Abstract
Reported relationships among Helicobacter pylori infection, white blood cell (WBC) count and nonalcoholic fatty liver disease (NAFLD) are inconsistent and controversial. We, therefore, conducted a cross-sectional study to investigate the associations among the presence of NAFLD, WBC count and H pylori infection, as diagnosed using the 13C-urea breath test (UBT).
This study included 20,389 subjects enrolled at the International Health Care Center of the Second Affiliated Hospital of the Zhejiang University School of Medicine from January 2015 to December 2015. All participants underwent a 13C-UBT for the diagnosis of H pylori infection and ultrasonography for NAFLD as well as a blood test to determine WBC count. Multivariate logistic regression was then performed to evaluate the relationship among H pylori infection, WBC count and NAFLD.
H pylori infection was detected in 38.49% (7,848/20,389) of the subjects via the UBT, and NAFLD was present in 37.24% (7,592/20,389) of the subjects. The prevalence of H pylori infection was higher in the NAFLD group than in the control group (41.25% vs 36.85%, P <.001). Significant differences were found between various WBC quartiles and H pylori infection, age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), high-sensitivity C-reactive protein (HS-CRP), glycosylated hemoglobin (HbA1c), triglyceride (TG), low-density lipoprotein (LDL-C), fasting blood glucose (FPG), homeostasis model assessment of insulin resistance (HOMA-IR), and smoking. Multivariate logistic regression revealed that the combination of H pylori infection and WBC count (odds ratio [OR] = 1.067, 95% confidence interval [CI]: 1.014, 1.093; P = .007; OR = 1.165, 95% CI: 1.023, 1.488; P <.001; OR = 1.183, 95% CI: 1.085, 1.559; P <.001, respectively) was positively associated with NAFLD.
H pylori infection and WBC count may contribute to the pathogenesis of NAFLD.
Keywords: 13C-urea breath test, cross-sectional study, Helicobacter pylori, nonalcoholic fatty liver disease, white blood cell count
1. Introduction
Helicobacter pylori, a gram-negative bacterium, infects more than 50% of humans[1] and causes many gastrointestinal diseases, including peptic ulcers, chronic gastritis, gastric mucosa-associated lymphoid tissue lymphoma, and even gastric cancer.[2,3] Interestingly, H pylori infection is also associated with many diseases outside the stomach.[4] The Kyoto Global Consensus Meeting proposed that H pylori gastritis should be defined as an infectious disease.[5] Studies have suggested that H pylori infection increases systemic and vascular inflammation by producing inflammatory factors and regulating gastrointestinal hormone secretion, which results in the development of insulin resistance (IR) and metabolic syndrome (MetS).[6–8] Therefore, H pylori may be a risk factor for diabetes, cardiovascular disease, MetS and nonalcoholic fatty liver disease (NAFLD).[9–16]
NAFLD is a clinical and pathological syndrome that is characterized by excessive deposition of fat in liver cells, excluding that caused by alcoholic liver damage or other specific factors and is closely related to IR and genetic susceptibility to metabolic stress-induced liver damage.[17,18] Due to the prevalence of obesity and its associated MetS, NAFLD has become an important cause of chronic liver disease in developed countries and some wealthy regions in developing countries, where it affects 20% to 45% of the general population and 60% to 75% of obese individuals.[19]
The white blood cell (WBC) count is a stable, readily available and inexpensive marker of inflammation, and has become an important predictor of infectious diseases and of cardiovascular disease, diabetes, and NAFLD.[20–22]
Relationships between H pylori infection and NAFLD have been reported in several studies,[11–16] though other investigations have found conflicting results.[23,24] In most of these prior studies, H pylori infection was diagnosed based on the presence of H pylori IgG antibody in the serum[11,13,14,16,23]; however, serum IgG can persist after H pylori is eradicated, and therefore, the presence of H pylori IgG antibody may not reflect the current infection status.[25] Regardless, it is thought that only current infection status can lead to a systemic inflammatory response. The 13C-UBT is a noninvasive method for the convenient detection of current H pylori infection. The sensitivity and specificity of the UBT are approximately 0.96 (95% confidence interval [CI]: 0.95–0.97) and 0.93 (95% CI: 0.91–0.94), respectively.[26] To our knowledge, few studies have investigated the association among NAFLD, WBC count and H pylori infection by utilizing the UBT as a diagnostic procedure.
We conducted a cross-sectional study in a large Chinese population to investigate the associations among the presence of NAFLD, WBC count and H pylori infection, as diagnosed by the 13C-UBT.
2. Materials and methods
2.1. Study participants
Participants who voluntarily underwent a general health screening from January to December 2015 were recruited from the International Health Care Center of the Second Affiliated Hospital of Zhejiang University School of Medicine. Participants with any of the following characteristics were excluded from the study:
-
1)
the alcoholic consumption of 3 or more drink units per week;
-
2)
chronic liver disease;
-
3)
a history of gastric surgery;
-
4)
the use of bismuth, antibiotics, proton pump inhibitors or H2 blockers within the prior 4 weeks;
-
5)
severe infection;
-
6)
a significant mental or neurological disorder;
-
7)
a history of cancer; and
-
8)
patients on steatogenic medications such as methotrexate and corticosteroids, among others.
All subjects underwent a detailed physical examination, including 13C-UBT detection of H pylori infection. The data used in this study was reviewed and approved by the Ethics Committee of the 2nd Affiliated Hospital, School of Medicine, Zhejiang University (2014–325). All participants provided informed consent before the examination.
2.2. Questionnaires
The medical history of each participant was obtained from a questionnaire and included the history of the present illness, previous diagnoses of H pylori infection, history of anti-H pylori therapy, history of gastric surgery, history of significant mental or neurological disorders, history of cancer(s), use of bismuth, antibiotics, proton pump inhibitors or H2 blockers within the previous 4 weeks, alcohol intake, and cigarette smoking.
2.3. Data collection
Blood pressure measurements were obtained after at least 10 minutes of rest. The body mass index (BMI) was defined as weight divided by height squared (kg/m2). Fasting plasma WBC, high-sensitivity C-reactive protein (HS-CRP), fasting blood glucose (FPG), fasting insulin (FINS), glycosylated hemoglobin (HbA1c), alanine aminotransferase (ALT), γ-glutamyltranspeptidase (γ-GT), aspartate aminotransferase (AST), total cholesterol (TC), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), and triglyceride (TG) levels were measured after an 8-hour overnight fast (Beckman Coulter AU 5400). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the following formula: HOMA-IR=[FINS (μIU/mL)×FPG (mmol/L)]/22.5.[27]
2.4. Diagnosis of H pylori infection
After fasting for at least 2 hours, all participants underwent a 13C-UBT at our health care center. After a baseline breath sample had been collected, participants ingested a 13C-urea reagent that was dissolved in water. The second breath sample was collected 30 minutes later and analyzed. A delta over baseline (DOB) value ≥4.0 indicated a positive result for H pylori infection.
2.5. Definition of NAFLD
NAFLD was defined according to the guidelines published in 2012 by the American Association for the Study of Liver Diseases (AASLD), the American College of Gastroenterology (ACG), and the American Gastroenterological Association (AGA).[28] In this study, the diagnosis of NAFLD required the following:
-
(1)
hepatic steatosis detected by ultrasonography;
-
(2)
no significant alcohol consumption (to strictly exclude the influence of alcohol, we chose individuals with alcohol consumption of less than 3 drink units per week); and
-
(3)
no co-existing causes of chronic liver disease, such as hepatitis C, medications, parenteral nutrition, Wilson's disease or severe malnutrition.
2.6. Statistical analysis
The basic information and laboratory test results of the 2 groups (NAFLD and control group) were described and compared. Normally distributed data are described as the mean ± the standard deviation, and a t-test was used to compare groups. Data with a skewed distribution are described by the median (interquartile range), and groups were compared with Wilcoxon's rank-sum test. Qualitative data are described by frequency (percentage), and the chi-square test was used to compare groups. Additionally, WBC count (10∗9/L) was further categorized into separate quartiles: Q1: WBC <5.30, Q2: 5.30 ≤WBC <6.00, Q3:6.00 ≤WBC <7.00, and Q4: WBC ≥7.00. The F test or Kruskal–Wallis test was used to compare the data among the different quartiles of WBC count.
Furthermore, we used a stepwise forward fitting multivariate logistic regression model to build the prediction models of NAFLD. The following were considered covariates in the logistic regression analysis: age, gender, smoking, H pylori infection, WBC, HS-CRP, HbA1c, FPG, HOMA-IR, TG, LDL-C, systolic blood pressure (SBP), and diastolic blood pressure (DBP). The inclusion criterion for stepwise regression was a P value ≤.050, and the exclusion criterion was a P value >.050. All P values were determined using a bilateral hypothesis test. The level of significance was set at 5%, and the homogeneity of variance test level was set at 10%. The 95% CI was then calculated. The statistical analysis was performed using STATA 14.0 software (StataCorp, College Station, TX).
3. Results
3.1. Clinical and demographic characteristics
Overall, 20,389 Chinese individuals (11,969 males and 8420 females) were enrolled (Fig. 1, flow chart). The average age was 50.20 ± 12.13 years in the NAFLD group and 46.45 ± 13.60 years in the control group. The demographic data are listed in Table 1. The 13C-UBT for H pylori infection was positive in 38.49% (7848/20,389) of subjects. NAFLD was present in 37.24% (7592/20,389) of subjects. The prevalence of NAFLD increased with age, and most subjects with NAFLD were male. The prevalence of H pylori infection was higher in the NAFLD group than in controls (41.25% vs 36.85%, P <.001).
Figure 1.
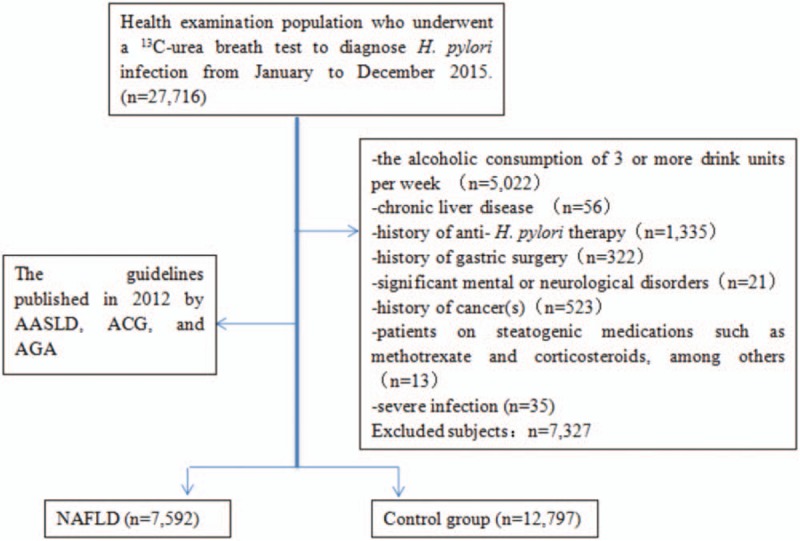
Flow diagram.
Table 1.
Characteristics of individuals with and without NAFLD.

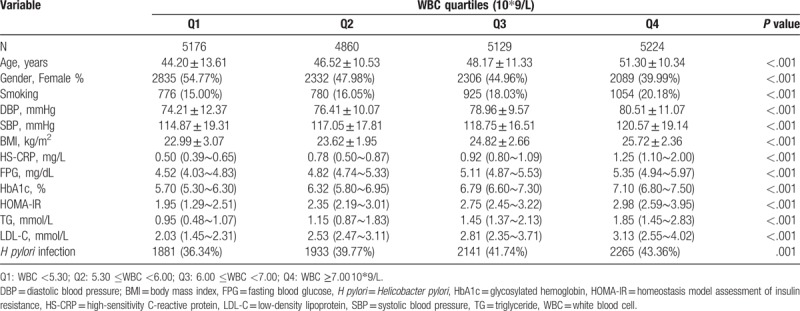
3.2. Characteristics of the subjects according to WBC quartile
The data were divided into 4 groups according to the quartiles of WBC count (Table 2). Significant differences were found between various WBC quartiles for age, gender, BMI, SBP, DBP, HS-CRP, HbA1c, TG, LDL-C, FPG, HOMA-IR, and smoking status (P <.001). A significant difference was also found between various WBC quartiles for H pylori infection (P = .001).
Table 2.
Characteristics according to WBC quartiles.
3.3. Multivariate logistic regression analysis of the NAFLD and control groups
We further built a multivariate logistic regression to predict NAFLD by considering the combination of H pylori infection status, WBC count and other metabolic parameters. The multivariate logistic regression revealed that when H pylori infection was negative, only the Q4 level of WBC count (odds ratio[OR] = 1.033, 95% CI: 1.025, 1.087; P = .002) was associated with NAFLD, but when H pylori infection was positive, the Q2, Q3, and Q4 levels of WBC count (OR = 1.067, 95% CI: 1.014, 1.093; P = .007; OR = 1.165, 95% CI: 1.023, 1.488; P <.001; OR = 1.183, 95% CI: 1.085, 1.559; P <.001, respectively) were positively associated with NAFLD (Table 3).
Table 3.
Multivariate logistic regression analysis of a combination of H pylori infection and WBC count and others as risk factors of NAFLD.

4. Discussion
The hypothesis of this study was that chronic H pylori infection induces higher inflammation as indicated by the WBC level and participates in the development of NAFLD.
Limited clinical data have been reported on the associations among H pylori infection, WBC count and NAFLD. Some studies found that H pylori infection was involved in the pathogenesis of IR,[9,10] which is important in the development of NAFLD, and certain pathogenic mechanisms have been proposed.[11,29,30] Pro-inflammatory factors such as TNF-α, interferon-γ, interleukin (IL)-1, IL-6, IL-8, IL-10, IL-12, and CRP are released during infection and were shown to be involved in the pathogenesis of IR. Moreover, 2 follow-up studies also demonstrated that higher WBC counts were associated with a greater risk for the development of incidental NAFLD.[21,22] These findings may explain the relationships among H pylori infection, WBC count and NAFLD.
Studies on the associations between H pylori infection and NAFLD are inconsistent and controversial.[11–16,23,24] Polyzos et al[11] demonstrated that patients with NAFLD had significantly higher anti-H pylori IgG, HOMA-IR, and tumor necrosis factor (TNF)-α levels than control patients. They further studied H pylori eradication, which showed a trend towards improvement in the NAFLD fibrosis score.[12] Takuma[13] and Doğan et al[14] also found that H pylori infection was an independent risk factor for the development of NAFLD. Furthermore, Zhou et al[15] demonstrated that H pylori infection induced hepatic IR via the c-Jun/miR-203/SOCS3 signaling pathway. A more recent study by Sumida et al[16] found that the prevalence of NASH was higher in H pylori-positive patients with NAFLD than in H pylori-negative patients. A histologic evaluation suggested an association of H pylori infection with hepatocyte ballooning but not with steatosis or liver fibrosis. However, some studies have shown that H pylori infection is not associated with NAFLD. Okushin et al[23] examined a total of 13,737 subjects in Japan, and a multivariable analysis revealed that H pylori infection was not associated with NAFLD. Baeg et al[24] examined 3663 individuals using the UBT for the diagnosis of H pylori infection, and a multivariable analysis showed that H pylori infection was not a risk factor for NAFLD, as indicated by the hepatic steatosis index (HSI) and NAFLD liver fat score (NAFLD-LFS).
We considered that the reason for the inconsistent results described above was the different screening methods of H pylori infection in various studies. Shin et al[31] found that MetS was more closely associated with histologic positivity for H pylori (adjusted OR = 1.26; 95% CI: 1.08–1.48) than with serologic positivity (adjusted OR = 1.12, 95% CI: 0.95–1.32). They suggested that serological positivity for H pylori does not necessarily indicate current infection and that the stronger association of MetS with histologic positivity than with serological positivity suggests that the effects of H pylori infection on the pathogenesis of cardiometabolic outcomes may be reversible.
In our study, we used a large sample of data (20,389 subjects), the UBT to diagnose H pylori infection and ultrasonography to diagnose NAFLD. Our study showed that WBC count alone was associated with NAFLD when it was at the higher level ≥7.00 (10∗9/L), but when combined with H pylori infection, other levels of WBC count (OR = 1.067, 95% CI: 1.014, 1.093; P =.007; OR = 1.165, 95% CI: 1.023, 1.488; P <.001; OR = 1.183, 95% CI: 1.085, 1.559; P <.001) were positively associated with NAFLD, which suggests that H pylori infection and WBC level may contribute to the pathogenesis of NAFLD.
This study had some limitations.
-
(1)
The subjects were recruited from the International Health Care Center and do not represent the general population. However, the majority of the population in our country participates in an examination each year, and our subjects represent a variety of occupational groups, such as civil servants, teachers, businessmen, bankers, medical personnel, factory workers, farmers, and housewives.
-
(2)
Ultrasound imaging was used to diagnose NAFLD in our study. Diagnosis by ultrasonography has the inevitable limitations of low sensitivity for mild steatosis and the inability to distinguish mild fibrosis from steatosis and to quantify fatty infiltration.[32] However, ultrasonography is still considered a first-line, noninvasive diagnostic tool for simple liver steatosis.
-
(3)
This is a cross-sectional study, and we can only draw conclusions about the association between H pylori infection and NAFLD.
5. Conclusions
Our results indicated that H pylori infection was more frequently observed in NAFLD patients than in controls and that H pylori infection with certain WBC levels may contribute to the pathogenesis of NAFLD. Future research should include prospective studies to show a cause-effect relationship between H pylori and NAFLD. Biochemical studies are also needed to better understand the pathophysiology behind the role of H pylori in NAFLD and should be included in future research. If confirmed, eradication H pylori infection may have particular therapeutic advantages for NAFLD treatment.
Acknowledgments
The authors thank Zhejiang Provincial Natural Science Foundation of China (LY18H090002), Zhejiang Provincial Education Department of China (Y201636053) and Zhejiang Provincial Medical Scientific and Technological Projects of China (2017KY387 and 2018KY413) for support. We thank Professor Qin-Dong Wu and Dr. Man-Li Huang for providing helpful comments regarding this paper. We also thank Jing-Kai Chen for his advice on statistics throughout the project.
Author contributions
Conceptualization: Jian-ting Cai.
Data curation: Zhen-ya Song.
Formal analysis: Yu-ling Tong.
Funding acquisition: Jing-hua Wang.
Investigation: Ying-ying Yu, Yu-ling Tong, Jing-hua Wang.
Methodology: Ying-ying Yu, Jing-hua Wang.
Project administration: Zhen-ya Song.
Resources: Ying-ying Yu, Zhen-ya Song.
Software: Yu-ling Tong.
Supervision: Zhen-ya Song.
Validation: Jian-ting Cai.
Writing – original draft: Ying-ying Yu.
Writing – review & editing: Jian-ting Cai.
Footnotes
Abbreviations: CI = confidence interval, BMI = body mass index, DBP = diastolic blood pressure, FINS = fasting insulin, FPG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HOMA-IR = homeostasis model assessment of insulin resistance, HS-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein, NAFLD = nonalcoholic fatty liver disease, OR = odds ratio, SBP = systolic blood pressure, TG = triglyceride, WBC = white blood cell.
All authors declare no conflict of interest.
References
- [1].Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- [2].Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- [4].Franceschi F, Zuccalà G, Roccarina D, et al. Clinical effects of Helicobacter pylori outside the stomach. Nat Rev Gastroenterol Hepatol 2014;11:234–42. [DOI] [PubMed] [Google Scholar]
- [5].Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Longo-Mbenza B, Nkondi Nsenga J, Vangu Ngoma D. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics. Int J Cardiol 2007;121:229–38. [DOI] [PubMed] [Google Scholar]
- [7].Oshima T, Ozono R, Yano Y, et al. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol 2005;45:1219–22. [DOI] [PubMed] [Google Scholar]
- [8].Pietroiusti A, Diomedi M, Silvestrini M, et al. Cytotoxin-associated gene-a–positive Helicobacter pylori strains are associated with atherosclerotic stroke. Circulation 2002;106:580–4. [DOI] [PubMed] [Google Scholar]
- [9].Polyzos SA, Kountouras J, Zavos C, et al. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter 2011;16:79–88. [DOI] [PubMed] [Google Scholar]
- [10].Chen TP, Hung HF, Chen MK, et al. Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: a cross-sectional study. Helicobacter 2015;20:184–91. [DOI] [PubMed] [Google Scholar]
- [11].Polyzos SA, Kountouras J, Papatheodorou A, et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 2013;62:121–6. [DOI] [PubMed] [Google Scholar]
- [12].Polyzos SA, Nikolopoulos P, Stogianni A, et al. Effect of Helicobacter pylori eradication on hepatic steatosis, NAFLD fibrosis score and HSENSI in patients with nonalcoholic steatohepatitis: a MR imaging-based pilot open-label study. Arq Gastroenterol 2014;51:261–8. [DOI] [PubMed] [Google Scholar]
- [13].Takuma Y. Helicobacter pylori infection and liver diseases. Gan To Kagaku Ryoho 2011;38:362–4. [PubMed] [Google Scholar]
- [14].Doğan Z, Filik L, Ergül B, et al. Association between Helicobacter pylori and liver-to-spleen ratio: a randomized-controlled single-blind study. Eur J Gastroenterol Hepatol 2013;25:107–10. [DOI] [PubMed] [Google Scholar]
- [15].Zhou X, Liu W, Gu M, et al. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol 2015;50:1027–40. [DOI] [PubMed] [Google Scholar]
- [16].Sumida Y, Kanemasa K, Imai S, et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J Gastroenterol 2015;50:996–1004. [DOI] [PubMed] [Google Scholar]
- [17].Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 2004;279:32345–53. [DOI] [PubMed] [Google Scholar]
- [18].Bhala N, Younes RI, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Curr Pharm Des 2013;19:5169–76. [DOI] [PubMed] [Google Scholar]
- [19].Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- [20].Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): a cohort study. Lancet 1999;353:1649–52. [DOI] [PubMed] [Google Scholar]
- [21].Chung GE, Yim JY, Kim D, et al. Associations between white blood cell count and the development of incidental nonalcoholic fatty liver disease. Gastroenterol Res Pract 2016;2016:7653689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang S, Zhang C, Zhang G, et al. Association between white blood cell count and non- alcoholic fatty liver disease in urban HanChinese: a prospective cohort study. BMJ Open 2016;6:e010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okushin K, Takahashi Y, Yamamichi N, et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: a large-scale cross-sectional study in Japan. BMC Gastroenterol 2015;15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baeg MK, Yoon SK, Ko SH, et al. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J Gastroenterol 2016;22:2592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miernyk KM, Bruden DL, Bruce MG, et al. Dynamics of Helicobacter pylori-specific immunoglobulin G for 2 years after successful eradication of helicobacter pylori infection in an American Indian and Alaska native population. Clin Vaccine Immunol 2007;14:85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol 2015;21:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592–609. [DOI] [PubMed] [Google Scholar]
- [29].Polyzos SA, Kountouras J. Novel advances in the association between Helicobacter pylori infection, metabolic syndrome, and related morbidity. Helicobacter 2015;20:405–9. [DOI] [PubMed] [Google Scholar]
- [30].Franceschi F, Annalisa T, Teresa DR, et al. Role of Helicobacter pylori infection on nutrition and metabolism. World J Gastroenterol 2014;20:12809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shin DW, Kwon HT, Kang JM, et al. Association between metabolic syndrome and Helicobacter pylori infection diagnosed by histologic status and serological status. J Clin Gastroenterol 2012;46:840–5. [DOI] [PubMed] [Google Scholar]
- [32].Festi D, Schiumerini R, Marzi L, et al. Review article: the diagnosis of non-alcoholic fatty liver disease – availability and accuracy of non-invasive methods. Aliment Pharmacol Ther 2013;37:392–400. [DOI] [PubMed] [Google Scholar]


