Abstract
The aim of this nonrandomized controlled study (level 3)was to evaluate whether preoperative denosumab treatment can reduce intraoperative blood loss, facilitate surgical treatment, and improve local control of sacral giant-cell tumor (GCT).
Surgical treatment of sacral GCT is very difficult due to extensive bone destruction and complex anatomical structures. The huge intraoperative blood loss may interrupt surgical management and judgment of tumor range. Denosumab can inhibit the differentiation of osteoclast-like giant cells and bone destruction by blocking RANKL-RANK pathway.
Study group (preoperative denosumab treatment) and control group (no denosumab treatment) were matched for age, gender, tumor site, staging, and tumor size. In study group, enhanced computed tomography (CT) was performed before and after denosumab treatment. The comparison parameters between 2 groups: CT enhancement rate, intraoperative blood loss, and oncologic outcome.
The mean preoperative time of denosumab treatment was 5.2 months in study group. The mean CT enhancement rate of study group was 2.60 before treatment and 1.37 after treatment (P = .012). The posttreatment CT enhancement rate of study group was significantly lower than that of control group (P = .007). The mean intraoperative bleeding of study group and control group was 2166.7 and 5240 mL, respectively (P = .040). The mean operative time of study group and control group was 268.3 and 268.5 minutes, respectively (P = .997). The recurrence rate of study group (66.7%) was significantly higher than that of control group (0%) (P = .046).
Preoperative denosumab treatment has the tendency to reduce blood supply and intraoperative bleeding of sacral GCT. But the sclerosis and bony separation can increase the difficulty of tumor curettage and lead to high recurrence rate after denosumab treatment. It is necessary to study the best surgical opportunity after denosumab treatment and precise method to judge tumor range.
Keywords: blood loss, denosumab, giant cell tumor, sacrum, surgery
1. Introduction
Giant-cell tumor (GCT) of bone is a primary intramedullary bone tumor with local aggressiveness. It accounts for about 5% of primary bone tumors. Most of tumors occur in the epiphysis of long bone.[1,2] The sacral tumor accounts for 2% to 8% of GCT.[1,3] Because of low incidence of sacral GCT, there were not many reports. In 1982, Sung et al[4] reported 208 cases of GCT and only 4 cases were sacral tumors. Turcotte et al[3] reported a big sample study with 26 cases of sacral GCT in 1993. The early clinical symptoms of sacral GCT are occult and maybe confused with other nonspecific diseases, so it is difficult to detect tumor. When tumor grows bigger with extensive bone destruction and sacral nerve is involved, obvious symptoms can appear. Therefore, most of sacral GCT was Campanacci stage 3 which was different from limb GCT.[3,5–7]
Surgical treatment of sacral GCT is difficult due to extensive bone destruction and complex anatomical structures. Moreover, the huge intraoperative blood loss may interrupt surgical management and the judgment of tumor range. The treatment of sacral GCT is still challenging and controversial.[8] Wide or marginal resection may improve local control, but resection can lead to unsatisfactory postoperative function and high complications. Intralesional curettage may avoid nerve roots injury and preserve the integrity of pelvic ring, but high local recurrence is induced.[3,8,9] Therefore, it is important that how to improve safety and reduce blood loss in the premise of removing tumor completely.
The formation and regulation mechanisms of osteoclast-like multinucleated giant cells in GCT have become clear with related study on receptor activator of nuclear factor-κB (RANK) and receptor activator of nuclear factor-κB ligand (RANKL).[10–12] Denosumab is a new type of humanized RANKL monoclonal antibody which can combine with RANKL specifically and block RANKL-RANK pathway. So denosumab can inhibit the differentiation and activation of osteoclast-like giant cells by blocking RANKL-RANK pathway. A phase 2 clinical trial[13] with 35 cases of recurrent or unresectable GCT showed 86% of the patients had effective response and clinical benefit. Pain relief and functional improvement was presented. In our clinical practices, we found an interesting tendency that enhanced computed tomography (CT) value of sacral GCT seemed to decrease after denosumab treatment. Therefore, we performed this study to evaluate whether denosumab can reduce blood loss, facilitate surgical treatment and improve local control of on sacral GCT.
2. Materials and methods
2.1. Patients’ enrollment
All cases were from the musculoskeletal tumor database of our department. Inclusion criteria were as follows: pathologic diagnosis confirmed as GCT of bone; sacral tumor; stage 3 tumor; extensive bone destruction; tumor curettage was performed in our department. Six patients who received denosumab treatment before surgery were eligible for study group and 10 patients who did not receive denosumab treatment were matched as control group. Study and control groups were matched for the following factors: age, gender, tumor site, staging, and tumor size (measured by CT). The treatment period of study group was from December 2015 to September 2016. As all similar cases at the same time received denosumab treatment, the control group was selected from earlier period when denosumab was not applied in our department. The treatment period of control group was from January 2014 to September 2015. There was no significant difference between these 2 groups (Tables 1 and 2).
Table 1.
Matching of 2 groups of patients.
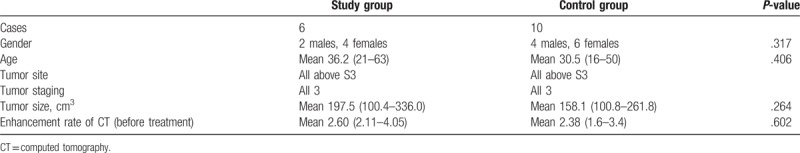
Table 2.
Clinical results of 2 groups of patients.

2.2. Denosumab treatment and CT evaluation procedures
All patients in study group complied with following conditions: patients were informed and consented for denosumab treatment; patients were adults or mature adolescents with GCT that was unresectable or surgical resection may result in severe morbidity; calcium and Vitamin D levels were normal; no contraindication of denosumab existed. Patients received subcutaneous denosumab 120 mg every 4 weeks, with additional doses administered on days 8 and 15 during the 1st month. Enhanced CT examination was performed before denosumab treatment and 12 weeks after treatment. If tumor was resectable or important structure can be retained, denosumab was stopped and the patient received surgery. If the above standard was not achieved, the patient continued treatment. All patients received CT examinations in the same CT machine. The injection volume, speed of contrast and CT scan times were same before and after treatment. About 4 to 8 levels of tumor were selected for evaluation. The CT value of the same tumor substance areas (liquid, cystic formation, and bone formation areas were excluded) were measured before and after treatment (Fig. 1). The CT enhancement rate was calculated as the ratio of enhanced CT value and unenhanced CT value of the same tumor area. It represents a percentage increase in tumor density on post- to precontrast CT images.
Figure 1.

The computed tomography (CT) changes of sacral giant-cell tumor before and after treatment in the same level of the tumor. The unenhanced CT (A) and enhanced CT (C) before treatment showed significant enhancement of lesion. The unenhanced CT (B) and enhanced CT (D) after treatment showed the increasing of sclerosis and decreasing of enhancement.
2.3. Surgical treatment
All patients received high selective tumor blood supply embolization preoperatively. A posterior approach was applied in operation. A median longitudinal incision was performed and the flap with fascia was opened. The gluteus maximus, erector spinae, and sacroiliac joint were exposed. Curettage of tumor was performed and high-speed burr was used until tumor was removed and normal bone was left. Tumor above S3 level received curettage. If tumor destructed sacrum below S3, distal part of tumor would be resected with clear margin.
2.4. Data record and follow-up
The following parameters were recorded and compared between 2 groups: CT enhancement rate; intraoperative blood loss; operative time; surgical treatment; and local recurrence. All patients were followed every 3 months postoperative. Physical examination, plain radiography, and sacral CT were performed every 3 months. The bone scanning and chest CT were performed every 6 months. Postoperative recurrence was recorded.
2.5. Statistical analysis
The data were analyzed by SPSS software (version 19.0; SPSS, Chicago, IL). The mean-value t test was performed to compare CT value, intraoperative blood loss, and operative time in different groups. The recurrence rates in different groups were compared by Chi-squared test or Fisher exact probability method. All statistical values were considered significant at P < .05.
3. Results
3.1. Denosumab treatment brought clinical benefits and had no significant side effects
The mean preoperative time of denosumab treatment was 5.2 (median 4.5, range 3–10) months in study group. The mean pre- and posttreatment visual analog scale (VAS) was 3.8 (2–6) and 0.7 (0–2), respectively (P = .001, F = 21.235). All 6 patients presented clinical benefits such as pain relief and increased function after treatment. All 6 patients tolerated well and no serious side effect was found.
3.2. Decreasing of CT enhancement rate and increasing of unenhanced CT value after denosumab treatment
The CT in study group showed mixed new bone formation with low-density areas in the lesion after denosumab treatment. Multiple uncommunicated bony sclerosis divisions were formatted in tumor. The sclerotic bony shell was found on the edge of tumor or soft-tissue mass (Fig. 1). The mean pre- and posttreatment unenhanced CT value was 42.0 (37–54) and 56.4 (41–70), respectively (P = .045, F = 5.431). The increasing of unenhanced CT value suggested the raising of osteogenesis and sclerosis.
The mean CT enhancement rate of study group was 2.60 (2.11–4.05) before treatment and 1.37 (1.11–1.65) after treatment (P = .012, F = 10.636) (Fig. 2). The decreasing of CT enhancement rate suggested the decreasing of tumor blood supply. The mean CT enhancement rate of control group was 2.38 (1.6–3.4). The pretreatment CT enhancement rate of study group was similar with control group (P = .602, F = 0.289); the posttreatment CT enhancement rate of study group was significantly lower than that of control group (P = .007, F = 10.829) (Fig. 3).
Figure 2.

The computed tomography enhancement rate of the 6 cases in the study group (before and after denosumab treatment, P = .012).
Figure 3.

The mean value of computed tomography (CT) enhancement rate of the study group (before and after denosumab treatment) and control group. The posttreatment CT enhancement rate of study group was significantly lower than that of control group (P = .007).
3.3. Denosumab treatment reduced intraoperative blood loss but increase the difficulty of tumor curettage
The mean intraoperative blood loss of study group and control group was 2166.7 (1300–3000) mL and 5240 (2500–14,000) mL, respectively (P = .040, F = 5.016). The mean operative time of study group and control group was 268.3 (210–360) minutes and 268.5 (180–4500) minutes, respectively (P = .997, F = 0.000). In study group, the increasing of sclerosis and bony separation brought some difficulties in operation: judgment of tumor boundary; tumor curettage; and separating tumor from sacral nerve.
3.4. Study group had higher recurrence rate
The study group was followed an average 12 (7–18) months and 4 patients had recurrence (66.7%). The control group was followed an average 35.3 (13–61) months and there was no recurrence. The recurrence rate of study group was significantly higher than that of control group (P = .046, χ2 = 4.000). The mean Musculoskeletal Tumor Society (MSTS) score of study group and control group was 25.9 (18–30) and 22 (17–27), respectively (P = .142, F = 2.505).
4. Discussion
The incidence rate of sacral GCT is low and surgical treatment is difficult.[1–4] Complex local structures and huge intraoperative blood loss make it very challenging. The local recurrence rate of sacral GCT is high.[6–9] Denosumab is a new type of humanized RANKL monoclonal antibody. It combines with RANKL specifically and block RANKL-RANK pathway. Thus it can interfere with the survival and differentiation of osteoclasts and inhibit osteoclast mediated bone destruction.[14] In our clinical practices, we found an interesting tendency that enhanced CT value of tumor seemed to decrease after denosumab treatment. Therefore, we performed this study to evaluate whether denosumab can reduce intraoperative blood loss, facilitate surgical treatment and improve local control of sacral GCT.
Our study showed significantly decreasing of VAS after denosumab treatment. All patients presented pain relief and clinical benefit. Thomas et al[13] reported a phase 2 study which showed 84% of patients had clinical benefit such as reduced pain or improvement in functional status. Martinbroto et al[15] reported another phase 2 study and most patients showed clinically relevant decreases in pain within 2 months of treatment. These previous reports had similar results with our study.
Our results showed mean intraoperative blood loss of study group and control group was 2166.7 and 5240 mL, respectively. The bleeding was significantly reduced after denosumab treatment. The significantly decreasing of CT enhancement rate (mean 2.60 vs 1.37) after denosumab treatment also supported the decreasing of tumor blood supply. As far as we know, there has been no quantifying enhanced CT analysis on the effect of denosumab treatment. The tumors in our study were aggressive and huge, while the blood loss in study group was relative small compared with that in previous reports. Ozaki et al[9] reported the mean intraoperative blood loss of sacral GCT was 6900 mL. In 2016, Guo et al[16] reported a big case series of sacral GCT and mean intraoperative blood loss was 3223.3 mL. In 2015, Domovitov et al[17] reported 24 sacral GCT who underwent conservative surgery (intralesional resection/curettage) and preoperative embolization may decrease the bleeding. The rich blood supply of tumor can lead to high risk of huge intraoperative bleeding. Less intraoperative bleeding can obtain clear visible surgical field and bring benefit for removing tumor thoroughly.
In the present study, the operative time of study group (mean 268.3 minutes) and control group (mean 268.5 minutes) was almost same. All tumors were located above sacral level 3 with extensive bone destruction, so the surgical treatment was difficult and challenging. The results showed preoperative denosumab treatment did not make surgery faster or easier from the perspective of time expenditure. In fact, although the relative less bleeding made surgeons unhurried, but new problems appeared and need to be resolved in operation. The mean pretreatment unenhanced CT value (mean 42.0) was significantly lower than posttreatment (mean 56.4). The increasing of CT value suggested the raising of osteogenesis and sclerosis. Müller et al[18] suggested denosumab can build a new-formed peripheral bone rim around tumor in their case series. Nishimura et al[19] reported the sacral GCT refractory to combination therapy with arterial embolization and zoledronic acid. CT also showed gradual appearance of bone sclerosis around sacrum after denosumab treatment. But our study showed tumor became hardening and bony septa increased after denosumab treatment, so it was difficult to judge tumor boundary and perform curettage. Focal sclerosis led to adhesion between tumor and sacral nerve. It is difficult to separate nerve from tumor.
After near 3 years follow-up, no local recurrence was found in control group. However, 4 of 6 cases (66.7%) in study group showed local recurrence after only 1 year follow-up. The main reason was that intralesional sclerosis and bony septa increased difficulty of tumor curettage. Another reason of high recurrence rate was that extensive curettage could not be performed in sacrum as that in limbs. Guo et al[16] reported the recurrence rate was 18.5% after marginal resection and curettage. In 2010, Pietro et al[20] reported 31 sacral GCT and the recurrence rate was 10%. The authors suggested that preoperative embolization and postoperative radiotherapy did not decrease the recurrence rate. Adjuvant treatment with phenol and liquid nitrogen was not associated with recurrence. Combined with previous reports before 2010, the recurrence rate of sacral GCT after intralesional curettage was about 20% to 40%.[3,21–25]
In 2015, Memorial Sloan Kettering Cancer Center[17] reported 24 cases received intralesional curettage and local recurrences rate was 30%. Radiation and preoperative embolization were associated with prolonged disease-free survival. There were no local recurrences among the 11 patients who were treated with both modalities. Another report[26] analyzed risk factor of recurrence and compared intracompartmental (T1) tumors and extracompartmental (T2) tumors in 2013. It showed that T1/T2 was the only risk factor of recurrence while age, gender, tumor site, tumor volume, and radiation were not. However, some reports[27–31] show that local recurrence rate after wide resection (20.3–38.9%) was not significantly decreased. Due to the low incidence and small sample report of sacral GCT, surgical treatment is still challenging and controversial.
There have been some clinical studies which reported oncologic results of GCT after denosamub treatment, but they did not focus on sacral tumor. Traub et al[32] reported a prospective nonrandomized study of 18 patients who received preoperative denosumab and intralesional surgery. The local recurrence rate was 17%. Müller et al[18] reported a local recurrence rate of 8.3% in 12 patients treated by curettage after denosumab treatment. The authors thought that tumor cells can remain in the newly formed bone induced by denosumab and newly formed bone makes curettage difficult. The new osseous matrix and thickened cortical bone raises a new surgical challenge by not allowing surgeon to delineate the true extent of tumor. The authors also considered that tumor cells can hide within the thickened bone matrix, which led to local recurrence when denosumab therapy discontinued. The high recurrence rate of study group in our study also supported this opinion. But up to now, there is no relevant report that recommends continuing denosumab therapy after surgery. Therefore, we suggest that it is important to curettage tumor thoroughly according to initial tumor boundary, but not the boundary after denosumab treatment.
The present study had some limitations. First, it was a retrospective small case series study. It was due to low incidence of sacral GCT. Second, all cases had aggressive huge tumor and selection bias may existed. The potential bias maybe caused high recurrence rate in study group. More cases were required for observing and evaluating the results of denosumab treatment on sacral GCT.
In conclusion, our study showed denosumab treatment had tendency to reduce blood supply and intraoperative blood loss of sacral GCT. However, because of the increasing of sclerosis and bony separation, it is difficult to judge and completely remove tumor. The high recurrence rate was shown. Therefore, caution should be taken when selecting sacral tumors for denosumab treatment. The positive and negative effect maybe balanced by controlling preoperative medication time. It is necessary to study the best preoperative medication duration and proper surgical opportunity, and also how to accurately judge tumor range during operation.
Author contributions
Conceptualization: Yongkun Yang, Yuan Li, Xiaohui Niu.
Data curation: Yongkun Yang, Weifeng Liu, Hairong Xu.
Formal analysis: Yongkun Yang, Weifeng Liu.
Investigation: Yongkun Yang.
Methodology: Yuan Li.
Project administration: Yuan Li.
Supervision: Xiaohui Niu.
Writing – original draft: Yongkun Yang.
Writing – review & editing: Xiaohui Niu.
Footnotes
Abbreviations: CT = computed tomography, GCT = giant-cell tumor, RANK = receptor activator of nuclear factor-κB, RANKL = receptor activator of nuclear factor-κB ligand, VAS = visual analog scale.
The work was supported by Beijing Talents Fund (2015000021469G181).
The authors have no conflicts of interest to disclose.
References
- [1].Campanacci M, Baldini N, Boriani S. Sudanese A: giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106–14. [PubMed] [Google Scholar]
- [2].Unni KK, Inwards CY. Dahlin's Bone Tumors: General Aspects and Data on 10,165 Cases. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- [3].Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop 1993;291:215–21. [PubMed] [Google Scholar]
- [4].Sung HW, Kuo DP, Shu WP, et al. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am 1982;64:755–61. [PubMed] [Google Scholar]
- [5].Campanacci M. Bone and Soft Tissue Tumors, 2nd ed. 1999;New York: Springer-Verlag, 99–136. [Google Scholar]
- [6].Smith J, Wixon D, Watson RC. Giant-cell tumor of the sacrum: clinical and radiologic features in 13 patients. J Can Assoc Radiol 1979;30:34–9. [PubMed] [Google Scholar]
- [7].Cheng JC, Johnston JO. Giant-cell tumor of bone: prognosis and treatment of pulmonary metastases. Clin Orthop 1997;338:205–14. [DOI] [PubMed] [Google Scholar]
- [8].Klenke FM, Wenger DE, Inwards CY, et al. Giant cell tumorof bone: risk factors for recurrence. Clin Orthop 2011;469:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ozaki T, Liljenqvist U, Halm H, et al. Giant cell tumor of the spine. Clin Orthop Relat Res 2002. 194–201. [DOI] [PubMed] [Google Scholar]
- [10].Tsuda E, Goto M, Mochizuki S, et al. Isolation of a novel cytokine from human fibroblasts that specifically inhibit s osteoclastogenesis. Biochem Biophys Res Comm 1997;234:137–42. [DOI] [PubMed] [Google Scholar]
- [11].Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin (OPG) ligand is a cytokine that regulates osteoclast different iation and activation. Cell 1998;93:165–76. [DOI] [PubMed] [Google Scholar]
- [12].Huang L, Xu J, Wood DJ, et al. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of nf-(B in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol 2000;156:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010;11:275–80. [DOI] [PubMed] [Google Scholar]
- [14].Atkinson J, Cranmer P, Saunders T, et al. AMG 162, a fully human RANK-L antibody, increases bone mass and bone strength in cynomolgus monkeys. J Bone Miner Res 2005;20:S29. [Google Scholar]
- [15].Martinbroto J, Cleeland CS, Glare PA, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncologica 2014;53:1173–9. [DOI] [PubMed] [Google Scholar]
- [16].Guo W, Yang Y, Ji T, et al. Outcomes of sacral giant cell tumor treatment with intralesional surgery. Chin J Bone Joint 2016;5:9–13. [Google Scholar]
- [17].Domovitov SV, Chandhanayingyong C, Boland PJ, et al. Conservative surgery in the treatment of giant cell tumor of the sacrum: 35 years’ experience. J Neurosurg Spine 2015;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Müller DA, Beltrami G, Scoccianti G, et al. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol 2016;14:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nishimura S, Hashimoto K, Tan A, et al. Successful treatment with denosumab in a patient with sacral giant cell tumor of bone refractory to combination therapy with arterial embolization and zoledronic acid: a case report. Mol Clin Oncol 2017;6:307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pietro R, Andreas FM, Giuseppe U, et al. Recurrence after and complications associated with adjuvant treatments for sacral giant cell tumor. Clin Orthop Relat Res 2010;468:2954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hosalkar HS, Jones KJ, King JJ, et al. Serial arterial embolization for large sacral giant-cell tumors: mid- to long-term results. Spine 2007;32:1107–15. [DOI] [PubMed] [Google Scholar]
- [22].Lin PP, Guzel VB, Moura MF, et al. Long-term follow up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer 2002;95:1317–25. [DOI] [PubMed] [Google Scholar]
- [23].Lackman RD, Khoury LD, Esmail A, et al. The treatment of sacral giant cell tumours by serial arterial embolisation. J Bone Joint Surg Br 2002;84:873–7. [DOI] [PubMed] [Google Scholar]
- [24].Marcove RC, Sheth DS, Brien EW, et al. Conservative surgery for giant-cell tumors of the sacrum: the role of cryosurgery as a supplement to curettage and partial excision. Cancer 1994;74:1253–60. [DOI] [PubMed] [Google Scholar]
- [25].McDonald DJ, Sim FH, McLeod RA, et al. Giant-cell tumor of bone. J Bone Joint Surg Am 1986;68:235–42. [PubMed] [Google Scholar]
- [26].Zhou M, Chen K, Yang H, et al. Analysis of risk factors for recurrence of giant cell tumor of the sacrum and mobile spine combined with preoperative embolization. Turkish Neurosurg 2013;23:645–52. [DOI] [PubMed] [Google Scholar]
- [27].van der Heijden L, van de Sande MA, van der Geest IC, et al. Giant cell tumors of the sacrum—a nationwide study on midterm results in 26 patients after intralesional excision. Eur Spine J 2014;23:1949–62. [DOI] [PubMed] [Google Scholar]
- [28].Li G, Fu D, Chen K, et al. Surgical strategy for the management of sacral giant cell tumors: a 32-case series. Spine J 2012;12:484–91. [DOI] [PubMed] [Google Scholar]
- [29].Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J 2010;30:69–75. [PMC free article] [PubMed] [Google Scholar]
- [30].Guo W, Ji T, Tang X, et al. Outcome of conservative surgery for giant cell tumor of the sacrum. Spine 2009;34:1025–31. [DOI] [PubMed] [Google Scholar]
- [31].Leggon RE, Zlotecki R, Reith J, et al. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res 2004. 196–207. [DOI] [PubMed] [Google Scholar]
- [32].Traub F, Singh J, Dickson BC, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer 2010;59:1–2. [DOI] [PubMed] [Google Scholar]


