Supplemental Digital Content is available in the text
Keywords: cancer antigen125, chronic obstructive pulmonary disease, high altitude, pleural effusions
Abstract
Chronic obstructive pulmonary disease (COPD) is the most frequently encountered progressive lung disease in clinical practice. This study sought to determine the predictive ability of the tumor biomarker cancer antigen-125 (CA-125) in the identification of COPD in a cohort of 284 patients with COPD living at high altitude (with an average elevation of over 2500 m).
Patients were classified by pleural effusion volumes into 4 categories and serum CA-125 concentrations were measured in each category. The analyses revealed that CA-125 concentrations were positively and significantly correlated with pleural effusion volume. CA-125 concentrations were also positively correlated with pulmonary heart disease and acute exacerbations of COPD, and negatively correlated with pulmonary hypertension.
The study evidence suggests that serum CA-125 concentrations are positively correlated with the risk of pleural effusions among patients with COPD living in high-altitude areas, and that CA-125 concentrations are also correlated with pulmonary heart disease, acute exacerbations, and pulmonary hypertension.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is the most frequently encountered progressive lung disease in clinical practice and is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious inhaled particles or gases.[1,2] The burden of COPD is substantial, as it is a major cause of chronic morbidity and mortality worldwide and is estimated to be the 3rd leading cause of death by 2020.[1,2]
Special consideration is warranted for patients with COPD native to high altitude (defined as beginning at 2400 m [8000 feet] above sea level[3]), with investigations showing that these patients have higher pulmonary artery pressure (PAP) values and right ventricle (RV) dimensions as compared with healthy volunteers living at the same altitude.[4] Notably, a significant fraction of these patients has possible or likely pulmonary hypertension (PH) based on echocardiographic measurements[4] and it is recognized that right-sided cardiac structural changes are associated with reduced exercise capacity in nonsevere COPD.[5] Even healthy children born and living at high altitudes display structural changes in RV measurements.[4] Interestingly, altitude itself does not apparently significantly influence COPD prevalence, although living at high altitude is linked to an increased risk of undiagnosed COPD.[6]
The tumor biomarker cancer antigen-125 (CA-125) may help to identify RV failure in patients with COPD. Elevated levels of CA-125 correlate with markers of RV dysfunction and PAP in COPD.[7] Moreover, serum CA-125 can be used to identify patients with COPD who have PH,[8] especially those with acute exacerbation of COPD,[9] and assist with risk stratification in COPD, by predicting long-term mortality.[10]
A pleural effusion is excessive fluid that accumulates in the pleural cavity, which can impair breathing[11] and is commonly found among patients presenting with respiratory symptoms.[12,13] Pleural effusions are caused by a variety of diseases, including pulmonary infections, pleural tumor metastasis, and tuberculous pleurisy[14]; the latter 2 conditions are difficult to differentiate in clinical practice. Accurate diagnosis of pleural effusion is extremely important for the treatment and prognosis of patients.
Pleural effusion can be diagnosed using the following imaging techniques: plain chest radiography (CXR), chest computed tomography (CT), or thoracic ultrasound. However, CXR can miss a large number of effusions, including as many as 10% of parapneumonic effusions[15]; CT is costly and not always easy to perform on patients, and moreover they are exposed to approximately 7 mSv of ionizing radiation.[16] Therefore, highly sensitive and specific biomarkers that are convenient and apply to differential diagnoses of pleural effusions are urgently needed.[14] Up until now, no clear association has been observed between serum CA-125 levels and radiological presentation, including pleural effusion, in patients with COPD.
The aim of this study was to determine whether serum CA-125 concentrations correlate with the development of pleural effusion in COPD, and whether CA-125 concentration plays any significant role in the risk stratification of patients with COPD residing in high-altitude areas. We classified patients with COPD by pleural effusion volumes and measured serum CA-125 concentrations in each group. We found that CA-125 concentrations correlated with pleural effusion volumes. These results suggest that CA-125 concentrations may be used to detect the pleural effusion of patients with COPD living in high-altitude areas, especially those who cannot tolerate invasive examinations such as thoracoscopy for distinguishing pleural effusions.[17]
2. Materials and methods
2.1. Subjects
This study recruited 284 patients who were diagnosed with stages I to IV COPD as according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 criteria[18] between August 2015 and June 2016 at outpatient clinics, the intensive care unit (ICU) and during hospitalization in Qinghai Red Cross Hospital, China. Inclusion criteria at baseline specified that patients had a postbronchodilator FEV1:FVC ratio of <70%; an improvement in FVC or FEV1 of <12% after inhaled β-agonist therapy; pleural effusions; and were aged more than 20 years. Exclusion criteria included active inflammatory disease, suspicion of malignancy, mental illness, pregnancy, and age below 20 years.
Healthy control subjects (n = 292) were people who regularly attended the physical examination center at the Qinghai Red Cross Hospital for Nationalities. Inclusion criteria specified that subjects did not have COPD and had never received standard COPD treatments; all participants had to be aged over 20 years. Exclusion criteria included any mental illness, cardiovascular disease, malignant tumor, hypertension, diabetes mellitus, renal or liver dysfunction, acute or chronic infection, pregnancy, and age under 20 years. All study subjects were born and living in a high-altitude plateau with an average elevation of over 2500 m. The study was approved by the Qinghai Red Cross Hospital Institutional Review Board (IRB number: 2017-11-29-104) and it was conducted in compliance with national legislation and the Declaration of Helsinki guidelines. All participants submitted informed written consent before enrollment.
2.2. Diagnosis of pleural effusion
Diagnostic criteria and pleural effusion volumes were determined using the Philips iE33 Ultrasound System (Andover, MA), according to previously described methodology.[11,15] Briefly, the ultrasonographic examination was first done with the patient flatly supine (no pillow or head rest) to obtain values for the supine formulae. With the transducer positioned perpendicular to the chest wall, the chest was insonated at the laterodorsal/posterolateral part of the chest wall through the intercostal spaces. Measurements were taken at maximum inspiration, with the patients holding their breath. The maximum perpendicular (interpleural) distance between the posterior surface of the lung (visceral pleura) and the posterior chest wall (parietal pleura) was obtained. Patients then sat in a fully erect position (no slouching or reclining) and measurements (in centimeters) were taken for the erect formulae. The dorsolateral/posterolateral aspect of the chest wall was insonated through the intercostal spaces with the transducer oriented longitudinally along the long axis of the chest. The craniocaudal extent (lateral height) of the effusion and the lung base-to-diaphragm distance were measured at end-expiration. Each measurement was repeated 3 times and the average value was recorded. All procedures were performed and findings interpreted by an experienced intensivist. Effusion volume estimates were calculated as follows: Erect: EV = X × 90, where EV = estimated effusion volume (mL); X = craniocaudal extent (cm) of the effusion at the dorsolateral chest wall measured in erect/sitting position with the probe oriented longitudinally; 90 = empirical factor/constant.[11]
The patients were classified into 4 categories by the volumes of pleural effusion: (I) minimal volume, <240 mL; (II) small volume, 300 to 500 mL; (III) median volume, 500 to 800 mL; and (IV) large volume, >800 mL.
2.3. Laboratory measurements
Subjects were asked to fast for at least 8 hours before a blood draw; all blood samples were analyzed within 24 hours. Serum protein concentrations of tumor biomarkers (carcinoembryonic antigen [CEA], alpha-fetoprotein [AFP], CA-125, CA-15-3, and CA-19-9) and inflammatory risk markers (B-type natriuretic peptide [BNP], and C-reactive protein [CRP]) were determined by chemiluminescence immunoassay (Simens Centaur XP, Tarrytown, NY), according to the manufacturer's instructions.
2.4. Statistical analysis
The analysis used SPSS Statistics 20.0 software package for Windows (IBM Corporation, Somers, NY). All values are expressed as the mean ± standard deviation. For normally distributed data sets, the Student t test and 1-way analysis of variance were used to compare differences between 2 variables or more than 2 groups. The Fisher exact test or Chi-squared test (χ2 test) was used to compare qualitative characteristics between multiple groups. In all cases, P-values of <.05 were defined as statistically significant.
3. Results
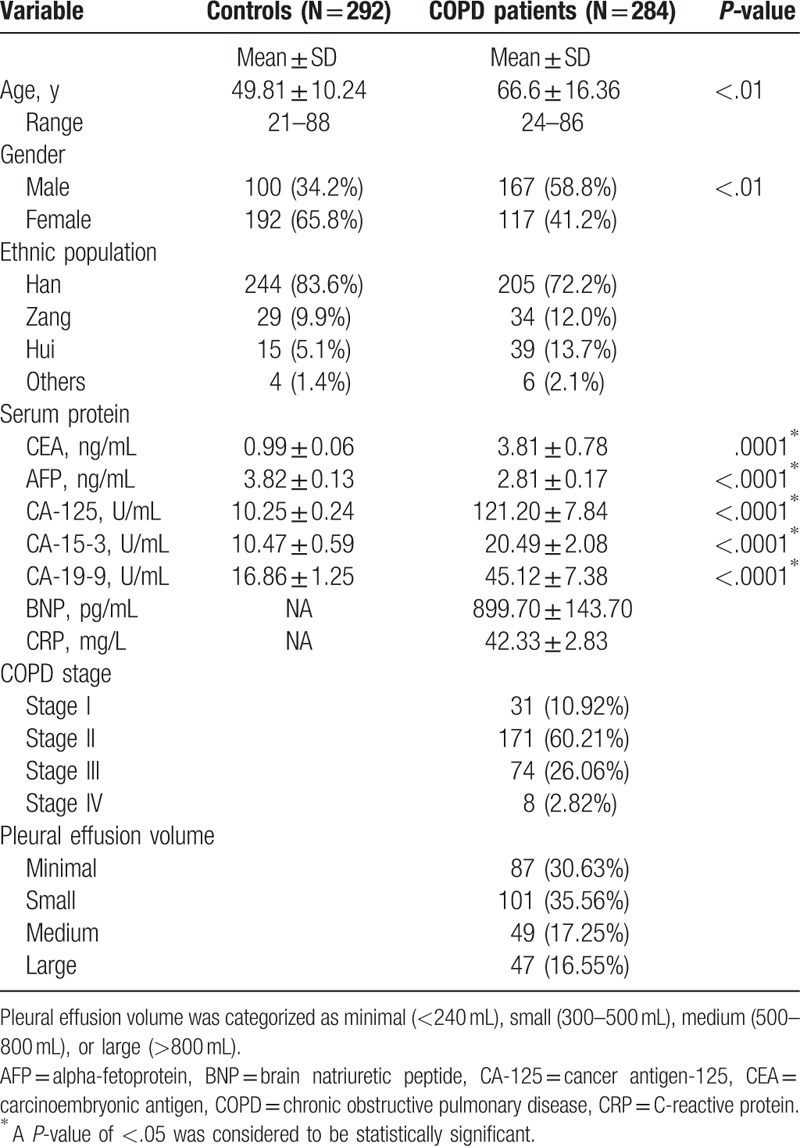
This study evaluated differences in the general demographic characteristics of 292 healthy controls and 284 patients with stages I to IV COPD; all participants were living in a high-altitude area with an average elevation of over 2500 m. The demographic characteristics of the participants are shown in Table 1. Compared with the patients, controls were significantly younger and had significantly fewer males. Most of the patients had moderate (stage II) stable COPD. Pleural effusion volumes were defined as minimal, small, medium, and large; around two-thirds of the COPD cohort had minimal or small pleural effusion volumes (Table 1). There was no correlation between age and CA-125 concentrations in either the controls or COPD cohort (Supplementary Fig. 1). Likewise, these analyses failed to find any correlations between gender and CA-125 concentrations (data not shown). We adjusted for the effects of age and gender by multiple logistic regression models in each of the following results.
Table 1.
Demographic and clinical characteristics for 284 Chinese patients with COPD and 292 healthy controls.
Different tumor-associated biomarkers, including CEA, AFP, CA-125, CA-15-3, CA-19-9, BNP, and CRP, were also investigated in this study. We found higher mean serum protein concentrations, particularly for CA-125, in patients with COPD than in controls (Table 1). We therefore sought to determine whether the high CA-125 expression correlated with pleural effusion volumes, by classifying study participants into 5 groups (i.e., control, minimal-, small-, medium- and large-volume groups). We found significant correlations between CA-125 concentrations and pleural effusion volumes in each group (Fig. 1A and Table 2). Further investigations revealed that this phenomenon did not differ by ethnicity (Fig. 1B–F).
Figure 1.
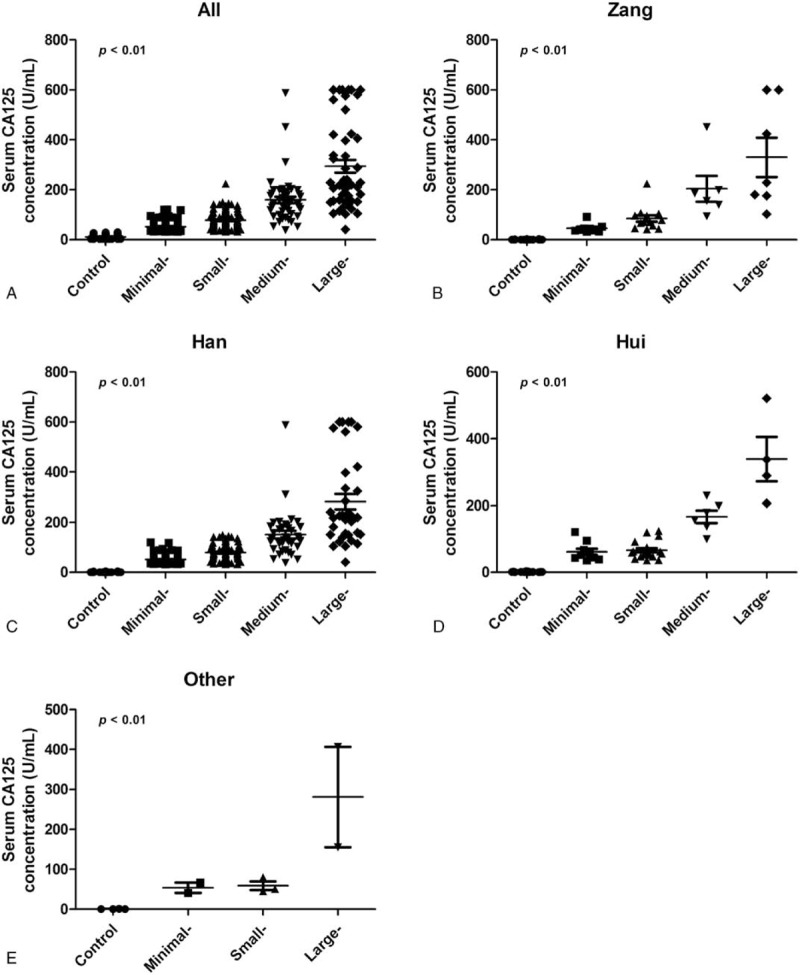
Correlation of serum cancer antigen-125 (CA-125) concentration with pleural effusion volume. Serum CA-125 levels in patients with chronic obstructive pulmonary disease pleural effusion in each group. (A) Overall population; (B) Zang ethnic population; (C) Han ethnic population; (D) Hui ethnic population; (E) other ethnic population.
Table 2.
Associations between pleural effusion volume, serum CA-125 concentrations, and disease-related complications in COPD patients.
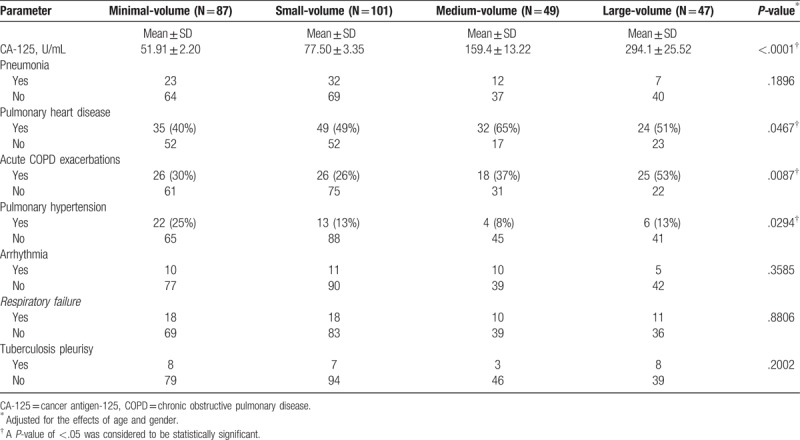
Pneumonia, pulmonary heart disease, acute exacerbations, PH, arrhythmia, respiratory failure, and tuberculosis pleurisy are all commonly reported complications of COPD. We therefore investigated the relationships between these complications and pleural effusion volume in our COPD cohort. As shown in Table 2, we found higher rates of both pulmonary heart disease and acute COPD exacerbations in the medium- and large-volume groups than in the minimal- and small-volume groups. Unexpectedly, we found lower rates of PH in the medium- and large-volume groups than in the minimal- and small-volume groups. In contrast, no correlations were seen between pneumonia, arrhythmia, respiratory failure, or tuberculosis pleurisy with pleural effusion volume (Table 2).
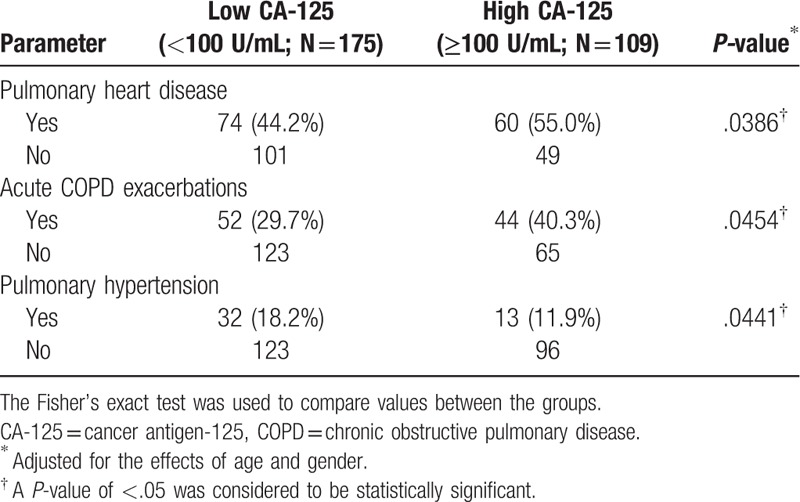
We further investigated the positive correlation between serum CA-125 concentrations and pleural effusion volume by classifying CA-125 concentrations as low or high (Table 3). We found that pulmonary heart disease and acute COPD exacerbations occurred significantly more often in the high CA-125 group than in the low CA-125 group. Interestingly, fewer patients in the high CA-125 cohort had PH, as compared with the low CA-125 cohort (Table 3). Pulmonary heart disease and acute COPD exacerbations appear to be positively correlated with serum CA-125 concentrations, while PH appears to be negatively correlated with serum CA-125 concentrations.
Table 3.
Demographic and selected clinical data of COPD patients categorized by serum CA-125 concentrations.
Our study results indicate that serum CA-125 concentrations may serve as a biomarker to determine those patients with COPD who are at risk of pleural effusions and other disease-related complications such as pulmonary heart disease, acute exacerbations, or PH.
4. Discussion
Although COPD is commonly observed worldwide, prevalence estimates vary widely by time, geography, or other factors beyond age and smoking, which can only partly explain its population variability.[18] Previous research has reported serum CA-125 concentrations may serve as an independent predictor of risk stratification and long-term mortality in patients with COPD.[8–10] Similarly, in this study, CA-125 concentrations were 12-fold higher among the patients with COPD as compared with healthy controls; rates of pulmonary heart disease and acute COPD exacerbations were significantly higher among the patients with COPD with high CA-125 concentrations (≥100 U/mL) than among those with low CA-125 concentrations (<100 U/mL). Thus, CA-125 may serve as a biomarker for disease severity in COPD.
Several studies have suggested that age may affect serum CA-125 concentrations,[19–21] especially in patients with COPD who are aged over 70 years.[21] However, in this study, we found that age did not significantly affect either CA-125 concentrations or volumes of pleural effusion, which is consistent with other studies from China.[22,23] Whether the differences between studies in relation to age and CA-125 concentrations are due to differences between worldwide regions or ethnicities needs to be explored in future research. We were also interested in knowing whether gender affects the predictive ability of CA-125 concentrations. Previously study has shown that the primary tumor site had no effect on the predictive ability of CA-125 concentration.[24] However, CA-125 concentration has been studied rarely in gender. Recently, one research mentioned that CA-125 has been evaluated as a marker of colorectal cancer, and its accuracy in men is controversial.[24] In our study, we found that average CA-125 concentrations in patients with COPD did not differ significantly between men and women (data not shown).
Pleural effusions are a common finding among patients presenting with respiratory symptoms[25] and also among patients with COPD admitted to a medical ICU,[15] which suggests that evaluating pleural effusion volume is crucial for patient management and prognosis. Many researchers have attempted to identify a reliable marker of pleural effusion. However, no existing diagnostic marker has high sensitivity and specificity,[26] especially for detecting pleural effusion volume. Here, we found that serum CA-125 concentrations were significantly associated with pleural effusion volumes in multi-ethnic patients with COPD living in high-altitude areas. This suggests that CA-125 concentrations may serve as a noninvasive marker of pleural effusion volume in patients with COPD and may be especially useful for those who cannot tolerate invasive examinations such as thoracoscopy.[17,26]
The COPD-related complications can greatly impact quality of life, morbidity, and mortality.[12,27–31] In this study, pulmonary heart disease and acute COPD exacerbations were positively correlated with pleural effusion volume as well as with CA-125 concentrations. Previous research has reported that some tumor biomarkers, such as CEA, AFP, CA-125, CA-15-3, and CA-19-9 are associated with COPD exacerbations[32] and that CA-125 concentrations are elevated in patients experiencing COPD exacerbations.[9,32] Similarly, our data demonstrate an association between serum CA-125 concentrations, COPD exacerbations and pleural effusion volume in patients with COPD living in high-altitude areas, regardless of ethnicity.
Commonly reported complications reported with worsening COPD include pneumonia, pulmonary heart disease, acute exacerbation, PH, arrhythmia, respiratory failure, and tuberculosis pleurisy.[12,27–31] A number of evidences suggested that altitude adversely influences health, vs medical conditions.[6,33,34] For instance, PH and right-sided heart failure are more prevalent in high-altitude areas[6,33] and patients with COPD living at high altitude have higher PAP values than their counterparts living at sea level.[34] Previous research has indicated that congestive heart failure increases CA-125 levels.[35] However, as our study had only 3 patients with COPD with congestive heart failure, we could not perform a comparative analysis of the relationship of congestive heart failure, pleural effusion volume, and CA-125 levels.
Interestingly, we found a negative correlation between pleural effusion volume and serum CA-125 concentrations with PH. These results differ from other studies, which have reported that patients with COPD with PH have significantly higher CA-125 concentrations than their counterparts without PH,[8,9] although none of those patients were living in high-altitude areas. Moreover, our findings might differ from those of other studies[8,9] might be due to the small number of patients in our study who had PH (only 15.8% of patients in our COPD cohort). Further research is needed to explain the discordant findings. Above all, our findings suggest that serum CA-125 concentrations correlate with pleural effusion volumes in patients with COPD living in high-altitude areas. Serum CA-125 may serve as a biomarker for pleural effusion volume, pulmonary heart disease, acute exacerbations, and PH.
There are some limitations of this study. First, it is a single-center, retrospective study. Second, some potentially important prognostic data are missing, such as nutritional status of the study participants, outpatient medications, functional status before admission, and smoking habits. Third, we may have underestimated the number of readmissions, as we did not include admissions from other hospitals. However, despite these omissions, the results of this study contribute important information about patients with COPD living in high-altitude areas.
5. Conclusion
We found that serum CA-125 concentrations correlated with pleural effusion volumes. Thus, CA-125 concentrations may be used to detect pleural effusions in patients with COPD living in high-altitude areas, especially in those unable to tolerate invasive examinations such as thoracoscopy. Using CA-125 as a marker to estimate the amount of pleural effusion might make testing and diagnosis safer, less expensive, easier, and faster.
Acknowledgments
The authors thank Iona J. MacDonald for careful reading of the manuscript and revision of the text. The authors also like to thank QingHai Science & Technology Department (2017-SF-120) for all the help of this study.
Author contributions
Conceptualization: Shengmei Li, Hsiao-Chi Tsai.
Data curation: Shengmei Li.
Formal analysis: Hsiao-Chi Tsai.
Investigation: Shengmei Li, Huiying Ma, Xiuqing Ma.
Methodology: Shangjie Wu.
Project administration: Huiying Ma.
Resources: Lijun Gan.
Supervision: Lijun Gan.
Validation: Mangui Li.
Writing – original draft: Hsiao-Chi Tsai.
Writing – review & editing: Chih-Hsin Tang.
Supplementary Material
Footnotes
Abbreviations: AFP = alpha-fetoprotein, BNP = B-type natriuretic peptide, CA-125 = cancer antigen-125, CEA = carcinoembryonic antigen, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CT = chest computed tomography, CXR = plain chest radiography, GOLD = chronic obstructive lung disease, ICU = intensive care unit, PAP = pulmonary artery pressure, PH = pulmonary hypertension, RV = values and right ventricle.
This work was supported by the QingHai Science & Technology Department (2017-SF-120).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology 2017;22:575–601. [DOI] [PubMed] [Google Scholar]
- [2].National Heart L, and Blood Institute, National Institutes of Health, USA, and the World Health Organization. The Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017. Accessed on November 16, 2016. Available at: http://goldcopd.org/. [Google Scholar]
- [3].Healy K, Labrique AB, Miranda JJ, et al. Dark adaptation at high altitude: an unexpected pupillary response to chronic hypoxia in Andean highlanders. High Alt Med Biol 2016;17:208–13. [DOI] [PubMed] [Google Scholar]
- [4].Guvenc TS, Kul S, Dogan C, et al. Assessment of right ventricular geometry and mechanics in chronic obstructive pulmonary disease patients living at high altitude. Int J Cardiovasc Imaging 2014;30:1305–13. [DOI] [PubMed] [Google Scholar]
- [5].Fenster BE, Holm KE, Weinberger HD, et al. Right ventricular diastolic function and exercise capacity in COPD. Respir Med 2015;109:1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Horner A, Soriano JB, Puhan MA, et al. Altitude and COPD prevalence: analysis of the PREPOCOL-PLATINO-BOLD-EPI-SCAN study. Respir Res 2017;18:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Falcao F, de Oliveira FRA, da Silva M, et al. Carbohydrate antigen 125: a promising tool for risk stratification in heart diseases. Biomark Med 2018;12:367–81. [DOI] [PubMed] [Google Scholar]
- [8].Rahimi-Rad MH, Rahimi P, Rahimi B, et al. Serum CA-125 level in patients with chronic obstructive pulmonary disease with and without pulmonary hypertension. Pneumologia 2014;63:164–6. [PubMed] [Google Scholar]
- [9].Zhang M, Li YL, Yang X, et al. Clinical significance of serum carbohydrate antigen 125 in acute exacerbation of chronic obstructive pulmonary disease [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 2016;36:1386–9. [PubMed] [Google Scholar]
- [10].Kaya H, Zorlu A, Yucel H, et al. Cancer antigen-125 levels predict long-term mortality in chronic obstructive pulmonary disease. Biomarkers 2015;20:162–7. [DOI] [PubMed] [Google Scholar]
- [11].Ibitoye BO, Idowu BM, Ogunrombi AB, et al. Ultrasonographic quantification of pleural effusion: comparison of four formulae. Ultrasonography 2018;37:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jernigan NL, Resta TC, Gonzalez Bosc LV. Altered redox balance in the development of chronic hypoxia-induced pulmonary hypertension. Adv Exp Med Biol 2017;967:83–103. [DOI] [PubMed] [Google Scholar]
- [13].Lee J, Lee YD, Lim JK, et al. Predictive factors and treatment outcomes of tuberculous pleural effusion in patients with cancer and pleural effusion. Am J Med Sci 2017;354:125–30. [DOI] [PubMed] [Google Scholar]
- [14].Gao Y, Ou Q, Wu J, et al. Potential diagnostic value of serum/pleural fluid IL-31 levels for tuberculous pleural effusion. Sci Rep 2016;6:20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care 2017;21:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med 2015;10:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoon DW, Cho JH, Choi YS, et al. Predictors of survival in patients who underwent video-assisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thorac Cancer 2016;7:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stolz D, Barandun J, Borer H, et al. Diagnosis, prevention and treatment of stable COPD and acute exacerbations of COPD: The Swiss Recommendations 2018. Respiration 2018;96:382–98. [DOI] [PubMed] [Google Scholar]
- [19].Pauler DK, Menon U, McIntosh M, et al. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev 2001;10:489–93. [PubMed] [Google Scholar]
- [20].Barcelo B, Ayllon O, Belmonte M, et al. Proposed reference value of the CA 125 tumour marker in men. Potential applications in clinical practice. Clin Biochem 2008;41:717–22. [DOI] [PubMed] [Google Scholar]
- [21].Sikaris KA. CA125 - a test with a change of heart. Heart Lung Circ 2011;20:634–40. [DOI] [PubMed] [Google Scholar]
- [22].Zhou H, Dong A, Xia H, et al. Associations between CA19-9 and CA125 levels and human epidermal growth factor receptor 2 overexpression in patients with gastric cancer. Oncol Lett 2018;16:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luo T, Chen W, Wang L, et al. CA125 is a potential biomarker to predict surgically incurable gastric and cardia cancer: a retrospective study. Medicine (Baltimore) 2016;95:e5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huang CJ, Jiang JK, Chang SC, et al. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore) 2016;95:e5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Botana-Rial M, Nunez-Delgado M, Leiro-Fernandez V, et al. Current management of pleural effusion: results of a national survey. Arch Bronconeumol 2018;pii: S0300-2896(18)30316-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [26].Ji M, Zhu X, Dong J, et al. Combination of procalcitonin, C-reaction protein and carcinoembryonic antigens for discriminating between benign and malignant pleural effusions. Oncol Lett 2018;16:1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Halpin DM, Miravitlles M, Metzdorf N, et al. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis 2017;12:2891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vozoris NT, Wang X, Austin PC, et al. Incident diuretic drug use and adverse respiratory events among older adults with chronic obstructive pulmonary disease. Br J Clin Pharmacol 2018;84:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shukla SD, Hansbro PM, Walters EH. Upregulated pneumococcal adhesion molecule (platelet-activating factor receptor) may predispose COPD patients to community-acquired pneumonia. Int J Chron Obstruct Pulmon Dis 2017;12:3111–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sangwan V, Chaudhry D, Malik R. Dyspnea, eosinopenia, consolidation, acidemia and atrial fibrillation score and BAP-65 score, tools for prediction of mortality in acute exacerbations of chronic obstructive pulmonary disease: a comparative pilot study. Indian J Crit Care Med 2017;21:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Irfan M. Post-tuberculosis pulmonary function and noninfectious pulmonary disorders. Int J Mycobacteriol 2016;5:S57. [DOI] [PubMed] [Google Scholar]
- [32].Barouchos N, Papazafiropoulou A, Iacovidou N, et al. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand J Clin Lab Invest 2015;75:126–32. [DOI] [PubMed] [Google Scholar]
- [33].Mirrakhimov AE, Strohl KP. High-altitude pulmonary hypertension: an update on disease pathogenesis and management. Open Cardiovasc Med J 2016;10:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang Y, Zha-Xi DJ, Mao W, et al. Comparison of echocardiographic parameters between healthy highlanders in Tibet and lowlanders in Beijing. High Alt Med Biol 2018;19:259–64. [DOI] [PubMed] [Google Scholar]
- [35].Stanciu AE, Stanciu MM, Vatasescu RG. NT-proBNP and CA 125 levels are associated with increased pro-inflammatory cytokines in coronary sinus serum of patients with chronic heart failure. Cytokine 2018;111:13–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


