Abstract
Background:
Early mobilization and physical exercise are considered fundamental components in cardiovascular surgery rehabilitation; however, occasionally they are inadequate for inhibiting functional decline. Neuromuscular electrical stimulation (NMES) is a promising tool in cardiovascular rehabilitation; however, to date, no randomized clinical trial has measured the effects of NMES on functional capacity and quality of life in patients who undergo routine cardiac surgery with a short intensive care unit (ICU) stay. Therefore, we aimed to investigate the effects of NMES on walking ability, muscle strength, functional independence, and quality of life in cardiac valve surgery patients in the immediate postoperative period.
Methods:
A randomized, parallel, controlled, 2-arm clinical trial with assessor blinding was conducted. Fifty-nine adult patients in the preoperative period after cardiac valve reconstruction and/or replacement were randomly assigned to a control or intervention group. The intervention group underwent NMES in the quadriceps and gastrocnemius, bilaterally, for 60 minutes, for up to 10 sessions. The primary outcome was ambulation ability, assessed through the Six-Minute Walk Test and Walking Speed Test at postoperative day 5 (5PO). Secondary outcomes were muscular strength (assessed through the Medical Research Council scale), functional independence measure (assessed through the Functional Independence Measurement Questionnaire), and quality of life (assessed through the Nottingham Health Profile) at baseline (preoperative) and at postoperative days 3 and 5.
Results:
The baseline characteristics were similar in both groups, except for body mass index. There was no statistically significant difference, with a small effect size, between both groups regarding the distance walked (95% CI, −64.87 to 65.97) and walking speed (95% CI, −0.55 to 0.57). There was a statistically significant difference in upper-limb muscle strength loss and decline in mobility at postoperative day 3, which had a tendency to recover to initial values at 5PO, in both groups. No significant between-group difference was noted for muscle strength, functional independence, and quality of life.
Conclusions:
The use of NMES had no effect on walking ability, strength, quality of life, or functional outcome in the postoperative period for patients that underwent regular valve replacement.
Keywords: ambulation, electric stimulation therapy, rehabilitation, thoracic surgery
1. Introduction
Patients with cardiac conditions tend to present with a decline in their performance of daily life activities because of a reduction in aerobic condition and muscle weakness.[1] This condition is aggravated by postoperative physical inactivity, in which a longer duration of bed rest leads to greater muscle strength loss and deconditioning.[2,3] Previous studies showed that even patients undergoing high-risk elective cardiac surgeries with good clinical outcome and short intensive care unit (ICU) stay commonly presented with acute muscle loss as a result of an imbalance between muscle atrophy and hypertrophy markers.[4] Muscle proteolysis is notably accelerated within 48 hours after cardiovascular surgery due to increased protein catabolism.[3,5] In addition, postoperative physical inactivity stimulates muscle wasting by slowing down protein synthesis, accelerating protein degradation and myonuclear apoptosis in the fibers, and promoting strength reduction and functional decline, which may compromise the quality of life.[6,7] This, and the fact that cardiovascular disease is the main cause of death worldwide suggests that more attention should be given to the rehabilitation of patients after cardiac surgery.[8]
Early mobilization and physical exercise are considered fundamental components in cardiovascular surgery rehabilitation, but occasionally they are inadequate for inhibiting functional decline.[3,9] Among a variety of resources, neuromuscular electrical stimulation (NMES), which is widely used as an adjunct tool in physical training, has been shown to be a promising tool in cardiovascular rehabilitation.
Studies show that NMES influences the improvement of maximal oxygen uptake,[1] fatigue tolerance, and ambulation ability in patients with heart failure[2] and can be safely applied to patients in the immediate postoperative period of cardiothoracic surgery.[6] NMES may even be used to attenuate muscle proteolysis and strength loss after cardiac surgery. [10]
No randomized clinical trial has measured the effects of NMES on functional capacity and quality of life in patients who undergo routine cardiac surgery with a short ICU stay. Thus, this study aimed to investigate the effects of NMES on walking ability, muscle strength, functional independence, and quality of life in cardiac valve surgery patients in the immediate postoperative period.
2. Material and methods
2.1. Study design
This was a randomized, parallel, 2-arm, controlled trial performed from February 2014 to December 2016. Adult patients in the immediate preoperative period after cardiac valve reconstruction and/or replacement were randomly assigned to an intervention or control group. The randomization was performed in a simple and confidential manner by an independent investigator using the electronic randomization system, http://random.org.
Considering the intervention protocol, it was not possible to blind patients and/or the investigator who performed the NMES. However, the investigator who recruited and assessed patients in the periods determined in the study was blinded. Variables were assessed in the preoperative period, at postoperative day 3 (3PO), and at the end of the NMES protocol at postoperative day 5 (5PO).
This study was approved by the Ethics and Research Committee of Tiradentes University, (approval number: 429.256) and written informed consent was obtained from each participant and/or their next of kin before enrolment in the study. This study was submitted to the Brazilian Registry of Clinical Trials (Registro Brasileiro de Ensaios Clínicos-REBeC; registration number: RBR-8vkw87).
2.2. Participants
Participants were recruited through the Cardiology Service of the Fundação de Beneficência Hospital de Cirurgia. Adult patients of both sexes scheduled to undergo preoperative cardiac valve reconstruction and/or replacement or bioprosthesis replacement were eligible for the research. Patients were excluded if they were <18 and >75 years old and had any psychiatric disorders, cognitive decline or dementia, recent or unresolved musculoskeletal or neuromuscular disorder limit in walking ability, mobility, or functional capacity, haemodynamic instability (mean arterial pressure <60 or >120 mmHg), dyspnoea with oxygen saturation below 90%, tachycardia or bradycardia, cardiac pacemakers, dermatitis, damaged skin or sensitivity changes, or if they refused to participate in the study. Patients were recruited for the research before surgery, and if surgery was canceled or the patient died in the perioperative period, the patient was excluded from the study.
Patients who needed reoperation, had a mechanical ventilation (MV) time longer than 24 hours, were discharged before 5PO, had postoperative cerebrovascular accident (neuromuscular disorder), were in an unstable medical condition that prevented assessment, and refused to continue in the study or died were discharged from the study.
2.3. Intervention
After randomization, participants in the experimental group received NMES, in addition to regular physiotherapy care, in the immediate postoperative period after admission to the ICU until 5PO. They underwent NMES twice a day (morning and evening), for a total of 10 sessions per patient. The 4-channel Neuromed 4082 IFC (Carci, Brazil) device was used, and 3 × 3-cm silicone-carbon electrodes were attached to the quadriceps and gastrocnemius muscle bellies, bilaterally, and fixed with adhesive tape. Functional electrical stimulation was applied with 50-Hz frequency, 400-ms pulse width, 3-second on-time, and 9-second off-time for 60 minutes.[11] The intensity was adjusted until visible muscle contraction occurred. For doubtful cases, contraction was confirmed by the palpation of the involved muscles. [12]
Participants in the control group received usual physiotherapy care from hospital physiotherapists twice a day, in the morning and evening.
2.4. Outcome measures
Assessments were performed at baseline (preoperative), at 3PO, and at the end of the intervention (5PO). Evaluators were blinded to patient allocation and participants were instructed not to disclose their group allocation. The demographic, physical, and clinical characteristics of patients were evaluated in the preoperative period, and information related to the surgical procedure, length of ICU stay, and time between surgery and hospital discharge was collected throughout the study.
The primary outcome was ambulation, which was quantified by measuring the distance traveled (in meters) during the Six-Minute Walk Test (6MWT). 6MWT was performed according to the recommendations of the American Thoracic Society,[13] with patients instructed to walk at their maximum tolerated speed for 6 minutes, on a 30-meter obstacle-free course, marked every 2 meters and at the end of course. Ambulation was also quantified by measuring the speed (in meters per second) during the 10-meter Walking Speed Test (T10). A 20-meter distance was marked with a straight line on a flat floor, and the patient was instructed to perform fast, non-running ambulation, at a comfortable pace, over 20 meters. The first and last 5 meters, which correspond to the period of gait acceleration and deceleration, respectively, were not measured.
The secondary outcomes were muscle strength, functional independence, and quality of life. Muscle strength was assessed by measuring the peak strength and representative maximum voluntary contraction through manual testing, ranging from 0 (no muscular contraction) to 5 (active movement against complete resistance) for 6 lower and upper limbs movements; thus, obtaining the total Medical Research Council (MRC) score. Functional independence was measured using the Functional Independence Measurement Questionnaire (FIM) and quality of life was assessed using the Nottingham Health Profile (NHP).
2.5. Data analysis
The sample size was calculated from an earlier study performed on the same population,[14] where ambulation ability was assessed in postoperative cardiac patients. Based on the results of this previous study, a sample size of 23 patients per group was derived, with a 2-sided significance level (α) of 0.05 and a power of 95%. The minimum clinically important difference was 20 meters based on the primary outcome variable (distance traveled in 6MWT).
Statistical analyses were performed with SPSS test version 15.0 (IBM Corp., Armonk, NY). All data were analyzed according to the intention-to-treat principle. Normality was assessed with the Shapiro–Wilk test. Normally distributed quantitative variables are expressed as mean (SD). To compare patient characteristics between groups, the chi-square test was used for categorical variables and the t test was used for independent samples.
To answer the research question, the distance traveled in 6MWT and the T10 walking speed were compared between groups using the t test for independent samples. The comparison of MRC, FIM, and NHP between both groups was performed using a 3 × 2 (time: preoperative period versus 3PO versus 5PO; group: NMES versus control) analysis of variance (ANOVA), and the Bonferroni post-hoc test was used to determine, when possible, where the differences occurred. The significance level was set at 0.05. All P values were 2-tailed. When comparing means between the 2 groups using t test, Hedges’ g (variation of Cohen's d) was used and the effect size was classified as small (>0.20), medium (>0.50) or large (>0.80).[15] When comparing means between the 2 groups using ANOVA, the eta-square was used and the effect size was classified as small (>0.01), medium (>0.06) or large (>0.014).[15]
3. Results
3.1. Flow of participants through the study
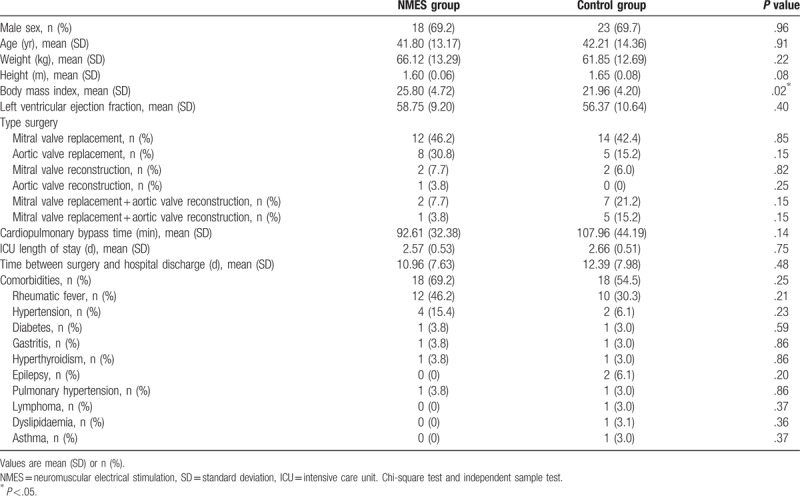
The analysis included 59 patients (NMES group, n = 26; control group, n = 33) from February 2014 to December 2016. The flow of participants through the study is summarised in Figure 1. All patients included in the analysis started the study in the NMES or control group; thus, the analysis is not limited to patients who completed the entire protocol. Patients who could not be assessed for any of the outcome measures at the end of the intervention were not included in the analysis. The patients’ characteristics are detailed in Table 1, and there was homogeneity between the 2 groups in relation to the studied variables, except for body mass index.
Figure 1.
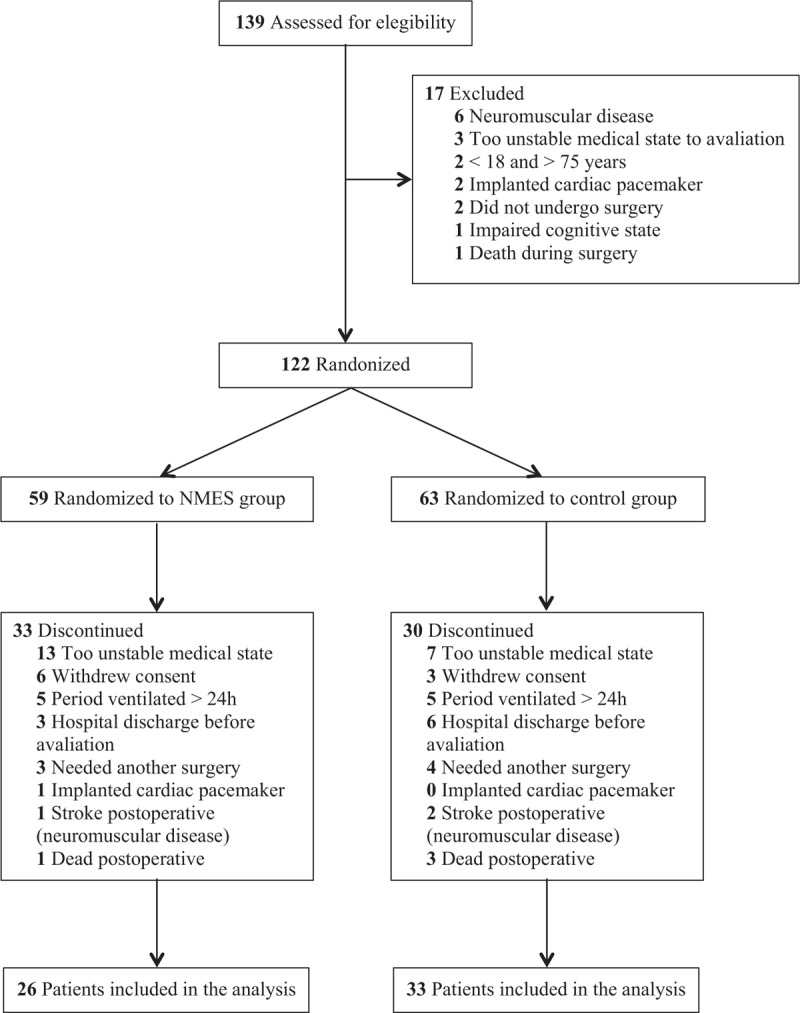
Flow of participants through the trial.
Table 1.
Baseline participants characteristics.
3.2. Compliance with the intervention
The median number of NMES sessions applied to the analyzed patients was 10 (range, 5–10), using an average intensity of 54.9 (range, 22.8–71.2) mA in the quadriceps and 49.5 (range, 17.5–79.1) mA in the gastrocnemius. Regarding adherence, only 20 of the 260 sessions planned were not performed (98.6% completion). Concerning adverse effects, 2 patients presented with hypotension during 1 NMES application and 1 patient reported pain.
3.3. Primary outcome
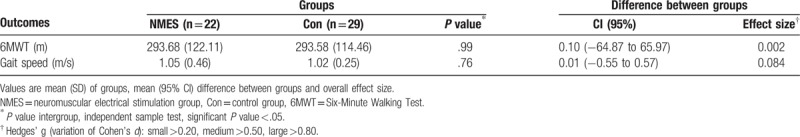
Eight patients did not undergo ambulation tests (NMES group, n = 4; control group, n = 4) because they presented with clinical contraindications to 6MWT at the time of assessment, such as precordial pain, resting heart rate above 120 beats per minute, systolic blood pressure >180 mmHg, and diastolic blood pressure >100 mmHg. There was no statistically significant difference, with a small effect size, between the groups regarding the distance walked (95% CI, −64.87 to 65.97) and walking speed (95% CI, −0.55 to 0.57), as shown in Table 2.
Table 2.
Ambulation ability outcome in both NMES and Control group.
3.4. Secondary outcome variables
Muscle strength was not measured in 1 patient at 5PO and in 2 patients at 3PO because they refused to exert maximum effort. All patients assessed in the preoperative period presented with normal mean upper-limb, lower-limb, and total MRC values, with no significant statistical difference between the 2 groups (P >.05 all). We observed a decrease in upper-limb MRC values at 3PO when compared to preoperative values in both groups, with a return to baseline values at 5PO in the control group (P = .35) and a statistical tendency to return to baseline values in the NMES group (P = .01), without a clinical difference in values in relation to the preoperative period. No significant between-group difference, with only a small effect size, was found for muscle strength in the upper-limb, lower-limb, and total MRC values (Table 3).
Table 3.
Strength muscle and independence functional outcome in both groups NMES and Control: Medical Research Council and Functional Independence Measure Questionnaire score.
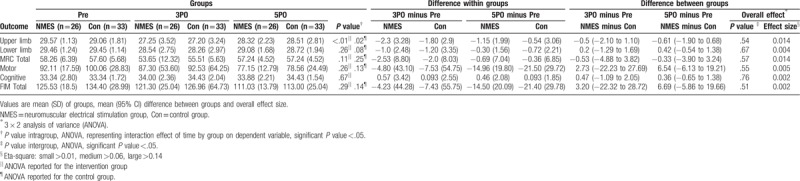
All patients were assessed for FIM, both at 3PO and 5PO, which showed no reduction during the study or significant differences between the groups (P >.05) (Table 3). This behavior was observed in the evaluation of the motor, cognitive, and total FIM, and their corresponding domains.
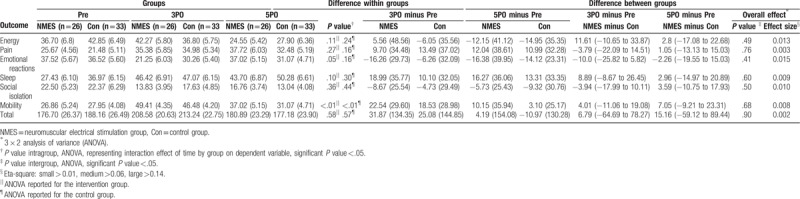
Regarding the quality of life, the total NHP and its domains were assessed in all patients at the 3-time points of the study, and no significant difference, with a small effect size, was found between the groups (P >.05) (Table 4). The score of the domain corresponding to mobility increased at 3PO (both groups, P <.01) and recovered at 5PO (control group, P =.61; NMES group, P =.17).
Table 4.
Quality of life outcome in both groups NMES and Control: Nottingham Health Profile.
4. Discussion
Our results suggest that the use of NMES during the immediate postoperative period in patients who underwent routine cardiac valve surgery and with a short ICU stay did not influence the ambulation ability, muscle strength, quality of life, and functional independence of this cohort. These findings may be associated with the short sedation period, MV, bed rest, and ICU stay, which favour the early active engagement of these patients in rehabilitation interventions, leading to better functional outcomes[16,17] and decreased vulnerability to atrophy and muscle weakness secondary to immobilization, for which NMES could provide greater benefits.[16]
The postoperative patients in this study showed maintenance of functional independence, quality of life, and overall muscle strength, confirming their lower vulnerability to functional decline. The decrease in the upper-limb MRC score and physical ability noted at 3PO in both groups, as shown by the increase in the score of this NHP domain and a tendency to recover to initial values at 5PO, was possibly related to sternotomy. Sternotomy limits the mobilization and evaluation of upper-limb muscle strength during the first postoperative days, suggesting that a longer time might be needed for better recovery.
NMES has shown its best results in patients who underwent longer sedation, MV, bed restriction, and ICU stays and in patients who could not engage in active rehabilitation interventions because of disease severity, deep sedation, delirium, and coma.[16] Muscular weakness acquired in the ICU affects 24% to 77% patients with an ICU stay >1 week, which was not seen in the present study population, whose average length of ICU stay was 2 to 3 days.[18]
Some studies indicate that NMES shows greater benefits when the stratification of certain diagnoses is taken into consideration. These benefits were noted in patients admitted to the ICU for respiratory complications such as chronic obstructive pulmonary disease, where NMES reduced the number of days needed to transfer the patient from bed to chair,[19] and showed an increase in the distance walked[20], as well as in cases of neurological complications.[21] Some publications have demonstrated the effectiveness of NMES in non-hospitalised cardiac patients with chronic heart failure in terms of exercise capacity,[22,23] distance traveled,[22–24] muscular strength, fatigue tolerance,[25] decreased levels of anaerobic enzymes, and transition from fast to slow fibers.[26,27]
A study reported that NMES, performed twice daily for 60 minutes and for an average of 16 days, promoted an increase in the distance travelled in 6MWT compared to the control group, in patients hospitalized for clinical compensation of heart failure.[11] This outcome differs from that of the present study, where the studied population included patients in the immediate postoperative period of cardiac surgery with a 5-day protocol of NMES application.
NMES has been reported as a useful tool for reducing protein catabolism in postoperative patients, but few studies show which molecular changes are accompanied by changes in strength, muscle mass, and functional outcome.[3,5,28]
In another randomized clinical trial, NMES did not promote any significant effect on muscle thickness and strength at the time of hospital discharge.[29] However, the MRC scores showed that patients in the NMES group regained strength 4.5 times faster than patients in the control group. In addition, patients did not recover their mobility levels, and their FIM score at the time of hospital discharge did not depend on the allocated group. The study only applied NMES to critically ill patients who had a median ICU stay of 6 to 7 days and longer MV periods, while those with ICU stays shorter than 48 hours were excluded. This is in contrast with our study where participants had a regular postoperative period and a mean ICU stay of 2 to 3 days, with NMES showing no impact on strength and functional independence.
Finally, NMES has been the subject of several published studies, including a wide variety of protocols. However, there is no consensus on parameters, which makes comparison difficult. Further, many authors found positive correlations between fluid balance and muscle mass change during the first 3 postoperative days, suggesting that edema, which is predominant during this initial phase in surgical patients, may affect current dissipation and decrease muscle contraction quality[29,30]. This may also have influenced our results, although visualization or palpation of muscle contraction during NMES sessions was possible.
This study was limited by the absence of a preoperative evaluation of the distance covered in 6MWT and T10 because the patients were in a serious health state, debilitated, and with preoperative restrictions. Other methods for measuring muscle strength, such as dynamometry, could have been more sensitive in assessing small muscle strength losses, and electromyography could have been used to better investigate NMES effects. Future studies should perform further follow-up NMES and patient evaluation to better investigate the effects of NMES.
In conclusion, the use of NMES showed no effect on ambulation ability, strength, quality of life, and functional outcome in patients in the regular postoperative period of cardiac valve surgery.
Acknowledgments
The authors thank the staff of the Cardiology Service of the Fundação de Beneficência Hospital de Cirurgia for their invaluable collaboration in this study and the members of the search group LAPERF (Laboratório de Pesquisa em Reintegração Funcional).
Author contributions
Conceptualization: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau, Gessica Uruga Oliveira, Walderi Monteiro da Silva Júnior, Vitor Oliveira Carvalho, José Teles de Mendonça, Valter Joviniano de Santana Filho.
Data curation: Telma Cristina Fontes Cerqueira, Gessica Uruga Oliveira.
Formal analysis: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau, Gessica Uruga Oliveira, Walderi Monteiro da Silva Júnior, Vitor Oliveira Carvalho, José Teles de Mendonça, Valter Joviniano de Santana Filho.
Funding acquisition: Manoel Luiz de Cerqueira Neto.
Investigation: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau.
Methodology: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau, Gessica Uruga Oliveira, Walderi Monteiro da Silva Júnior, Vitor Oliveira Carvalho, José Teles de Mendonça, Valter Joviniano de Santana Filho.
Project administration: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto.
Resources: Manoel Luiz de Cerqueira Neto.
Supervision: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Valter Joviniano de Santana Filho.
Validation: Telma Cristina Fontes Cerqueira.
Visualization: Telma Cristina Fontes Cerqueira.
Writing – original draft: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau, Gessica Uruga Oliveira, Walderi Monteiro da Silva Júnior, Vitor Oliveira Carvalho, José Teles de Mendonça, Valter Joviniano de Santana Filho.
Writing – review & editing: Telma Cristina Fontes Cerqueira, Manoel Luiz de Cerqueira Neto, Lucas de Assis Pereira Cacau, Gessica Uruga Oliveira, Walderi Monteiro da Silva Júnior, Vitor Oliveira Carvalho, José Teles de Mendonça, Valter Joviniano de Santana Filho.
Footnotes
Abbreviations: 3PO = postoperative day 3, 5PO = postoperative day 5, 6MWT = Six-Minute Walk Test, ANOVA = analysis of variance, FIM = Functional Independence Measurement Questionnaire, ICU = intensive care unit, MRC = Medical Research Council score, MV = mechanical ventilation, NHP = Nottingham Health Profile, NMES = neuromuscular electrical stimulation, T10 = 10-meter Walking Speed Test.
This work was supported by the Foundation for Research and Technological Innovation Support of the State of Sergipe (Notice MS/CNPQ/FAPITEC/SE/SES N° 02/2013 PPSUS Sergipe).
The authors have no conflicts of interest.
References
- [1].Savage PA, Shaw AO, Miller MS, et al. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc 2011;43:1379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Killewich LA. Strategies to minimize postoperative deconditioning in elderly surgical patients. J Am Coll Surg 2006;203:735–45. [DOI] [PubMed] [Google Scholar]
- [3].Santos KMS, Cerqueira Neto ML, Carvalho VO, et al. Evaluation of peripheral muscle strength of patients undergoing elective cardiac surgery: a longitudinal study. Rev Bras Cir Cardiovasc 2014;29:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bloch SA, Lee JY, Wort SJ, et al. Sustained elevation of circulating growth and differentiation factor-15 and dynamic imbalance in mediators of muscle homeostasis are associated with the development if acute muscle wasting following cardiac surgery. Crit Care Med 2013;41:982–9. [DOI] [PubMed] [Google Scholar]
- [5].Iida Y, Yamazaki T, Kawabe T, et al. Postoperative muscle proteolysis affects systemic muscle weakness in patients undergoing cardiac surgery. Int Cardiol 2014;172:595–7. [DOI] [PubMed] [Google Scholar]
- [6].Iwatsu K, Yamada S, Iida Y, et al. Feasibility of neuromuscular electrical stimulation immediately after cardiovascular surgery. Arch Phys Med Rehab 2015;96:63–8. [DOI] [PubMed] [Google Scholar]
- [7].Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically. Crit Care Med 2009;37:S337–46. [DOI] [PubMed] [Google Scholar]
- [8].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Riedi C, Mora CTR, Driessen T, et al. Relação do comportamento da força muscular com as complicações respiratórias na cirurgia cardíaca. Rev Bras Cir Cardiovasc 2010;25:500–5. [DOI] [PubMed] [Google Scholar]
- [10].Iwatsu K, Iida Y, Kono Y, et al. Neuromuscular electrical stimulation may attenuate muscle proteolysis after cardiovascular surgery: a preliminary study. J Thorac Cardiovasc Surg 2017;153:373–9. [DOI] [PubMed] [Google Scholar]
- [11].Araújo CJS, Gonçalves FS, Bittencourt HS, et al. Effects of neuromuscular electrostimulation in patients with heart failure admitted to ward. J Cardiothorac Surg 2012;7:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Routsi C, Gerovasili V, Vasileiades I, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 2010;14:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- [14].Cacau LAP, Oliveira GU, Maynard LG, et al. The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Braz J Cardiovasc Surg 2013;28:281–9. [DOI] [PubMed] [Google Scholar]
- [15].Maher JM, Markey JC, Ebert-May D. The other half of the story: effect size analysis in quantitative research. CBE Life Sci Educ 2013;12:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kho ME, Truong AD, Zanni JM, et al. Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham controlled, pilot trial with blinded outcome assessment. J Crit Care 2015;30:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Latronico N, Boldon CN. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931–41. [DOI] [PubMed] [Google Scholar]
- [19].Zanotti E, Felicetti G, Maini M, et al. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 2003;124:292–6. [DOI] [PubMed] [Google Scholar]
- [20].Abdellaoui A, Prefaut C, Gouzi F, et al. Skeletal muscle effects electrostimulation after COPD exacerbation. Eur Respir J 2011;31:781–8. [DOI] [PubMed] [Google Scholar]
- [21].Maffiuletti NA, Roig M, Karatzanos E, et al. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med 2013;11:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dobsák P, Nováková M, Fiser B, et al. Electrical stimulation of skeletal muscle. An alternative to aerobic exercise training in patients with chronic heart failure? Int Heart J 2006;47:441–53. [DOI] [PubMed] [Google Scholar]
- [23].Nuhr MJ, Pette D, Berger R, et al. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J 2004;25:136–43. [DOI] [PubMed] [Google Scholar]
- [24].Karavidas A, Parissin JT, Arapi S, et al. Effects of functional electrical stimulation on quality of life and emotional stress in patients with chronic heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy: a randomized, placebo-controlled trial. Eur J Heart Fail 2008;10:709–13. [DOI] [PubMed] [Google Scholar]
- [25].Quittan M, Sochor A, Wiesinger GF, et al. Strength improvement of knee extensor muscles in patients with chronic heart failure by neuromuscular electrical stimulation. Artif Organs 1999;23:432–5. [DOI] [PubMed] [Google Scholar]
- [26].Nuhr M, Crevenna R, Gohlsch B, et al. Functional and biochemical properties of chronically stimulated human skeletal muscle. Eur J Appl Physiol 2003;89:202–8. [DOI] [PubMed] [Google Scholar]
- [27].Hambrecht R, Fiehn E, Yu J, et al. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 1997;29:1067–73. [DOI] [PubMed] [Google Scholar]
- [28].Srasser EM, Stättner S, Karner J, et al. Neuromuscular electrical stimulation skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner. Ann Surg 2009;249:738–43. [DOI] [PubMed] [Google Scholar]
- [29].Fischer A, Spiegl M, Altmann K, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care 2016;20:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Segers J, Hermans G, Bruyninckx F, et al. Feasibility of neuromuscular electrical stimulation in critically ill patients. J Crit Care 2014;29:1082–8. [DOI] [PubMed] [Google Scholar]


