Abstract
Systemic inflammatory response syndrome (SIRS) is an important process associated with the pathogenesis of multiple organ failure resulting from heat stroke (HS). Alterations in the levels of circulating cytokines during the progression of SIRS have been well established. However, only a small number of studies have demonstrated the responses of lymphocytes during HS, and no studies have investigated immune-regulatory cells, such as regulatory T cells (Tregs). Tregs have been revealed to be important in numerous inflammation-associated diseases, and have exhibited promising therapeutic effects in both experimental and clinical trials. In the present study, the splenic Treg response in a classic HS mouse model was investigated, and the results demonstrated that total numbers of splenic Tregs were significantly decreased at 0, 24 and 72 h time intervals post-heat stress. Furthermore, the immunosuppressive capacity of splenic Tregs on cluster of differentiation (CD)4+T cell expansion was revealed to be suppressed following heat stress. In addition, HS was demonstrated to downregulate the expression levels of surface inhibitory molecules (CD39, CD73 and cytotoxic T-lymphocyte associated protein 4), as well as anti-inflammatory cytokines [interleukin (IL)-10, transforming growth factor-β and IL-35], in Tregs. It was hypothesized that the aforementioned Treg responses may contribute to SIRS during HS. To the best of our knowledge, the present study is the first study to investigate the response of Tregs to HS, and the results demonstrated that there were significant alterations regarding to the total number, and function, of splenic Tregs, as well as the expression levels of inhibitory surface molecules and secretory cytokines. These results may highlight a novel mechanism underlying the pathogenesis of HS, as well as identify a potential therapeutic target for SIRS in patients suffering from HS.
Keywords: heat stroke, systemic inflammatory response syndrome, regulatory T cells
Introduction
Heat stroke (HS) is a life-threatening disease that is characterized by central nervous system dysfunction, hyperthermia and rapid progression to multiple organ failure (MOF). Despite important developments regarding rapid cooling and multi-organ function support, a large proportion of patients experience permanent neurological impairment or succumb to mortality post-HS (1). A previous study revealed that marked levels of cytokine release following extreme hyperthermia may lead to systemic inflammatory response syndrome (SIRS) (2), and both the absolute number and percentage of T helper cells are significantly decreased during HS (3). Regulatory T cells (Tregs) are a specialized lineage of suppressive cluster of differentiation (CD)4 T cells. The most important characteristic of natural and induced Tregs is the expression of forkhead/winged-helix transcription factor (Foxp)3 that functions as an important negative regulator of inflammation in numerous biological contexts, including sepsis (4), which may have a similar mechanism to HS. A previous study demonstrated that natural Tregs may attenuate collateral tissue damage induced by vigorous antimicrobial immune responses during sepsis (5), which suggests that Tregs may have a potential role in the regulation of rapidly progressing MOF induced by SIRS during early HS. However, to the best of our knowledge, the response of Tregs to HS has not yet been reported.
To investigate the response of Tregs to HS, the aim of the present study was to determine the total number and function of splenic Tregs during the early stage of HS. Considering that Tregs suppress the activation of adaptive and innate immune cells via association with contact-dependent or soluble mediators, the expression levels of surface molecules, such as cytotoxic T-lymphocyte associated protein 4 (CTLA4) and CD39/CD73, and anti-inflammatory cytokines, such as interleukin (IL)-10, transforming growth factor (TGF)-β and IL-35, were determined to investigate the mechanism underlying the association between Tregs and HS.
Materials and methods
Animals
C57/BL6 mice (age, 8–12 weeks; weight, 20–25 g; n=20) were purchased from the Animal Center of the Chinese PLA General Hospital (Beijing, China). Mice were housed in cages at 23°C with 55% humidity and 12-h light/dark cycles. Mice had free access to standard food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee of Chinese PLA General Hospital and Chinese PLA Military Medical College.
Experimental setup
Mice were divided into an HS group (n=10) and a negative control (NC) group (n=10). HS modeling was performed according to previously published protocol (6). Briefly, mice were exposed to an incubator at a temperature of 43±0.2°C and a humidity of 60±5% in the absence of food and water, and rectal temperatures of the mice were determined every 10 min until a maximum temperature of 42.7°C was reached. Following this, mice were removed from the incubator and provided with food and water ad libitum during undisturbed recovery at 25±0.5°C for 6 h. The NC group was exposed to an incubator with a temperature of 25±0.5°C in the absence of food and water, and subsequently provided with food and water at the same time as the HS group.
Cell preparation
Single-cell harvest and preparation using spleen tissues was performed as described previously (7). Blood was flushed out using ice-cold PBS prior to the spleen being harvested. Following this, spleen tissue was removed, incubated in PBS with 1% bovine serum albumin (BSA; cat. no. 16010159; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 4°C for 10 min, and then lysed with red blood cell lysis buffer (TBD Science, Tianjin, China) at room temperature for 1–2 min, according to the manufacturer's protocol.
Flow cytometry
Non-specific binding was blocked using 5% BSA for 1 h at room temperature. Surface staining was performed using antibodies against fluorescein isothiocyanate (FITC)-CD4 (1:10; cat. no. 553729; BD Biosciences, Franklin Lakes, NJ, USA), allophycocyanin (APC)-CD25 (1:10; cat. no. 557192; BD Biosciences), phycoerythrin (PE)-CTLA-4 (1:10; cat. no. 561718; BD Biosciences), PE-CD39 (1:80; cat. no. 12-0391-80; eBioscience; Thermo Fisher Scientific, Inc.) and PE-CD73 (1:10; cat. no. 550741; BD Biosciences) at room temperature for 30 min in accordance with the manufacturers' instructions. Intracellular staining of Foxp3 using APC-Foxp3 (1:20. cat. no. 17-5773-80; eBioscience; Thermo Fisher Scientific, Inc.) at room temperature for 45 min was also performed according to the manufacturer's instructions. Following three washes with PBS, cells were observed using BD FACS Calibur flow cytometry (BD Biosciences) and analyzed using FlowJo 7.6 software (Tree Star, Inc., Ashland, OR, USA).
In vitro inhibition analysis
Splenic cells in NC and HS groups were harvested at 24 h post-heat stress. FITC-CD4 and APC-CD25 monoclonal fluorescent antibodies were added to splenic cells prior to sorting using BD FACSCalibur. Sorted cells had a purity of >95%. For Treg functional analysis, purified Tregs were cultured at 37°C with CD4+T cells (5×104) at ratios of 0:1, 1:1, 2:1 and 4:1 for 44 h in 96-well plates pre-coated with anti-CD3 antibodies (5 µg/ml; cat. no. 555273; BD Pharmingen; BD Biosciences). Soluble anti-CD28 antibodies (5 µg/ml; cat. no. 553295; BD Pharmingen; BD Biosciences) were added, and cells were subsequently incubated at 37°C for 44 h prior to proliferation assessment using bromodeoxyuridine (BrdU) staining via flow cytometry. Cells were then incubated at 37°C with 10 mM BrdU for a further 4 h prior to fixing with 4% paraformaldehyde at room temperature for 10 min. Following rinsing with PBS, cells were treated at room temperature with 2 M HCl for 30 min. Non-specific binding was blocked using 5% BSA for 1 h at room temperature. Cells were then separately incubated with mouse anti-BrdU (1:200; cat. no. MAB3222; EMD Millipore, Billerica, MA, USA) overnight at 4°C. Following three washes with PBS, cells were incubated with Cy5-conjugated goat anti-mouse IgG H&L pre-adsorbed secondary antibodies (1:500; cat. no. ab6563; Abcam, Cambridge, UK) for 1 h at room temperature. Following three washes with PBS, cells were observed using BD FACS Calibur flow cytometry (BD Biosciences) and analyzed using FlowJo 7.6 software (Tree Star, Inc., Ashland).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (2 µg) was used for cDNA synthesis, which was performed using a RevertAid First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc.). RNA was incubated with primers at 42°C for 60 min, followed by enzyme inactivation for 10 min at 95°C and storage of the samples on ice. The IQ5 detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and SYBR Green Real time PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) were used for qPCR. The thermocycling conditions used for qPCR were as follows: Denaturation for 5 min at 95°C; followed by 40 cycles of denaturation for 30 sec at 90°C, annealing for 40 sec at 60°C and extension for 40 sec at 72°C. Fold changes were calculated via relative quantification using the 2−ΔΔCq method (8). Each experiment was performed in triplicate. qPCR primers used are listed in Table I.
Table I.
List of nucleotides used for cDNA amplification.
| Gene | Direction | Sequence | Length (bp) |
|---|---|---|---|
| IL-10 | Forward | 5′-GCCAGAGCCACATGCTCCTA-3′ | 144 |
| Reverse | 5′-GATAAGGCTTGGCAACCCAAGTA-3′ | ||
| IL-35 | Forward | 5′-CTGTGCCTTGGTAGCATCTATG-3′ | 166 |
| Reverse | 5′-GCAGAGTCTCGCCATTATGA-3′ | ||
| TGF-β | Forward | 5′-AACAATTCCTGGCGTTACCTT-3′ | 119 |
| Reverse | 5′-TGTATTCCGTCTCCTTGGTTC-3′ | ||
| β-actin | Forward | 5′-TGTTACCAACTGGGACGACA-3′ | 139 |
| Reverse | 5′-CTGGGTCATCTTTTCACGGT-3′ |
IL, interleukin; TGF-β, transforming growth factor-β.
Statistical analysis
Data analysis was performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Comparisons of multiple groups of parametric data were performed using one-way analysis of variance followed by the Student-Newman-Keuls post-hoc test. Student's t-tests were used for comparisons between two groups. *P<0.05 was considered to indicate a statistically significant difference. Graphs were produced using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Numbers of splenic Tregs are significantly decreased following HS
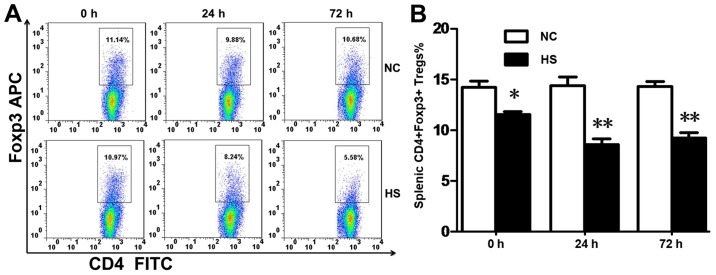
To observe the morphology of splenic Tregs, Foxp3 staining via FACs was performed. The results demonstrated that the percentage of CD4+Foxp3+cells in splenic tissues (Fig. 1A and B) was decreased at 0, 24 and 72 h time intervals following heat stress compared with the NC group.
Figure 1.
Total numbers of splenic Tregs are significantly decreased during heat stroke. (A) Splenic Tregs are defined as CD4+Foxp3+ splenocytes in FACs. (B) The percentage of Tregs decreased in the HS group at 0, 24 and 72 h time intervals post-heat stress compared with the NC group. Data are expressed as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. NC. NC, negative control; HS, heatstroke; CD, cluster of differentiation, Foxp3, forkhead/winged-helix transcription factor; Tregs, T regulatory cells; APC, allophycocyanin; FITC, fluorescein isothiocyanate.
Immunosuppressive capacity of splenic Tregs is suppressed following HS
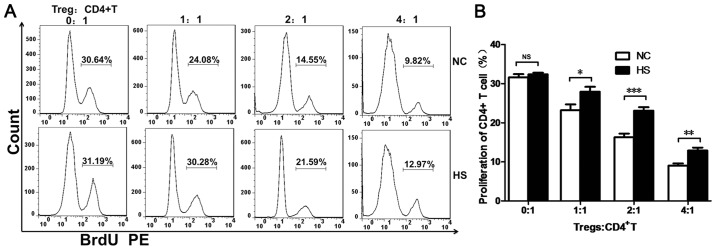
To investigate the immunosuppressive capacity of Tregs in response to heat stress, splenic Tregs (CD4+CD25+) and CD4+T cells were purified at 24 h post-heat stress via flow cytometry. BrdU staining demonstrated that the immunosuppressive capacity of splenic Tregs in the HS group was decreased at every ratio compared with the NC group (Fig. 2A and B).
Figure 2.
Heat stroke impairs the immunosuppressive capacity of splenic Tregs. (A) Splenocytes were harvested at 24 h post-heat stress, and splenic Tregs and CD4+T cells were sorted via FACS analysis. The proliferation rate of CD4+T cells was defined by BrdU+CD4+T cells. (B) The proliferation rate of CD4+T cells was increased in the HS group compared with NC group at different ratios. Data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001. NS, not significant; NC, negative control; HS, heat stroke; CD, cluster of differentiation; Tregs, T regulatory cells; BrdU, bromodeoxyuridine; PE, phycoerythrin.
HS downregulates CTLA4 and CD39/CD73 expression levels in splenic Tregs
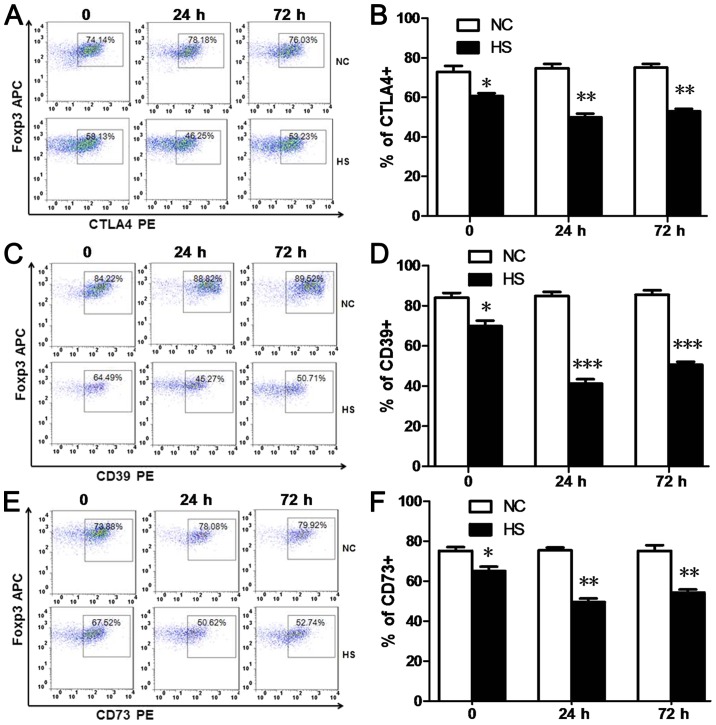
Constitutive expression of CTLA-4 in Tregs is important for the immunosuppressive function of Tregs. CD39 and CD73 have been revealed to be highly expressed on the surface of Tregs and are associated with the generation of extracellular adenosine, which may also have an important role in the suppressive function of Tregs (9,10). In the present study, the numbers of CTLA4+Tregs (Fig. 3A and B), CD39+Tregs (Fig. 3C and D) and CD73+Tregs (Fig. 3E and F) detected via FACs analyses were significantly decreased at 0, 24 and 72 h time intervals post-heat stress, compared with the NC group.
Figure 3.
Heat stroke downregulates the expression levels of CTLA4 and CD39/CD73 in splenic Tregs. (A and B) Splenic CTLA4+, (C and D) CD39+ and (E and F) CD73+Tregs were defined as CD4+Foxp3+ CTLA4+, CD4+Foxp3+ CD39+ and CD4+Foxp3+ CD73+splenocytes in FACS. Percentages of CTLA4+, CD39+ and CD73+Tregs were decreased in the HS group at 0, 24 and 72 h time intervals post-heat stress compared with the NC group. Data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs. NC. NC, negative control; HS, heat stroke; Foxp3, forkhead/winged-helix transcription factor; CD, cluster of differentiation; Tregs, T regulatory cells; CTLA4, cytotoxic T-lymphocyte associated protein 4; APC, allophycocyanin; PE, phycoerythrin.
HS decreases the secretion of inhibitory cytokines by splenic Tregs
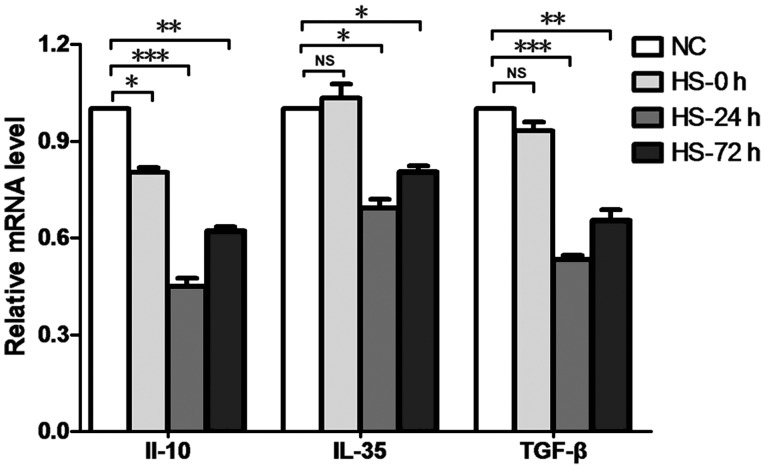
Anti-inflammatory cytokines, such as IL-10, TGF-β and IL-35, have been suggested to be associated with the physiological functioning of Tregs, and have been established to be associated with Treg-mediated immunosuppression (11). In the present study, RT-qPCR was performed to determine the expression levels of such cytokines, and results revealed that the expression of IL-10 in splenic Tregs in the HS group was decreased at 0, 24 and 72 h time intervals, and the secretion levels of TGF-β and IL-35 in splenic Tregs in the HS group were downregulated at 24 and 72 h time intervals compared with the NC group (Fig. 4).
Figure 4.
Heat stroke decreases the secretion of inhibitory cytokines in splenic Tregs. Splenocytes were harvested at 24 h post-heat stress, and splenic Tregs were sorted by FACS analysis. Reverse transcription-quantitative polymerase chain reaction analyses demonstrated that IL-10 production was decreased at 0, 24 and 72 h time intervals following heat stress, and IL-35 and TGF-β secretion levels were decreased at 24 and 72 h following heat stress, compared with the NC group. Data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 and ***P<0.001. NS, not significant; NC, negative control; HS, heat stroke; IL, interleukin; TGF-β, transforming growth factor-β; Tregs, T regulatory cells.
Discussion
To the best of our knowledge, the present study is the first to demonstrate that exposure to HS may deplete numbers of Tregs. Furthermore, HS was demonstrated to decrease the expression levels of functional surface molecules (CTLA4, CD39 and CD73) as well as the production of inhibitory cytokines (IL-35, IL-10 and TGF-β), which may contribute to the inhibitory effect of Tregs on CD4+ T cell proliferation. The alterations of splenic frequency and function may contribute to enhanced levels of SIRS, and thus may represent a potential therapeutic target for the treatment of HS.
Tregs are specialized immune cells that have an important role in maintaining immune self-tolerance and preventing autoimmune disease (4,5). Tregs are characterized by Foxp3 expression in cell nuclei (11). During infection, Tregs may attenuate inflammation and collateral tissue damage; however, this may also suppress the capability of bacterial clearance (5). Recent studies demonstrated that high numbers of circulating CD39+ Tregs predict poor survival for patients with sepsis (12) and levels of Foxp3+Tregs increased in all CD4+T cells during murine sepsis (13). However, depletion of Tregs has been previously revealed, either via use of anti-CD25 antibodies (14) or a DEREG mouse model (13), to contribute towards a negative clinical outcome in early phase sepsis. In addition, a number of drugs may improve survival in mice with septic shock, partially via facilitating the proliferation of IL-10+Tregs in septic mice (15). The aforementioned studies suggested that Tregs may attenuate collateral tissue damage induced by vigorous antimicrobial immune responses during sepsis. Considering that SIRS associated with HS share a similar mechanism with sepsis, it was hypothesized that Tregs may also be associated with HS-induced multiple organ dysfunction, which was subsequently investigated in the present study.
In the present study, the numbers of splenic Tregs were decreased at 0, 24 and 72 h time intervals post-heat stress. To further investigate the Treg response in HS, splenic Tregs were harvested at 24 h post-heat stress to determine their immunosuppressive capacity on CD4+T cells via BrdU staining. In vitro inhibition analysis demonstrated that the immunosuppressive capacity of splenic Tregs in the HS group was decreased at every ratio compared with the NC group. Therefore, decreased levels of splenic Tregs, as well as suppressed immunosuppressive capacity of splenic Tregs, may markedly affect the SIRS during the early phase of HS.
Numerous surface markers have been demonstrated to have an important role in the immunosuppressive capacity of Tregs. CTLA4 (CD152) is a well-characterized negative regulator expressed on T cells (16). Tregs exhibit enhanced levels of CTLA4 expression (11) and may require CTLA4 to suppress immune responses via regulation of the potency of antigen-presenting cells to activate other T cells (17), as well as the inhibition of IL-2 expression, which represents an important cytokine for T-cell expansion (16). A previous study revealed that peripheral blood samples obtained from patients with acute liver failure exhibited higher numbers of CD4+CTLA4+T cells, and patients with infections exhibited the greatest overall numbers of CD4+CTLA4+T cells (16). Thus, in severe sepsis, suppression of CTLA4 may improve survival in patients (18). In the present study, the number of CTLA4+Tregs decreased following heat stress, thus suggesting potential abatement of immunosuppressive capacity of Tregs on CD4+T cell proliferation. CD39 and CD73 are important components of cell surface enzymes associated with the purinergic system, and may exert anti-inflammation effects by decreasing the ATP/ADP ratio and increasing adenosine availability (9). Therefore, high levels of circulating CD39+Tregs may be used to predict poor survival for patients with sepsis (13). However, emerging data have demonstrated that expression levels of CD39 (9) and CD73 (10) improve the survival of patients with microbial sepsis by attenuating systemic inflammation (9) and liver dysfunction (19). In the present study, levels of both CD39+and CD73+Tregs were decreased in the HS group, which may be associated with suppressed immunosuppressive capacities exhibited by Tregs against CD4+T cell proliferation. Based on the present study, adenosine signaling may represent a potential therapeutic strategy for patients with HS-induced SIRS and sequential organ dysfunction.
Tregs may also exhibit immunoregulatory effects via the release of inhibitory cytokines, such as IL-10, TGF-β and IL-35 (20). A recent study established a novel and effective method for the generation of human porcine-specific Tregs exhibiting high expression levels of IL-10, TGF-β1 and IL-35; which suggests that Tregs have an important role in immunomodulation (21). IL-10-producing Tregs constitute a Treg cell subset characterized by the production of enhanced levels of IL-10, cytokine-mediated immunosuppressive capabilities and selective migration to peripheral tissues in order to suppress local immune responses (20,22). Decreased production of IL-10 in Tregs may lead to the exacerbation of tissue injury in numerous inflammation (23,24) and autoimmune-associated diseases (25). IL-35 exhibits strong immunosuppressive properties and is predominantly secreted by Tregs (20). IL-35-producing Tregs, which predominantly produce IL-35, preferentially localize to the T cell zone of secondary lymphoid organs and have a role in the suppression of anti-tumor responses (20). Decreased production of IL-35 in Tregs may attenuate autoimmune and inflammatory responses, such as ulcerative colitis (26) and type-1 diabetes (27). TGF-β has an important role in the suppression of immune responses, and Tregs are its predominant source (27). TGF-β originating from Tregs regulates immunomodulation and cell apoptosis. Firstly, Tregs may utilize TGF-β to block cell activation and differentiation, which subsequently suppresses the immune response. Secondly, TGF-β may convert naïve T cells into induced-Tregs (28). Lastly, TGF-β produced by Tregs may protect Tregs against apoptosis and destabilization by inducing surrounding inflammation and providing constant stimulation (28). Thus, peripheral Tregs and serum TGF-β reduction may induce type 1 diabetes mellitus (29), and inhibition of TGF-β1 expression may represent a novel strategy for the improvement of host immunosuppression therapy following sepsis (30). In the present study, the expression levels of IL-35, IL-10 and TGF-β in splenic Tregs were significantly decreased compared with the NC group at 24 h post-heat stress, which may also be attributed to the suppressed immunosuppressive capacity of splenic Tregs. Furthermore, the downregulation of TGF-β exhibited by splenic Tregs may have also contributed to the reduced numbers of splenic Tregs via promotion of Treg apoptosis during HS, which will be investigated in our future studies.
In conclusion, to the best of our knowledge, this is the first study to investigate the response of Tregs to HS, as well as its potential underlying mechanisms, to determine a novel therapeutic target of HS. The results of the present study revealed that the total numbers and the immunosuppressive capacity of splenic Tregs were suppressed during HS. Furthermore, it was demonstrated that downregulation of the expression levels of surface molecules (CTLA4, CD39 and CD73), as well as secretory anti-inflammatory cytokines (IL10, TGF-β and IL-35), may have also contributed to the aforementioned effects, and thus may serve as potential therapeutic targets for the treatment of patients suffering from HS.
Acknowledgements
Not applicable.
Funding
The present study was supported by two grants from the National Natural Science Foundation of China (grant nos. 81501642 and 81671966).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
JH conceived and designed the study. CL, PH and M-MY performed the experiments and analyzed the data. H-JK and F-HZ obtained the reagents, materials and analysis tools. JH wrote the manuscript. All authors read and approved the final study.
Ethics approval and consent to participate
All procedures were approved by the Institutional Animal Care and Use Committee of Chinese PLA General Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Leon LR, Helwig BG. Heat stroke: Role of the systemic inflammatory response. J Appl Physiol (1985) 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- 2.Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5:611–647. doi: 10.1002/cphy.c140017. [DOI] [PubMed] [Google Scholar]
- 3.Hammami MM, Bouchama A, Shail E, Aboul-Enein HY, Al-Sedairy S. Lymphocyte subsets and adhesion molecules expression in heatstroke and heat stress. J Appl Physiol (1985) 1998;84:1615–1621. doi: 10.1152/jappl.1998.84.5.1615. [DOI] [PubMed] [Google Scholar]
- 4.van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS, Rudensky AY. Memory of inflammation in regulatory T cells. Cell. 2016;166:977–990. doi: 10.1016/j.cell.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 6.Leon LR, DuBose DA, Mason CW. Heat stress induces a biphasic thermoregulatory response in mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R197–R204. doi: 10.1152/ajpregu.00046.2004. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q, et al. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84:521–531. doi: 10.1038/ki.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Csóka B, Németh ZH, Törő G, Koscsó B, Kókai E, Robson SC, Enjyoji K, Rolandelli RH, Erdélyi K, Pacher P, Haskó G. CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J. 2015;29:25–36. doi: 10.1096/fj.14-253567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haskó G, Csóka B, Koscsó B, Chandra R, Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virág L, Gergely P, et al. Ecto-5′-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187:4256–4267. doi: 10.4049/jimmunol.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: Markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–2276. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Xu R, Lin F, Bao C, Wang S, Ji C, Li K, Jin L, Mu J, Wang Y, et al. High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int J Infect Dis. 2015;30:57–63. doi: 10.1016/j.ijid.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Tatura R, Zeschnigk M, Hansen W, Steinmann J, Vidigal PG, Hutzler M, Pastille E, Westendorf AM, Buer J, Kehrmann J. Relevance of Foxp3(+) regulatory T cells for early and late phases of murine sepsis. Immunology. 2015;146:144–156. doi: 10.1111/imm.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo R, Wang L, Wang X, Zhao Y, Wang Y, Zhao X, Chang L, Liu SL, Tong D, Zhang H, Huang Y. Removal of regulatory T cells prevents secondary chronic infection but increases the mortality of subsequent sub-acute infection in sepsis mice. Oncotarget. 2016;7:10962–10975. doi: 10.18632/oncotarget.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Feng Y, Shen X, Pan G, Fan G, Gao X, Han J, Zhu Y. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol. 2018;211:358–365. doi: 10.1016/j.jep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Khamri W, Abeles RD, Hou TZ, Anderson AE, El-Masry A, Triantafyllou E, Bernsmeier C, Larsen FS, Singanayagam A, Kudo N, et al. Increased expression of cytotoxic T-lymphocyte-associated protein 4 by T cells, induced by B7 in Sera, reduces adaptive immunity in patients with acute liver failure. Gastroenterology. 2017;153:263–276.e8. doi: 10.1053/j.gastro.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 18.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savio LEB, de Andrade Mello P, Figliuolo VR, de Avelar Almeida TF, Santana PT, Oliveira SDS, Silva CLM, Feldbrügge L, Csizmadia E, Minshall RD, et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol. 2017;67:716–726. doi: 10.1016/j.jhep.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J, Zhou X. Reciprocal expression of IL-35 and IL-10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep. 2017;21:1853–1869. doi: 10.1016/j.celrep.2017.10.090. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Eckl J, Geiger C, Schendel DJ, Pohla H. A novel and effective method to generate human porcine-specific regulatory T cells with high expression of IL-10, TGF-β1 and IL-35. Sci Rep. 2017;7:3974. doi: 10.1038/s41598-017-04322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujio K, Yamamoto K, Okamura T. Overview of LAG-3-expressing, IL-10-producing regulatory T Cells. Curr Top Microbiol Immunol. 2017;410:29–45. doi: 10.1007/82_2017_59. [DOI] [PubMed] [Google Scholar]
- 23.Toyama M, Kudo D, Aoyagi T, Miyasaka T, Ishii K, Kanno E, Kaku M, Kushimoto S, Kawakami K. Attenuated accumulation of T cells and reduced production of interleukin 10 lead to the exacerbation of tissue injury in a mouse model of acute respiratory distress syndrome. Microbiol Immunol. 2018;62:111–123. doi: 10.1111/1348-0421.12564. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Liu LX, Wu FL, Zhang X, Li YY, Shi T, Li DZ, Han TT. The changes of Th17/Treg and related cytokines: IL-17, IL-23, IL-10, and TGF-β in respiratory syncytial virus bronchiolitis rat model. Iran J Allergy Asthma Immunol. 2017;16:386–395. [PubMed] [Google Scholar]
- 25.Li F, Ji L, Wang W, Hua F, Zhan Y, Zou S, Yuan L, Ke Y, Min Z, Song D, et al. Insufficient secretion of IL-10 by Tregs compromised its control on over-activated CD4+ T effector cells in newly diagnosed adult immune thrombocytopenia patients. Immunol Res. 2015;61:269–280. doi: 10.1007/s12026-014-8620-2. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadnia-Afrouzi M, Hosseini AZ, Khalili A, Abediankenari S, Amari A, Aghili B, Nataj HH. Altered microRNA Expression and immunosuppressive cytokine production by regulatory T cells of ulcerative colitis patients. Immunol Invest. 2016;45:63–74. doi: 10.3109/08820139.2015.1103749. [DOI] [PubMed] [Google Scholar]
- 27.Singh K, Kadesjö E, Lindroos J, Hjort M, Lundberg M, Espes D, Carlsson PO, Sandler S, Thorvaldson L. Interleukin-35 administration counteracts established murine type 1 diabetes-possible involvement of regulatory T cells. Sci Rep. 2015;5:12633. doi: 10.1038/srep12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran DQ. TGF-β: The sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 29.Qiao YC, Shen J, Hong XZ, Liang L, Bo CS, Sui Y, Zhao HL. Changes of regulatory T cells, transforming growth factor-beta and interleukin-10 in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. Clin Immunol. 2016;170:61–69. doi: 10.1016/j.clim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Luan YY, Yin CF, Qin QH, Dong N, Zhu XM, Sheng ZY, Zhang QH, Yao YM. Effect of regulatory T cells on promoting apoptosis of T lymphocyte and its regulatory mechanism in sepsis. J Interferon Cytokine Res. 2015;35:969–980. doi: 10.1089/jir.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.