Scholar]
- [13].Gomis R, Espadero R-M, Jone R, et al. Efficacy and safety of initial combination therapy with linagliptin and piogltazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind placebo-controlled study. Diabetes Obes Metab 2011;13:653–61. [DOI] [PubMed] [Google Scholar]
- [14].Henry RR, Staels B, Fonseca VA, et al. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone—a factorial study. Diabetes Obesity Metab 2014;16:223–30. [DOI] [PubMed] [Google Scholar]
- [15].Pratley RE, Reusch JE, Fleck PR, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 2009;25:2361–71. [DOI] [PubMed] [Google Scholar]
- [16].Kaku K, Itayasu T, Hiroi S, et al. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab 2011;13:1028–35. [DOI] [PubMed] [Google Scholar]
- [17].Rosenstock J, Kim SW, Baron MA, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with componentmonotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007;9:175–85. [DOI] [PubMed] [Google Scholar]
- [18].Yoon KH, Steinberg H, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and pioglitazone in patients with type 2 diabetes: a 54-week study. Diabetes Obes Metab 2012;14:745–52. [DOI] [PubMed] [Google Scholar]
- [19].Rosenstock J, Inzucchi SE, Seufert J, et al. Combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes. Diabetes Care 2010;33:2406–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bundhun PK, Janoo G, Teeluck AR, et al. Adverse drug effects observed with vildagliptin versus pioglitazone or rosiglitazone in the treatment of patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol 2017;18:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].LeBras MH, Barry AR, Koshman SL. Cardiovascular safety outcomes of new antidiabetic therapies. Am J Health Syst Pharm 2017;74:970–6. [DOI] [PubMed] [Google Scholar]
- [22].Cersosimo E, Solis-Herrera C, Trautmann ME, et al. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev 2014;10:2–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


