Abstract
β-amyloid (Aβ) aggregation and tau hyperphosphorylation are considered to be the primary pathological hallmarks of Alzheimer's disease (AD). Targeted inhibition of these pathological processes may provide effective treatments for AD. Accumulating evidence has demonstrated that ellagic acid (EA) exerts neuroprotective effects in several diseases. The present study investigated the effects of EA on AD-associated learning and memory deficits on APP/PS1 double transgenic mice and the underlying mechanisms. APP/PS1 mice or wild-type C57BL/6 mice were intragastrically administered EA (50 mg/kg/day) or vehicle for 60 consecutive days. The learning and memory abilities of mice were investigated using the Morris water maze test. Hippocampal regions were examined for the presence of amyloid plaques, neuronal apoptosis and tau phosphorylation. Expression levels of APP, Aβ, RAC-αserine/threonine-protein kinase and glycogen synthase kinase (GSK)3β in the hippocampus were determined by western blot analysis and ELISA. The results demonstrated that EA treatment ameliorated spatial learning and memory impairment in APP/PS1 mice and significantly reduced neuronal apoptosis and Aβ deposition in the hippocampus (P<0.05 and P<0.01). In addition, EA significantly inhibited the hyperphosphorylation of tau and significantly decreased the activity of glycogen synthase kinase (GSK)3β (P<0.01), which is involved in tau phosphorylation. Overall, these findings indicated that the beneficial effects of EA on AD-associated cognitive impairments may be attributed to the inhibition of Aβ production and tau hyperphosphorylation, and its beneficial action may be mediated in part, by the RAC-α serine/threonine-protein kinase/GSK3β signaling pathway.
Keywords: Alzheimer's disease, ellagic acid, cognitive impairment, tau hyperphosphorylation, glycogen synthase kinase 3β
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disease that frequently occurs in the elderly population and is characterized by progressive learning dysfunction and memory impairment (1). Pathologically, accumulation of senile plaques, neurofibrillary tangles (NFTs) and neuronal loss have been observed in the brain cortex and hippocampus of patients with AD (2). Although the underlying mechanisms have not been fully elucidated, β-amyloid (Aβ) aggregation and tau hyperphosphorylation have been revealed to exhibit key roles in the pathogenesis of AD (3,4), as they are primary components of senile plaques and NFTs, respectively (5,6) and are associated with neuronal loss and memory deficits (7,8). Therefore, potential pharmacological agents targeting the inhibition of these pathological processes may be promising therapeutic approaches in treating AD.
Ellagic acid (EA) is a natural polyphenol present in several types of fruits and nuts, including strawberries, pomegranates and walnuts (9). EA has been reported to possess multiple pharmacological effects, including antioxidant, anti-inflammatory, antitumor and antifibrotic properties (10,11). A previous study demonstrated the neuroprotective role of EA in several central nervous system diseases (12). Farbood et al (13) reported that EA alleviates brain injury-induced cognitive dysfunction via inhibition of neuroinflammation. Notably, Sarkaki et al (14) demonstrated that EA mitigates the symptoms of Parkinson's disease induced by 6-hydroxydopamine, and this effect may be attributed to its antioxidant properties (9). Furthermore, Feng et al (15) indicated that EA reduces Aβ42-induced neurotoxicity, which suggested a beneficial role of EA in AD. In accordance with these results, Kiasalari et al (16) further revealed that EA ameliorated learning and memory deficits in a rat model of AD induced by Aβ25–35 and suggested the underlying mechanisms were associated with its inhibition of oxidative stress and the nuclear factor (NF)-κB/NF erythroid 2 like 2/Toll-like receptor 4 signaling pathway. However, whether EA affects Aβ production and tau hyperphosphorylation remains unclear.
In the present study, the APP/PS1 double-transgenic mouse model was employed to evaluate the efficacy of EA in the treatment of AD. Brain hippocampi were examined for alterations in neuronal apoptosis, Aβ plaque formation and tau hyperphosphorylation. In addition, the RAC-α serine/threonine-protein kinase (AKT)/glycogen synthase kinase (GSK) 3β signaling pathway, which is involved in the regulation of tau hyperphosphorylation, was also investigated.
Materials and methods
Animals and treatment
Male APP/PS1 double-transgenic and wild-type (WT) C57BL/6 mice (6-months-old, weighing 24–28 g, n=12 per group) were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Prior to the experiment, all the mice were acclimatized for 1 week and were kept at a 12-h light/dark cycle at room temperature (21–23°C) with 55% humidity and access to food and water ad libitum. Mice were randomly divided into four groups (n=12 mice per group), namely the WT, WT+EA, APP/PS1 and APP/PS1+EA groups. The mice were intragastrically administered EA (50 mg/kg/day, cat. no. E102710; Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China) or the same volume of 10% DMSO for 60 consecutive days. The dosage of EA used was based on previous data (14,17), which demonstrated that EA at this dose exerted protective effects in a model of Parkinson's disease. The animal experiments were performed in accordance with the guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Heilongjiang University of Chinese Medicine (Harbin, China).
Morris water maze (MWM) test
Following 60 days of EA treatment, the spatial learning and memory abilities of the mice were assessed using the MWM test, as previously described (18). The apparatus included a black circular tank (180 cm in diameter) filled with water (temperature, 22–24°C). A platform (9 cm in diameter) was placed at the targeted quadrant 1.5 cm below the water surface. Mice were randomly placed into one of the four quadrants facing the maze wall and allowed to search for the platform. If the mouse did not find the platform within 120 sec, it was guided to the platform for 30 sec. The training lasted for 5 consecutive days, with four sessions per day. The time required to find the hidden escape platform was recorded. The probe trial was performed following 5 days of hidden platform trials. Mice were allowed to swim freely in the water without the platform for 120 sec. The frequency of crossing the platform location, the time spent in the target quadrant and the swimming tracks were monitored.
Tissue preparation
Following the behavioral tests, the mice were euthanized. Part of the resected hippocampal tissues were fixed in 10% formalin at 4°C for 24 h, embedded in paraffin and sliced into 5-µm sections. The remaining tissues were homogenized for western blot analysis and ELISA.
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) assay
TUNEL staining (In Situ Cell Death Detection kit; Roche Diagnostics, Indianapolis, IN, USA) was used to assess neuronal apoptosis in the hippocampus. The TUNEL assay was performed according to standard procedures. Hippocampal slices (5-µm thick) were deparaffinized, rehydrated, permeabilized and blocked in 3% H2O2 for 10 min at room temperature. Following washing, the slices were incubated in TUNEL reaction mixture for 60 min at 37°C and processed with 50 µl converter-peroxidase for 30 min at 37°C. Sections were stained using the diaminobenzidine substrate kit (cat no. DA1010; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 3 min at room temperature for color development and counterstained with hematoxylin for 3 min at room temperature. Apoptotic cells were assessed under a light microscope (magnification, ×400). The number of apoptotic cells was calculated in six different visual fields of each section.
Thioflavin-S staining
Amyloid plaque formation was measured by thioflavin-S staining (cat no. T1892; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the manufacturer's instructions. Hippocampal slices (5-µm thick) were deparaffinized, rehydrated and stained in thioflavin-S solution at room temperature for 8 min. Subsequently, the slices were successively rinsed in 50% alcohol and distilled water. Amyloid deposition was evaluated under a fluorescent microscope (magnification, ×100 and ×400).
Immunohistochemical analysis
The expression of tau protein in the hippocampus was determined using immunohistochemical analysis. The procedures were performed as previously described (19) with slight modifications. Briefly, hippocampal sections were subjected to heat-induced epitope retrieval by immersing slides in a boiling solution for 10 min, followed by 3% H2O2 treatment to block endogenous peroxidases for 15 min at room temperature. The sections were then blocked with 10% goat serum (cat no. SL038; Beijing Solarbio Science & Technology Co., Ltd.) for 15 min at room temperature, followed by incubation with anti-tau antibody (cat no. D155045; 1:50; Sangon Biotech, Co., Ltd., Shanghai, China) at 4°C overnight. Following washing three times in PBS, the sections were incubated with a biotinylated anti-rabbit IgG antibody (cat no. A0277, 1:200; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min at 37°C, followed by incubation with the avidin biotinylated horseradish peroxidase (cat no. A0303; 1:200; Beyotime Institute of Biotechnology) for 30 min at 37°C. Color was developed using the diaminobenzidine substrate kit (cat no. DA1010; Beijing Solarbio Science & Technology Co., Ltd.) for 3 min at room temperature and counterstained with hematoxylin for 3 min at room temperature. Images of the stained sections were analyzed under a light microscopy (magnification, ×400).
ELISA
The concentration of Aβ40 and Aβ42 in the hippocampus was quantified using commercial ELISA kits (cat no. CEA864Mu and cat no. CEA946Mu; USCN Business Co., Ltd., Wuhan, China). ELISA was performed according to the manufacturer's protocol.
Western blot analysis
Western blot analysis was performed according to standard protocols with some modifications (20). Briefly, total protein from each tissue sample were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) following the manufacturer's instructions. Protein concentrations were measured using the BCA method. A total of 20 µg proteins were separated by SDS-PAGE (8, 11 or 15% gels) and transferred to polyvinylidene difluoride membranes. Following blocking with 5% skimmed milk at room temperature for 1 h, the membrane was incubated with a specific primary antibody at 4°C overnight. The primary antibodies used in the study included anti-cleaved caspase-3 antibody (cat no. ab2302; 1:1,000), anti-pSer199-tau antibody (cat no. ab81268; 1:10,000) (both from Abcam, Cambridge, UK), anti-amyloid precursor protein (APP) antibody (cat no. D260097; 1:500; Sangon Biotech, Co., Ltd.), anti-pThr668-APP antibody (cat no. D155082; 1:500; Sangon Biotech, Co., Ltd.), anti-β secretase (BACE) 1 (cat no. D220305; 1:500; Sangon Biotech, Co., Ltd.), anti-pSer396-tau antibody (cat no. D155045; 1:500; Sangon Biotech, Co., Ltd.), anti-pSer473-AKT antibody (cat no. KG11054; 1:500; Nanjing KenGen Biotech, Co., Ltd., Nanjing, China), anti-AKT antibody (cat no. KG21054; 1:500; Nanjing KenGen Biotech, Co., Ltd.), anti-pTyr216-GSK3β (cat no. KG11301-2; 1:500; Nanjing KenGen Biotech, Co., Ltd), anti-GSK3β antibody (cat no. KG21002-2; 1:500; Nanjing KenGen Biotech, Co., Ltd.), anti-tau antibody (cat no. KG21099-2; 1:500; Nanjing KenGen Biotech, Co., Ltd.) and anti-β-actin antibody (cat no. bsm-33139M; 1:500; Bioss Antibodies, Beijing, China). The membrane was subsequently incubated with goat anti-rabbit IgG horseradish peroxidase-conjugated antibody (cat no. A0208; 1:5,000; Beyotime Institute of Biotechnology) at 37°C for 45 min. An enhanced chemiluminescence detection reagent (Beyotime Institute of Biotechnology) was used for enhanced blot detection. The band intensity was assessed and analyzed with Gel-Pro-Analyzer software 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. Analyses were performed using one-way analysis of variance and the Bonferroni post-hoc test with GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
EA improves learning and memory deficits in APP/PS1 transgenic mice
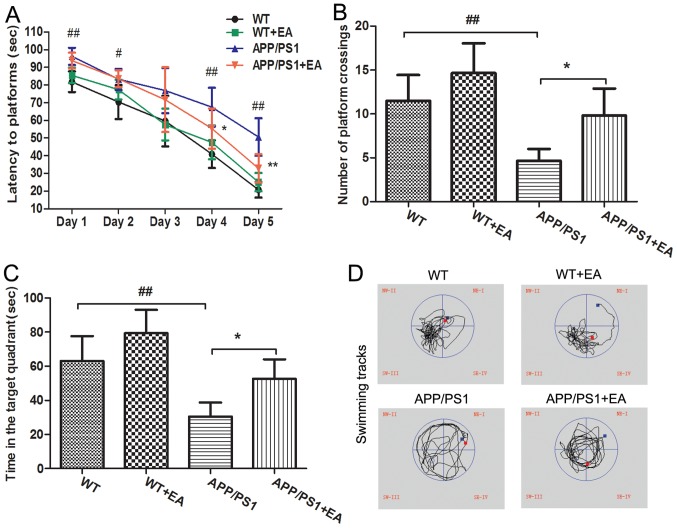
APP/PS1 transgenic mice begin to exhibit memory impairment and amyloid plaque deposition at ~6 months of age (21). In the present study, the MWM test was performed to evaluate the learning and memory abilities of the mice. In the hidden platform trials, the APP/PS1 model group exhibited significantly longer escape latencies at days 1, 2, 4 and 5 of the trial compared with the WT group (P<0.01; Fig. 1A). EA treatment significantly decreased the escape latencies at day 4 and 5 of the trial compared with the APP/PS1 group (P<0.05), reflecting the improved learning ability with EA treatment. In the probe trials, following removal of the platform, the mice randomly swam in the pool for 120 sec. In addition, the number of platform crossings and the time spent in the targeted quadrant for the APP/PS1-vehicle treated mice was significantly decreased compared with the WT group (P<0.01; Fig. 1B and C). By contrast, the EA-treated APP/PS1 mice crossed the position of the platform more frequently and spent more time searching for the platform in the target quadrant compared with the APP/PS1-vehicle treated mice (P<0.05). Furthermore, the swimming tracks of EA-treated APP/PS1 mice were similar to those of the WT group (Fig. 1D). Taken together, the MWM test results suggested that EA treatment improved the spatial learning and memory abilities in APP/PS1 transgenic mice.
Figure 1.
EA treatment improves learning and memory impairment in APP/PS1 mice. Mice were intragastrically administered EA (50 mg/kg/day) or vehicle for 60 consecutive days. The learning and memory abilities of the mice were evaluated using the Morris Water Maze test. (A) Escape latencies of mice for finding the platform in the hidden platform trials. (B) Number of mice crossing the platform location in the probe test. (C) Time spent in the target quadrant in the probe test. (D) Swimming tracks of mice in the probe test. The circle in the left lower quadrant represents the location of the hidden platform. Data are presented as the mean ± standard deviation. #P<0.05 or ##P<0.01 vs. WT mice; *P<0.05 or **P<0.01 vs. APP/PS1 transgenic mice. EA, ellagic acid; WT, wild-type.
EA reduces neuronal cell apoptosis in the hippocampus of APP/PS1 transgenic mice
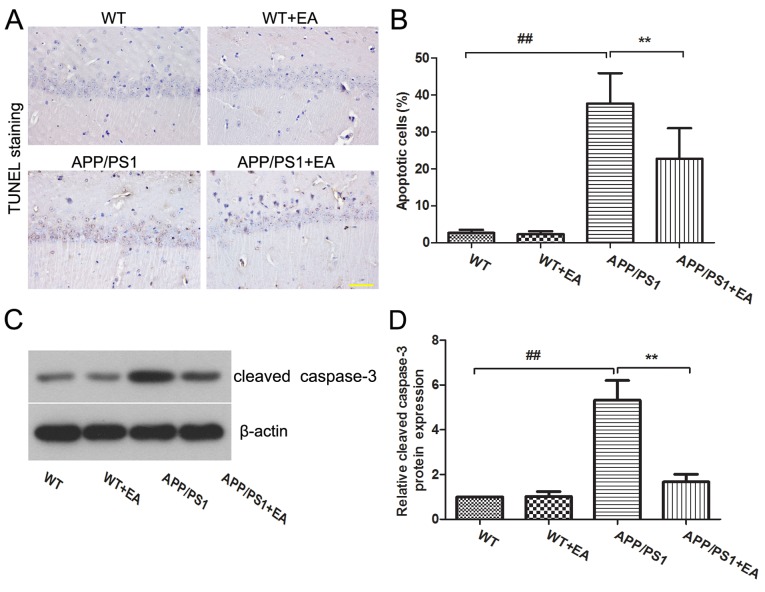
Neuronal loss is responsible for the learning and memory deficits in patients with AD (22). The present study further evaluated the effects of EA on neuronal apoptosis in the hippocampus. TUNEL staining indicated that the APP/PS1 group exhibited an increased proportion of apoptotic cells in the hippocampus compared with the WT group, whereas EA treatment reduced the numbers of apoptotic cells compared with the APP/PS1-vehicle group (Fig. 2A). Furthermore, the differences between the percentage of apoptotic cells exhibited in the hippocampus of mice in these groups were statistically significant (P<0.01; Fig. 2B). Western blot analysis also revealed that the expression of cleaved caspase-3 was upregulated in APP/PS1 mice, and EA treatment reduced the expression level of caspase-3 in the hippocampus compared with that in the APP/PS1-vehicle group (Fig. 2C). Statistical analysis indicated these differences were significant (P<0.01; Fig. 2D).
Figure 2.
Effects of EA on neuronal apoptosis in the hippocampus of APP/PS1 transgenic mice. (A) TUNEL staining of the hippocampus from WT, WT+EA, APP/PS1 and APP/PS1+EA groups. Magnification, ×400; scale bar, 50 µm. (B) Percentage of apoptotic cells in the hippocampus. (C) Western blotting and (D) quantitative analysis of cleaved caspase-3 expression in the hippocampus; β-actin served as an internal control for grayscale analysis. Data are presented as the mean ± standard deviation. ##P<0.01 vs. WT mice; **P<0.01 vs. APP/PS1 transgenic mice. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; EA, ellagic acid; WT, wild-type.
EA reduces Aβ deposition and Aβ protein expression levels in the hippocampus of APP/PS1 transgenic mice
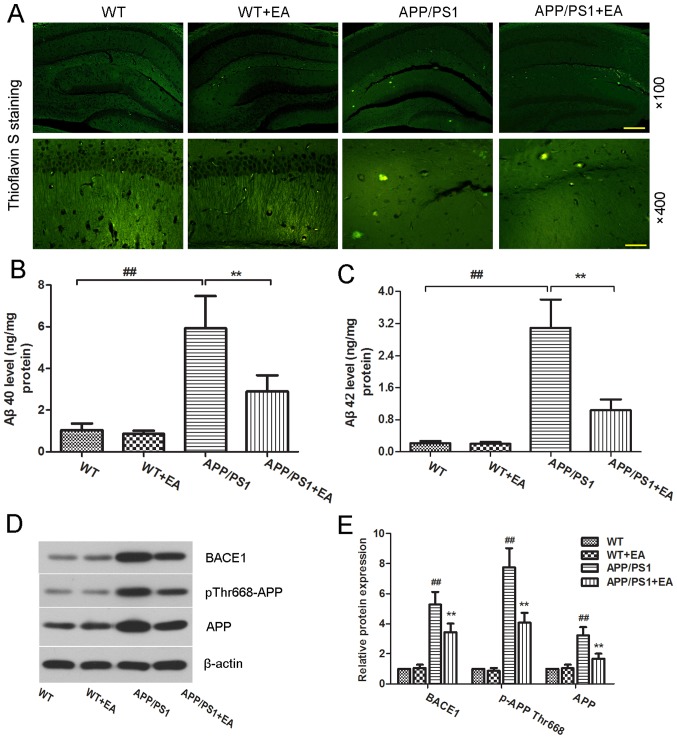
Accumulation of Aβ in the brain is considered to be a primary hallmark of AD (23). Deposited amyloid plaques in the hippocampus may further induce neuronal loss and cognitive impairment deficits (20,24). The present study further evaluated the effect of EA on Aβ deposition. Aβ plaques were stained with thioflavin-S (Fig. 3A). Compared with the WT group, the APP/PS1 group exhibited increased amyloid staining in the hippocampus, whereas EA treatment reduced the amyloid aggregates in the hippocampus. ELISA also revealed significantly increased levels of Aβ40 and Aβ42 in the APP/PS1 group, whereas EA treatment effectively reversed this increase (P<0.01; Fig. 3B and C).
Figure 3.
Effect of EA on β-amyloid plaque deposition in the hippocampus of APP/PS1 mice. Following completion of the behavioral tests, the brain tissues from each group were subjected to analysis. (A) Thioflavin S-stained Aβ plaques in the hippocampus. Magnification, ×100; scale bar, 200 µm (upper panels) and magnification, ×400; scale bar, 50 µm (lower panels). Concentration of (B) Aβ40 and (C) Aβ42 in the hippocampal tissues was determined using ELISA kits. (D) Western blotting and (E) quantitative analysis of APP and pThr668-APP protein expression levels in the hippocampus. β-actin served as an internal control for grayscale analysis. Data are presented as the mean ± standard deviation. ##P<0.01 vs. WT mice; **P<0.01 vs. APP/PS1 transgenic mice. EA, ellagic acid WT, wild-type; APP, amyloid precursor protein; Aβ, β-amyloid; BACE1, β-secretase 1.
Aβ peptides are produced from the APP; phosphorylation of APP and BACE1 kinase are responsible for Aβ generation (25,26). To evaluate whether EA affects Aβ production, the protein expression levels of pThr668-APP and BACE1 were determined. The western blot analysis results revealed that pThr668-APP and BACE1 protein expression levels in the APP/PS1 group were increased compared with the WT group, whereas EA treatment reduced these expression levels (Fig. 3D). Notably, these differences were indicated to be statistically significant (P<0.01; Fig. 3E). These results suggested that EA reduced Aβ deposition by suppressing Aβ production.
EA inhibits tau hyperphosphorylation in the hippocampus of APP/PS1 transgenic mice
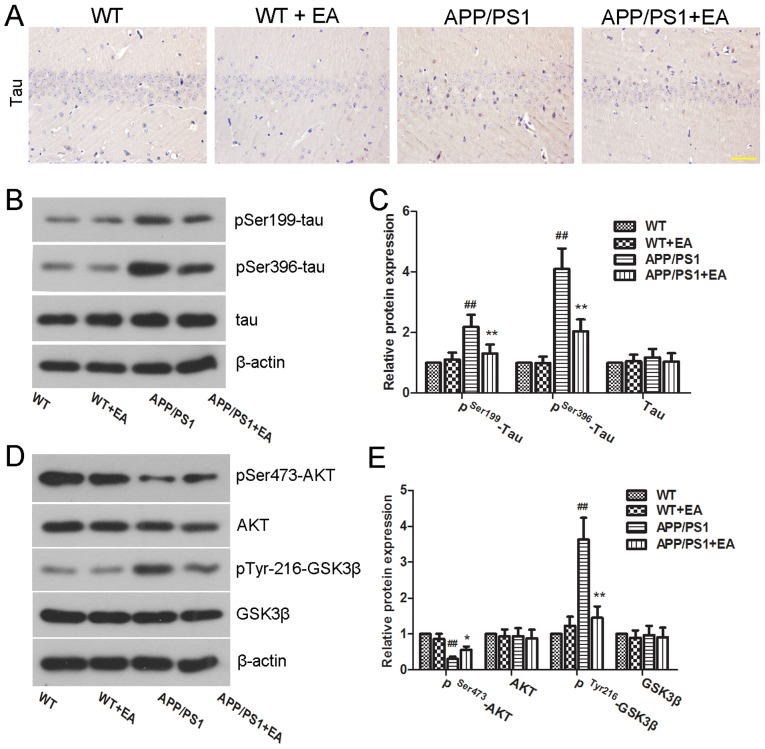
In addition to Aβ deposition, tau hyperphosphorylation is another characteristic feature of the brain in APP/PS1 mice (27). In the present study, the extent of Tau hyperphosphorylation in the hippocampus was determined by immunohistochemical staining and western blot analysis. Immunohistochemical results revealed high expression of pSer396-tau in the APP/PS1 group compared with the WT group. Notably, EA treatment reduced the expression of pSer396-tau in the hippocampus (Fig. 4A). Furthermore, western blot analysis also determined that EA downregulated the protein expression levels of pSer199-tau and pSer396-tau in the APP/PS1 group (Fig. 4B). Furthermore, the difference between the protein expression levels of pSer199-tau and pSer396-tau in the APP/PS1 group compared with the APP/PS1+EA group was indicated to be statistically significant (P<0.01; Fig. 4C). Taken together, these results indicated that the beneficial role of EA in AD may be associated with the downregulation of tau phosphorylation.
Figure 4.
Effect of EA on tau hyperphosphorylation in APP/PS1 transgenic mice. (A) Protein expression of pSer396-tau in the hippocampus was detected by immunohistochemical staining (magnification, ×400; scale bar, 50 µm). (B) Western blotting and (C) quantitative analysis of pSer199-tau, pSer396-tau and tau protein expression levels in the hippocampus. β-actin served as an internal control for grayscale analysis. (D) Western blotting and (E) quantitative analysis of pSer473-AKT, pTyr216-GSK3β, AKT, and GSK3β protein expression levels in the hippocampus. β-actin served as an internal control for grayscale analysis. Data are presented as the mean ± standard deviation. ##P<0.01 vs. WT mice; *P<0.05 or **P<0.01 vs. APP/PS1 transgenic mice. EA, ellagic acid; WT, wild-type; GSK3β, glycogen synthase kinase 3β; AKT, RAC-α serine/threonine-protein kinase.
The AKT/GSK3β signaling pathway has been demonstrated to have an important role in tau hyperphosphorylation. Activated GSK3β has been demonstrated to facilitate tau phosphorylation and NFT formation (28). To further investigate the underlying mechanism of action of EA, the effect of EA on the regulation of the AKT/GSK3β signaling pathway was examined by western blot analysis. As presented in Fig. 4D, mice in the APP/PS1 group exhibited low protein expression levels of pSer473-AKT and high expression levels of pTyr216-GSK3β compared with those in the WT group. Notably, these differences were indicated to be statistically significant (P<0.01; Fig. 4E). These results suggested GSK3β activation. Conversely, EA treatment significantly upregulated the protein expression levels of pSer473-AKT and decreased the protein expression levels of pTyr216-GSK3β in APP/PS1 mice (P<0.05 and P<0.01, respectively; Fig. 4E), which indicated activation of AKT and deactivation of GSK3β following EA treatment. These results suggested that EA inhibited tau phosphorylation in the hippocampus of APP/PS1 mice and that this inhibitory effect may be partially mediated by the AKT/GSK3β signaling pathway.
Discussion
AD, which is the most common form of dementia, severely affects the life quality of elderly patients, and there is currently no effective treatment (29). To further understand the underlying mechanisms and develop novel pharmacological agents for AD, animal models of AD have been employed. A growing body of evidence has demonstrated that APP/PS1 transgenic mice exhibit similar pathological alterations to those of patients with AD (30). In APP/PS1 transgenic mice, the memory impairment and amyloid deposition appear at 4–6 months of age and progress in an age-dependent manner (31,32). In the present study, APP/PS1 mice were selected to investigate the therapeutic efficacy of EA in AD. The results demonstrated that administration of EA (50 mg/kg/day for 60 days) ameliorated the learning and memory deficits in APP/PS1 mice, significantly reduced neuronal apoptosis and amyloid deposition, and also significantly inhibited tau hyperphosphorylation in the hippocampus, which suggested a beneficial role of EA in AD. A previous study also reported the beneficial role of EA in an Aβ-induced AD mouse model (16). Notably, the present findings further demonstrated the therapeutic efficacy of EA in APP/PS1 mice and the beneficial effects were indicated to be associated with the inhibition of Aβ production and tau hyperphosphorylation.
Aβ aggregates exhibit crucial roles in AD-associated cognitive impairment and neuronal loss (24,33). Targeted inhibition of Aβ production has been indicated to exert beneficial effects on APP/PS1 mice (34). In the present study, APP/PS1 mice exhibited significant deficits in their learning and memory abilities alongside increased neuronal apoptosis and Aβ deposition. Learning and memory impairments of APP/PS1 mice in the present study were in line with those reported by other studies (35,36). Notably, EA treatment improved the learning and memory abilities, and significantly decreased Aβ deposition and Aβ expression levels in the hippocampus. Furthermore, EA treatment significantly reduced the expression levels of pThr668APP and BACE1. As previously reported, the phosphorylation of APP at the Thr668 site promotes Aβ generation (25) and BACE1 is the key enzyme for Aβ generation through cleavage of APP (26,37). The present results revealed that EA treatment alleviated cognitive impairment and decreased Aβ production. These results were consistent with a previous study reporting the anti-amyloidogenic effect of EA on potassium sorbate and glucose-induced human serum albumin fibril formation (38).
Hyperphosphorylated tau is the primary component of intracellular NFTs, which have an important role in AD pathology (2,39). It has been demonstrated that hyperphosphorylated tau may also result in neuronal death and memory impairment (5,40,41). A number of serine phosphorylation sites of tau are elevated in AD mice, including Ser199 and Ser396 (42). Targeting inhibition of tau phosphorylation may therefore provide an effective therapeutic approach to AD (43). In the present study, phosphorylation of tau at Ser199 and Ser396 was significantly increased in the hippocampus of APP/PS1 mice, whereas EA treatment efficiently inhibited tau phosphorylation. Since the phosphorylation of tau is regulated by multiple signals, including AKT/GSK3β signaling (35,44), the present study further investigated the effect of EA on the AKT/GSK3β signaling pathway. The results demonstrated that EA significantly increased the phosphorylation of AKT at the Ser473 site and significantly decreased the phosphorylation of GSK3β at the Tyr216 site, indicating de-activation of GSK3β (45). These results suggested that EA inhibited tau phosphorylation in APP/PS1 mice and that the inhibitory effect of EA may be partially mediated by the AKT/GSK3β signaling pathway. However, several other kinases are involved in the regulation of tau phosphorylation and further investigation is required to identify the precise mechanisms of action of EA in AD.
In conclusion, the present study demonstrated the beneficial effects of EA on spatial learning and memory in APP/PS1 mice, which included inhibition of neuronal death, Aβ production and tau hyperphosphorylation. Furthermore, the beneficial effects of EA were indicated to be partially mediated by the AKT/GSK3β signaling pathway. These findings suggest that EA may be a promising therapeutic agent for the treatment of AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Natural Science Foundation of Heilongjiang Province (grant no. QC2015101 and QC2015102), the Postdoctoral Foundation of Heilongjiang Province Government (grant no. LBH-Z14196 and LBH-Z15207), the Postdoctoral Science Foundation of China (grant nos. 2015M581496 and 2016M591565) and the National Natural Science Foundation of China (grant no. 81403288).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LZ, HL and ZS contributed to the conception and design of the study, wrote and revised the manuscript. LZ, HL and WZ conducted the studies and analyzed and interpreted the data. XL, BJ and HF analyzed the data and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal experiments were performed in accordance with the guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Heilongjiang University of Chinese Medicine (Harbin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/JAD-2001-3111. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom GS. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 5.Ruan YY, Zhai W, Shi XM, Zhang L, Hu YL. Safflower yellow ameliorates cognition deficits and reduces tau phosphorylation in APP/PS1 transgenic mice. Metab Brain Dis. 2016;31:1133–1142. doi: 10.1007/s11011-016-9857-3. [DOI] [PubMed] [Google Scholar]
- 6.Sobów T, Flirski M, Liberski PP. Amyloid-beta and tau proteins as biochemical markers of Alzheimer's disease. Acta Neurobiol Exp (Wars) 2004;64:53–70. doi: 10.55782/ane-2004-1491. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta U, Nilson AN, Kayed R. The role of Amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Li Q, Chu X, Sun S, Chen S. Salidroside reduces tau hyperphosphorylation via up-regulating GSK-3β phosphorylation in a tau transgenic Drosophila model of Alzheimer's disease. Transl Neurodegener. 2016;5:21. doi: 10.1186/s40035-016-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amakura Y, Okada M, Tsuji S, Tonogai Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J Chromatogr A. 2000;896:87–93. doi: 10.1016/S0021-9673(00)00414-3. [DOI] [PubMed] [Google Scholar]
- 10.Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26:3601–3606. [PubMed] [Google Scholar]
- 11.Mansouri MT, Hemmati AA, Naghizadeh B, Mard SA, Rezaie A, Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol. 2015;47:292–298. doi: 10.4103/0253-7613.157127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed T, Setzer WN, Nabavi SF, Orhan IE, Braidy N, Sobarzo-Sanchez E, Nabavi SM. Insights into effects of ellagic acid on the nervous system: A mini review. Curr Pharm Des. 2016;22:1350–1360. doi: 10.2174/1381612822666160125114503. [DOI] [PubMed] [Google Scholar]
- 13.Farbood Y, Sarkaki A, Dianat M, Khodadadi A, Haddad MK, Mashhadizadeh S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015;124:120–127. doi: 10.1016/j.lfs.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Sarkaki A, Farbood Y, Dolatshahi M, Mansouri SM, Khodadadi A. Neuroprotective effects of ellagic acid in a rat model of Parkinson's disease. Acta Med Iran. 2016;54:494–502. [PubMed] [Google Scholar]
- 15.Feng Y, Yang SG, Du XT, Zhang X, Sun XX, Zhao M, Sun GY, Liu RT. Ellagic acid promotes Abeta42 fibrillization and inhibits Abeta42-induced neurotoxicity. Biochem Biophys Res Commun. 2009;390:1250–1254. doi: 10.1016/j.bbrc.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 16.Kiasalari Z, Heydarifard R, Khalili M, Afshin-Majd S, Baluchnejadmojarad T, Zahedi E, Sanaierad A, Roghani M. Ellagic acid ameliorates learning and memory deficits in a rat model of Alzheimer's disease: An exploration of underlying mechanisms. Psychopharmacology (Berl) 2017;234:1841–1852. doi: 10.1007/s00213-017-4589-6. [DOI] [PubMed] [Google Scholar]
- 17.Baluchnejadmojarad T, Rabiee N, Zabihnejad S, Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson's disease: Possible involvement of ERbeta/Nrf2/HO-1 signaling. Brain Res. 2017;1662:23–30. doi: 10.1016/j.brainres.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZX, Zhao RP, Wang DS, Wang AN. Fuzhisan ameliorates Aβ production and tau phosphorylation in hippocampal of 11 month old APP/PS1 transgenic mice: A Western blot study. Exp Gerontol. 2016;84:88–95. doi: 10.1016/j.exger.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Gao Y, Jiang L, Chao FL, Huang W, Zhou CN, Tang W, Zhang L, Huang CX, Zhang Y, et al. Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer's disease mice. Oncotarget. 2017;8:27676–27692. doi: 10.18632/oncotarget.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Niikura T, Tajima H, Kita Y. Neuronal cell death in Alzheimer's disease and a neuroprotective factor, humanin. Curr Neuropharmacol. 2006;4:139–147. doi: 10.2174/157015906776359577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 24.Jahn H. Memory loss in Alzheimer's disease. Dialogues Clin Neurosci. 2013;15:445–454. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi S, Refolo LM, Sambamurti K. Amyloid precursor protein compartmentalization restricts beta-amyloid production: Therapeutic targets based on BACE compartmentalization. J Mol Neurosci. 2004;24:137–143. doi: 10.1385/JMN:24:1:137. [DOI] [PubMed] [Google Scholar]
- 27.Kurt MA, Davies DC, Kidd M, Duff K, Howlett DR. Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol Dis. 2003;14:89–97. doi: 10.1016/S0969-9961(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 29.Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer's disease: Consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol. 2011;11:21. doi: 10.1186/1471-2377-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Persp Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parthsarathy V, McClean PL, Hölscher C, Taylor M, Tinker C, Jones G, Kolosov O, Salvati E, Gregori M, Masserini M, Allsop D. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPswe/PS1DeltaE9 mouse model of Alzheimer's disease. PLoS One. 2013;8:e54769. doi: 10.1371/annotation/57e0a947-8600-4658-b04c-cf7a45c8bd8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herran E, Perez-Gonzalez R, Igartua M, Pedraz JL, Carro E, Hernandez RM. Enhanced Hippocampal Neurogenesis in APP/Ps1 Mouse Model of Alzheimer's Disease After Implantation of VEGF-loaded PLGA Nanospheres. Curr Alzheimer Res. 2015;12:932–940. doi: 10.2174/1567205012666151027121622. [DOI] [PubMed] [Google Scholar]
- 33.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 34.Dionísio PA, Amaral JD, Ribeiro MF, Lo AC, D'Hooge R, Rodrigues CM. Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol Aging. 2015;36:228–240. doi: 10.1016/j.neurobiolaging.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Bao XQ, Li N, Wang T, Kong XC, Tai WJ, Sun H, Zhang D. FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer's disease via decreasing beta-amyloid production and tau hyperphosphorylation. PloS One. 2013;8:e78033. doi: 10.1371/journal.pone.0078033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Guo XD, Lei M, Wu JY, Jin JZ, Shi XF, Zhu ZY, Rukachaisirikul V, Hu LH, Wen TQ, Shen X. Thamnolia vermicularis extract improves learning ability in APP/PS1 transgenic mice by ameliorating both Aβ and Tau pathologies. Acta Pharmacol Sin. 2017;38:9–28. doi: 10.1038/aps.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang KA, Kim HS, Ha TY, Ha JW, Shin KY, Jeong YH, Lee JP, Park CH, Kim S, Baik TK, Suh YH. Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol Cell Biol. 2006;26:4327–4338. doi: 10.1128/MCB.02393-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taghavi F, Habibi-Rezaei M, Bohlooli M, Farhadi M, Goodarzi M, Movaghati S, Maghami P, Taghibiglou C, Amanlou M, Haertlé T, Moosavi-Movahedi AA. Antiamyloidogenic effects of ellagic acid on human serum albumin fibril formation induced by potassium sorbate and glucose. J Mol Recognit. 2016;29:611–618. doi: 10.1002/jmr.2560. [DOI] [PubMed] [Google Scholar]
- 39.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q, Ruan YY, Xu H, Shi XM, Wang ZX, Hu YL. Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid beta-induced impairment of learning and memory in rats. Biomed Pharmacother. 2015;76:153–164. doi: 10.1016/j.biopha.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Maas T, Eidenmüller J, Brandt R. Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem. 2000;275:15733–15740. doi: 10.1074/jbc.M000389200. [DOI] [PubMed] [Google Scholar]
- 43.Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP, LaFerla FM, Gozes I, Aisen PS. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer's disease at early pathological stage. J Mol Neurosci. 2007;31:165–170. doi: 10.1385/jmn/31:02:165. [DOI] [PubMed] [Google Scholar]
- 44.Ryder J, Su Y, Ni B. Akt/GSK3beta serine/threonine kinases: Evidence for a signalling pathway mediated by familial Alzheimer's disease mutations. Cell Signal. 2004;16:187–200. doi: 10.1016/j.cellsig.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Gassowska M, Czapski GA, Pajak B, Cieslik M, Lenkiewicz AM, Adamczyk A. Extracellular α-synuclein leads to microtubule destabilization via GSK-3β-dependent Tau phosphorylation in PC12 cells. PloS One. 2014;9:e94259. doi: 10.1371/journal.pone.0094259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.