Abstract
Background:
Published studies about passive smoking and cervical cancer have found inconsistent results. Hence, the present meta-analysis was performed to assess this association.
Methods:
A systematical search was performed to identify eligible cohort and case–control studies in PubMed, Scopus, Elsevier ScienceDirect, and Web of Science databases (up to March, 2018). The quality of included studies was assessed by the Newcastle–Ottawa quality scale (NOS). The random effects model (REM) was used to calculate the pooled odds ratio (ORs). Subgroup and sensitivity analyses were performed. Publication bias was assessed by funnel plot, using Begg's test and Egger's test.
Results:
Around 14 eligible studies were included for analysis, which included a total of 384,995 participants. The pooled ORs of passive smoking with cervical cancer risk was 1.70 (95% CI: 1.40–2.07, I2 = 64.3%). Subgroups stratified by continent, study design, quality score, and cervical cancer types/phases suggested that the result was robust. For instance, the pooled ORs for the cohort and case–control studies was 1.37 (95% CI: 1.16–1.62, I2 = 0%) and 2.09 (95% CI: 1.52–2.89, I2 = 76.6%), respectively. The pooled ORs ranged from 1.61 (95%CI: 1.34–1.92) to 1.77 (95%CI: 1.44–2.16) after one study was removed each time in the sensitivity analyses, indicating that the result was stable. Publication bias was detected by funnel plot and Egger's tests. The recalculated ORs were 1.33 (95% CI: 1.21–1.47).
Conclusions:
This meta-analysis provides evidence that passive smoking is associated with an increased risk of cervical cancer.
Keywords: cervical cancer, meta-analysis, passive smoking, publication bias
1. Introduction
Cervical cancer was one of the most common cancers among women in the world, a total of 527,600 new cases occurred each year, of which 265,700 were dead. It was the second highest incidence in female malignancies and the fourth in mortality.[1] Human papillomavirus (HPV) has been acknowledged as the most essential causative factor for cervical cancer or cervical intraepithelial neoplasia (CIN).[2] Previous several epidemiologic studies have identified some factors associated with the high risk of cervical cancer: use of oral contraceptive, sexual promiscuity and cigarette smoking.[3–5]
Smoking was a main public health problem all over the world. Thousands of studies have investigated the health effects of active smoking, and the overall toxic effects of active smoking have been generally recognized .[6] In contrast, the impacts of passive smoking on health were not fully understood. Cigarettes contain large amounts of carcinogens or toxic substances such as nicotine, benzopyrene, and carbon monoxide. Despite global effort to combat the cigarette epidemic, smoking remains the primary avoidable cause of morbidity and mortality, killing approximately 6 million people each year all over the world.[7]
It has been widely acknowledged that smoking is undoubtedly an important risk factor for cancer, respiratory, and cardiovascular diseases.[8–10] However, there were inconsistent results of existing studies about passive smoking and cervical cancer.[11] A previous meta-analysis based on 3230 cases and 2982 controls by Zeng et al[12] from 11 studies published up to 2012 summarized that passive smoking was associated with a significantly increased the risk of cervical cancer. To provide the latest quantitative estimation of the passive smoking-cervical cancer association, we performed a meta-analysis to investigate the association between passive smoking and risk of cervical cancer.
2. Methods
2.1. Data searches
We conducted a related literature search (up to March 2018) of PubMed, Scopus, Elsevier ScienceDirect, and Web of Science databases for studies describing the association of passive smoking and cervical cancer. The searching terms (subject word and random word) were (smoking behaviors OR smoking habits OR passive smoking OR involuntary smoking OR second-hand tobacco smoke OR cigarette smoking OR environmental tobacco smoke) and (cervical neoplasm, uterine OR neoplasms, cervical OR neoplasms, cervix OR cancer of the uterine cervix OR cervical cancer OR uterine cervical cancer OR cervix cancer). In addition, we also searched the reference lists of retrieved articles, reviews, and meta-analyses.
2.2. Study selection and extraction
Two investigators independently evaluated the literature suitability; differences were resolved by agreement or determined by a third investigator. First of all, we inspected the repeatability, and removed the repeated papers. Then, titles and abstracts of the papers were perused carefully. Finally, reading the full articles to include the appropriate studies. Studies were included if they met all of the following criteria, the study design was prospective and retrospective studies. The odds ratio (OR), relative ratio (RR), hazard ratio (HR), and the corresponding 95% CIs, or reported data to calculate them. Studies were published in English.
Cervical cancer comprised different phases and types: unspecified histology (CC), ≥CIN2, high-grade squamous intraepithelial lesion (HSIL), squamous cell carcinoma of the cervix (SCCA), invasive cervical cancer (ICC), and cervical carcinoma in situ. We extracted the information about the studies: study features (study name, authors, year of publication and number of participants), participants’ features (mean age or age range), smoking status (never and passive), and disease outcomes.
2.3. Quality assessment
The quality of studies was independently assessed by the Newcastle–Ottawa quality scale (NOS), which is a specific scale to assess the quality of non-randomized studies in meta-analyses. It consisted of 3 parts which were the selection of study groups, the comparability of study groups, and the assessment of exposure or outcomes. We gave points if the studies met related condition. Studies with scores of 0–3, 4–6, 7–9 were, respectively, considered as low, moderate, and high quality.
2.4. Statistical analysis
The Q test and I2 statistic were used to evaluate the heterogeneity among selected studies.[13] If the heterogeneity was obvious (I2 >50%), the random effects model (REM) was used. If the heterogeneity was low (I2≤50%), the fixed effects model (FEM) was used.
Sensitivity analysis was performed to detect whether a single study had significantly affected the pooled result by removing one study in each turn. We conducted the subgroup analyses to identify potential confounding factors. Study design, NOS scores, continent, and cervical cancer types/phases for study were used in the subgroup analysis.
The possibility of publication bias was estimated by visual inspection of the funnel plot using Begg's test and Egger's test. The “fill and trim” method[14] was used to further evaluate the possible effect of publication bias on the pooled OR. All reported probabilities (P-value) were 2-sided and P < .05 was considered statistically significant. STATA version 14.0 was employed to conduct all statistical analyses. The ethics committee was not applicable to this meta-analysis.
3. Results
3.1. Literature search
Figure 1 showed the study selection process. The search strategy identified 729 papers. Around 87 articles were removed because they were duplicates. After systematic examining the titles and abstracts of the articles, 510 articles were excluded. The reasons for removing other 148 articles included that they were not cohort or case–control studies, unable to obtain data, the outcome was not cervical cancer, and no control group, after reading the full text of the articles. Finally, 14 studies were elected for inclusion in the meta-analysis.
Figure 1.
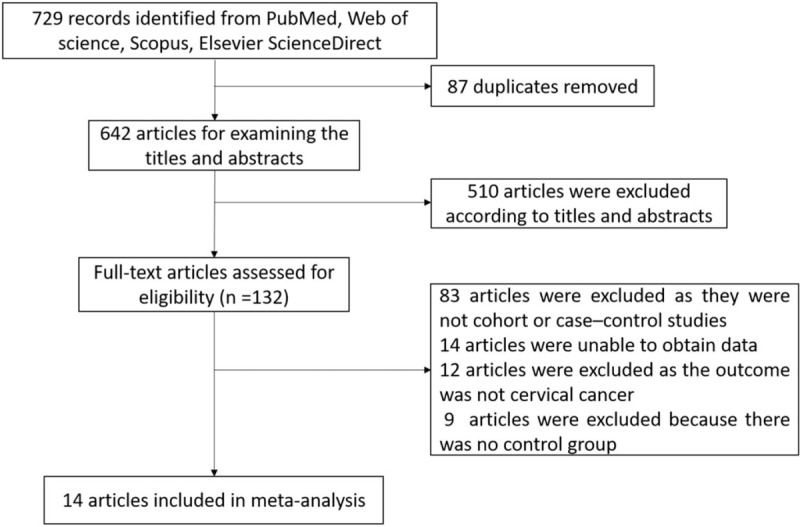
The flowchart of searching and selecting literatures.
3.2. Study characteristics
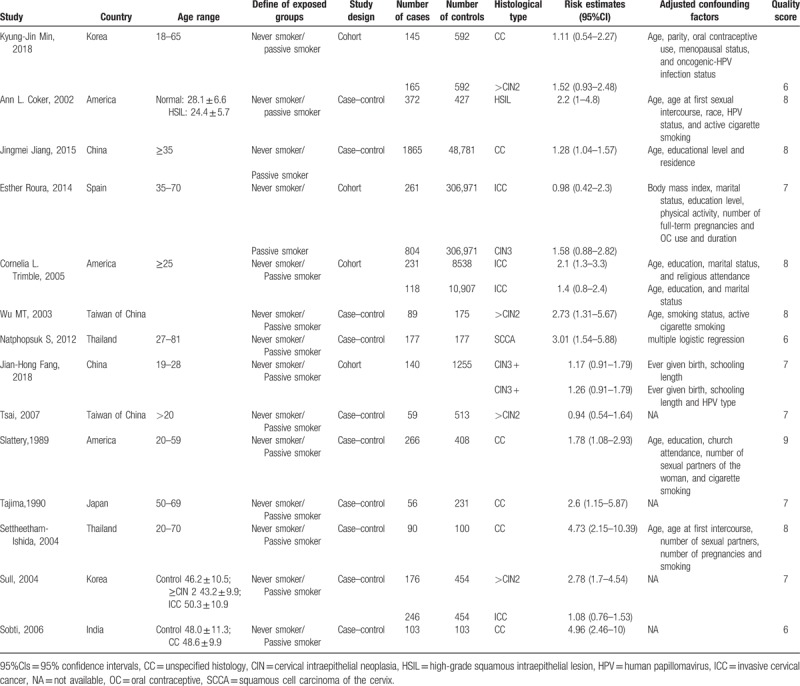
The main characteristics of 14 articles were listed in Table 1. The studies were conducted in Asia, Europe, and North America. In addition, adjustment for potential confounding factors differed according to the corresponding studies, and some main adjusted factors were age, parity, race, marital status, educational level and residence, age at first intercourse, HPV status, oral contraceptive use, body mass index (BMI), physical activity, number of sex partners, etc. Based on the quality assessment of NOS, 11 studies[5,15–24] were of high quality while the other 3 studies[25–27] were in moderate quality.
Table 1.
Main characteristics of studies.
3.3. Meta-analysis
As shown in Figure 2, the overall OR derived from the 14 studies indicated that a positive association between passive smoking and risk of cervical cancer (REM: I2 = 64.3%, OR = 1.70, 95%CI: 1.40–2.07, P < .01). However, statistically significant heterogeneity (I2 = 64.3%, P < .01) was detected between passive smoking and cervical cancer.
Figure 2.
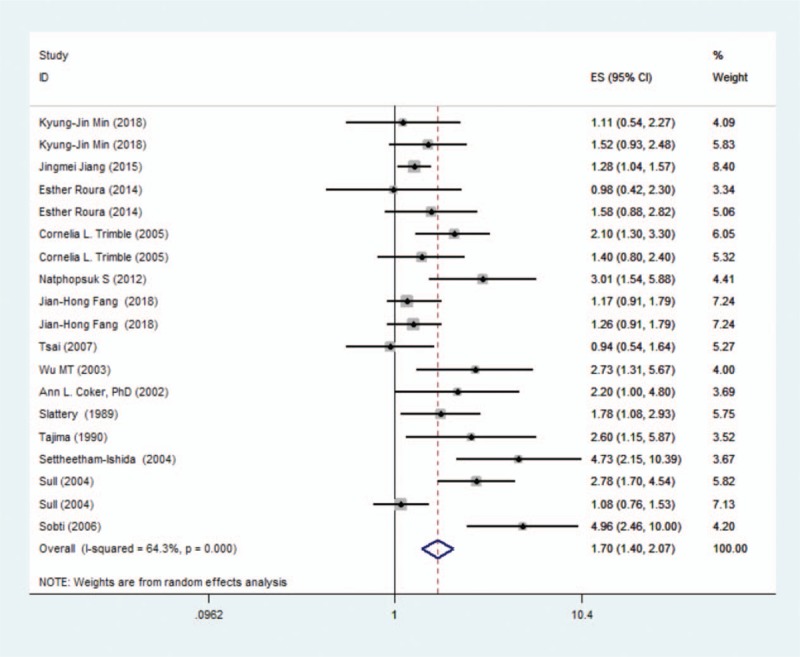
Forest graph on the association of passive smoking with cervical cancer risk. The risk estimates and 95% CI from each study are shown by a square and segments, respectively. The overall summary relative risk is represented by a rhombus.
3.4. Subgroup analyses
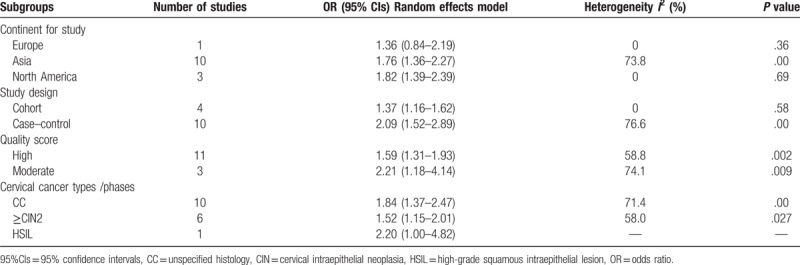
As shown in Table 2, stratification of the studies by continent showed that the OR for Europe was 1.36 (95% CI: 0.84–2.19, I2 = 0%, P = .36), for Asia was 1.76 (95% CI: 1.36–2.27, I2 = 73.8%, P < .01), for North America was 1.82 (95% CI: 1.39–2.39, I2 = 0%, P = .69). Stratification by study design showed that the OR was 1.37 (95% CI: 1.16–1.62, I2 = 0%, P = .58) for cohort studies, and 2.09 (95% CI: 1.52–2.89, I2 = 76.6%, P < .01) for case–control studies. Stratification by quality score showed that the OR was 2.21 (95% CI: 1.18–4.14, I2 = 74.1%, P = .009) for moderate quality, and 1.59 (95% CI: 1.31–1.93, I2 = 58.8%, P = .002) for high quality. Stratification of the studies by cervical cancer types/phases showed that OR was 1.84 (95% CI: 1.37–2.47, I2 = 71.4%, P < .01) for CC, was 1.52 (95% CI: 1.15–2.01, I2 = 58.0%, P = .027) for ≥CIN2.
Table 2.
Association of passive smoking with cervical cancer risk for different subgroups.
3.5. Sensitivity analysis
The results of sensitivity analysis were presented in Figure 3. The pooled ORs ranged from 1.61 (95%CI: 1.34–1.92) to 1.77 (95%CI: 1.44–2.16) after one study was removed each time, indicating that no individual study substantially influenced the pooled ORs.
Figure 3.
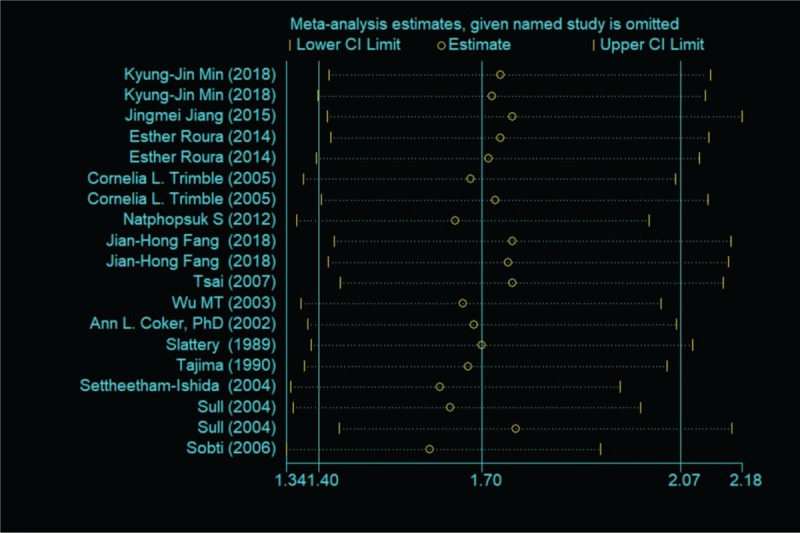
The result of sensitivity analysis.
3.6. Publication bias
Figure 4 showed the funnel plot was obviously asymmetrical, indicating that publication bias was found in the studies, as suggested by Begg's test (P = .054) and Egger's test (P = .011). In addition, the “fill and trim” method identified 5 hypothetical studies. The recalculated overall result continued to display a positive association between passive smoking and cervical cancer (OR = 1.33, 95% CI: 1.21–1.47).
Figure 4.
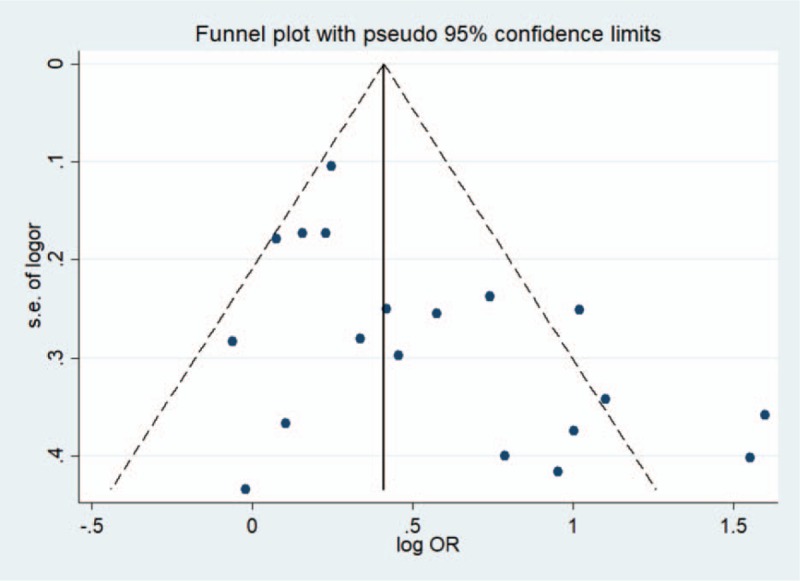
Funnel plots on the association of passive smoking with cervical cancer risk.
4. Discussion
This meta-analysis takes passive smoking and cervical cancer into consideration to explore the association of passive smoking with cervical cancer. The result of this meta-analysis suggested that passive smoking was associated with increased risk of incident cervical cancer (OR = 1.70). Additionally, the association persisted across almost subgroups stratified by continent, study design, quality score, and cervical cancer types/phases.
Most previous research have focused on the risk of active smoking and cervical cancer, but more and more evidence has suggested that second-hand smoke may also be a concern.[25] One meta-analysis[12] has recently summarized the data for this topic, but they did not include cohort studies, and included some cross-sectional studies in this analysis. A recent analysis[28] estimated that more than 30% of never smokers worldwide were exposed to second-hand tobacco smoke, accounting for 0.7% of disease burden and 1% of global total mortality. Nevertheless, cervical cancer was not included in that analysis of disease burden.
This meta-analysis was conducted on 4 cohort studies and 10 case–control studies with 384,995 participants altogether to provide robust and reliable results. The heterogeneity in our analysis may be caused by differences in the study population, study design, and follow-up duration. The sensitivity analysis demonstrated that our results were reliable and stable. We also explored the publication bias by using the method of “fill and trim,” with the exception of funnel plot.
The biological mechanisms linking passive smoking with cervical neoplasm are not well characterized. Nevertheless, several mechanisms are considered to have a significant role. First, incessant smoking may weaken the immune function,[29] thus increasing the risk of HPV infection. HPV is regarded as the most essential causative factor for cervical cancer.[30,31] Second, nicotine has been proved to promote tumor development.[32] Finally, pharmacokinetic interactions with smoke may well have a significant impact on the efficacy and toxicity of anticancer drugs.[33]
Several limitations of this meta-analysis should be acknowledged. Firstly, the state of smoking in most studies was mainly self-reported and was not confirmed by biochemical analysis, so there was recall bias. We were unable to assess the impact of changes in smoking status on the risk of cervical cancer during follow-up. Secondly, this meta-analysis only included English publications, and the possibility of unpublished reports was not yet recognized. Both literature screening and data extraction were directed independently by 2 researchers, thus selection bias was improbable. However, we have tried a variety of sources to screen for appropriate studies, and the method of “fill and trim” proposed that publication bias does not materially alter these associations. Thirdly, we did not study the association between different cigarette exposure levels and the risk of cervical cancer, because there was not enough dose-response relationship information in our studies.
In conclusion, this meta-analysis provides evidence that passive smoking is associated with an increased risk of cervical cancer. There are still some limitations in this meta-analysis. For further research, the dose-response relationship is necessary to be studied. We suggest that legislation to limit smoking in indoor spaces is implemented to protect women, as has been implemented in several countries such China, America. Given the pervasiveness of smoking in numerous countries and the growing number of patients with cervical carcinoma in the world, decreasing cigarette use should be listed as a vital public health strategy to prevent and control the worldwide cervical carcinoma epidemic. In order to reduce the current tobacco consumption among smokers, further efforts are approved to enforce the provisions of WHO Framework Convention on Tobacco Control.[34] Smoke free policy can offer protections for never smokers and may increase the number of successful quitters.
Acknowledgments
We appreciate the work of editors and anonymous reviewers.
Author contributions
Conceptualization: Wen Qin.
Data curation: Feng Xue.
Formal analysis: Xiaomin Wei.
Methodology: Qiangdong Guan.
Software: Wenchong Jiang.
Supervision: Shue Wang.
Visualization: Mengmeng Xu.
Writing – original draft: Benyu Su.
Writing – review & editing: Sufang Yu.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ORs = odds ratios, NOS = Newcastle–Ottawa quality scale, REM = random effects model.
Authors’ note: BS and WQ contributed equally to this work.
Funding: This study was funded by National Natural Science Funding of China (No. 81372969).
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [2].Min K-J, Lee J-K, So KA, et al. Association between passive smoking and the risk of cervical intraepithelial neoplasia 1 in Korean women. J Epidemiol 2017;28:48–53. advpub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bond S. Large prospective study finds no association between oral contraceptive use and breast cancer but increased risk for cervical cancer. J Midwifery Womens Health 2014;59:218–9. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez D, Suarez EL, Ortiz AP. Cervical cancer screening and sexual risky behaviors among a population of hispanic origin. Womens Health Issues 2015;25:254–61. [DOI] [PubMed] [Google Scholar]
- [5].Roura E, Castellsague X, Pawlita M, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer 2014;135:453–66. [DOI] [PubMed] [Google Scholar]
- [6].Akl EA, Gaddam S, Gunukula SK, et al. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol 2010;39:834–57. [DOI] [PubMed] [Google Scholar]
- [7].WHO. WHO global report: mortality attributable to tobacco 2012. 2013. http://www.who.int/tobacco/publications/ [Google Scholar]
- [8].Lee W, Hwang SH, Choi H, et al. The association between smoking or passive smoking and cardiovascular diseases using a Bayesian hierarchical model: based on the 2008–2013 Korea Community Health Survey. Epidemiol Health 2017;39:e2017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jung KJ, Jeon C, Jee SH. The effect of smoking on lung cancer: ethnic differences and the smoking paradox. Epidemiol Health 2016;38:e2016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Churg A, Hall R, Bilawich A. Respiratory bronchiolitis with fibrosis-interstitial lung disease: a new form of smoking-induced interstitial lung disease. Arch Pathol Lab Med 2015;139:437–40. [DOI] [PubMed] [Google Scholar]
- [11].Louie KS, Castellsague X, de Sanjose S, et al. Smoking and passive smoking in cervical cancer risk: pooled analysis of couples from the IARC multicentric case–control studies. Cancer Epidemiol Biomarkers Prev 2011;20:1379–90. [DOI] [PubMed] [Google Scholar]
- [12].Zeng XT, Xiong PA, Wang F, et al. Passive smoking and cervical cancer risk: a meta-analysis based on 3,230 cases and 2,982 controls. Asian Pac J Cancer Prev 2012;13:2687–93. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [15].Jiang JM, Pang HY, Liu BQ, et al. Effects of active, passive, and combined smoking on cervical cancer mortality: a nationwide proportional mortality study in Chinese urban women. Cancer Causes Control 2015;26:983–91. [DOI] [PubMed] [Google Scholar]
- [16].Trimble CL, Genkinger JM, Burke AE, et al. Active and passive cigarette smoking and the risk of cervical neoplasia. Obstetr Gynecol 2005;105:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu MT, Lee LH, Ho CK, et al. Lifetime exposure to environmental tobacco smoke and cervical intraepithelial neoplasms among nonsmoking Taiwanese women. Arch Environ Health 2003;58:353–9. [PubMed] [Google Scholar]
- [18].Fang JH, Yu XM, Zhang SH, et al. Effect of smoking on high-grade cervical cancer in women on the basis of human papillomavirus infection studies. J Cancer Res Ther 2018;14supplement:S184–9. [DOI] [PubMed] [Google Scholar]
- [19].Tsai HT, Tsai YM, Yang SF, et al. Lifetime cigarette smoke and second-hand smoke and cervical intraepithelial neoplasm—a community-based case–control study. Gynecol Oncol 2007;105:181–8. [DOI] [PubMed] [Google Scholar]
- [20].Coker AL, Bond SM, Williams A, et al. Active and passive smoking, high-risk human papillomaviruses and cervical neoplasia. Cancer Detect Prev 2002;26:121–8. [DOI] [PubMed] [Google Scholar]
- [21].Slattery ML, Robison LM, Schuman KL, et al. Cigarette smoking and exposure to passive smoke are risk factors for cervical cancer. JAMA 1989;261:1593–8. [PubMed] [Google Scholar]
- [22].Tajima K, Hirose K, Ogawa H, et al. Hospital epidemiology—a comparative case control study of breast and cervical cancers. Japan J Cancer Clin 1990;Spec No:351–64. [PubMed] [Google Scholar]
- [23].Settheetham-Ishida W, Singto Y, Yuenyao P, et al. Contribution of epigenetic risk factors but not p53 codon 72 polymorphism to the development of cervical cancer in Northeastern Thailand. Cancer Lett 2004;210:205–11. [DOI] [PubMed] [Google Scholar]
- [24].Sull JW, Jee SH, Yi S, et al. The effect of methylenetetrahydrofolate reductase polymorphism C677T on cervical cancer in Korean women. Gynecol Oncol 2004;95:557–63. [DOI] [PubMed] [Google Scholar]
- [25].Min KJ, Lee JK, So KA, et al. Association between passive smoking and the risk of cervical intraepithelial neoplasia 1 in Korean women. J Epidemiol 2018;28:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Natphopsuk S, Settheetham-Ishida W, Sinawat S, et al. Risk factors for cervical cancer in northeastern Thailand: detailed analyses of sexual and smoking behavior. Asian Pac J Cancer Prev 2012;13:5489–95. [DOI] [PubMed] [Google Scholar]
- [27].Sobti RC, Kaur S, Kaur P, et al. Interaction of passive smoking with GST (GSTM1, GSTT1, and GSTP1) genotypes in the risk of cervical cancer in India. Cancer Genet Cytogenet 2006;166:117–23. [DOI] [PubMed] [Google Scholar]
- [28].Oberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet (London, England) 2011;377:139–46. [DOI] [PubMed] [Google Scholar]
- [29].Sabra S, Gratacos E, Gomez Roig MD. Smoking-induced changes in the maternal immune, endocrine, and metabolic pathways and their impact on fetal growth: a topical review. Fetal Diagn Ther 2017;41:241–50. [DOI] [PubMed] [Google Scholar]
- [30].Wardak S. Human Papillomavirus (HPV) and cervical cancer. Med Dosw Mikrobiol 2016;68:73–84. [PubMed] [Google Scholar]
- [31].Song D, Li H, Li HB, et al. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer (Review). Oncol Lett 2015;10:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Warren GW, Singh AK. Nicotine and lung cancer. J Carcinog 2013;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Condoluci A, Mazzara C, Zoccoli A, et al. Impact of smoking on lung cancer treatment effectiveness: a review. Future Oncol 2016;12:2149–61. [DOI] [PubMed] [Google Scholar]
- [34].Organization WH. WHO Framework Convention on Tobacco Control. 2009. http://www.who.int/fctc/en/ [Google Scholar]


