Supplemental Digital Content is available in the text
Keywords: cisplatin, efficacy, lipoplatin, meta-analysis, safety
Abstract
Background:
While liposomal cisplatin has shown enhanced drug tolerability and higher targeting property as compared with the conventional cisplatin, the doubt remains whether lipoplatin could improve its anticancer efficacy. What's more, there is still no systematic evaluation of the safety profiles of lipoplatin comparing with original cisplatin. Thus, we performed a systematic literature search for randomized clinical trials directly comparing efficacy and safety of liposomal cisplatin versus its conventional nonliposomal cisplatin.
Methods:
The electronic search was conducted in PubMed, Embase, The Cochrane Library, and ClinicalTrials.gov from inception to February 10, 2018. The pooled odds ratio (OR) and 95% confidence intervals (CIs) of progressive disease (PD), partial response (PR), stable disease (SD), and adverse events (AEs) were obtained to assess the efficacy and safety. Heterogeneity was estimated using the I2 test (I2 > 50%, significant heterogeneity).
Results:
The search yielded 5 clinical trials that meet inclusion criteria, with a total of 523 patients. We found that the liposome encapsulated cisplatin was more clinical efficacious than cisplatin as assessed by PD rate (OR, 0.46; 95% CI, 0.28–0.74; P = .002), while subgroup analysis of the only nonsmall cell lung cancer (NSCLC) patients showed higher response rates in PR (OR, 0.46; 95% CI, 0.28–0.74; P = .002) and PD (OR, 0.46; 95% CI, 0.28–0.74; P = .002) simultaneously. In addition, the toxicity meta-analysis revealed lipoplatin was much less toxic than the original cisplatin, with respect to grade 3 to 4 neurotoxicity (OR, 0.18; 95% CI, 0.04–0.74; P = .02), grade 3 to 4 leukopenia (OR, 0.47; 95% CI, 0.26–0.85; P = .01), grade 3 to 4 neutropenia (OR, 0.26; 95% CI, 0.09–0.71; P = .009), grade 1 and 2 nausea/vomiting (OR, 0.50; 95% CI, 0.32–0.77; P = .002), and grade 3 and 4 asthenia (OR, 0.11; 95% CI, 0.03–0.42; P = .001).
Conclusions:
This meta-analysis revealed that with both NSCLC and squamous cell carcinoma of the head and neck (SCCHN) patients, liposomal cisplatin-based chemotherapy offers significant advantages regarding the PD and reduced toxicities relative to conventional cisplatin.
1. Introduction
Chemotherapy is still one of the most effective approaches to treat cancers in the clinic, but the problems such as cytotoxicity and low bioavailability limit the future application of chemotherapeutic agents.[1] Nanoparticle formulations for packaging existing drugs have been widely used in the treatments of cancers for their tremendous therapeutic potential.[2,3] Passive extravasation of nano-carriers contributing to the “enhanced permeability and retention” (EPR) effect can increase tumor-targeted delivery while reducing normal tissue distribution,[4] theoretically enhancing the therapeutic efficiency and decreasing systemic toxicity.
Cisplatin, as a classical chemotherapeutical drug, has been recommended for the treatment of various cancers, such as metastatic testicular, ovarian, and transitional cell bladder cancer, nonsmall cell lung cancer (NSCLC), cervical cancer, and also head and neck cancer.[5] Unfortunately, its clinical use has been impeded by its severe toxicities, especially nephrotoxicity, neurotoxicity, nausea-vomiting, asthenia, and hematological toxicity.[6,7] Lipoplatin (Regulon Inc, Mountain View, CA), a novel FDA-approved liposomal formulation, is designed to enhance tumor targeting and reduce the systemic toxicity of cisplatin.[8,9] Many preclinical and clinical trials on lipoplatin have been carried out over the last 2 decades. Data resulting from the trials are promising in terms of toxicity profile, higher targeting properties, and longer half-life compared with cisplatin.[9–12] Theoretically, this enhanced drug tolerability and tumor drug delivery should result in increased anticancer efficacy. Yet, the pertinent clinical trials have not demonstrated clear evidence of superior efficacy of lipoplatin over conventional cisplatin (Fig. 1). One phase III clinical trial has shown lipoplatin appeared more effective than conventional cisplatin and had a more favorable safety profile, particularly regarding nephrotoxicity, neurotoxicity and asthenia,[13] whereas another phase III clinical study has demonstrated liposomal cisplatin reduced nephrotoxicity but had a similar antitumor efficacy compared with conventional formulation.[14] There is an urgent need to conduct a meta-analysis addressing pertinent evidence to evaluate whether liposomal cisplatin could enhance antitumor efficacy over conventional nonliposomal cisplatin. Furthermore, there is still no systematic evaluation of the safety profiles comparing lipoplatin with original cisplatin. Therefore, we performed a meta-analysis that incorporates all available clinical trials in this study to evaluate the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin.
Figure 1.
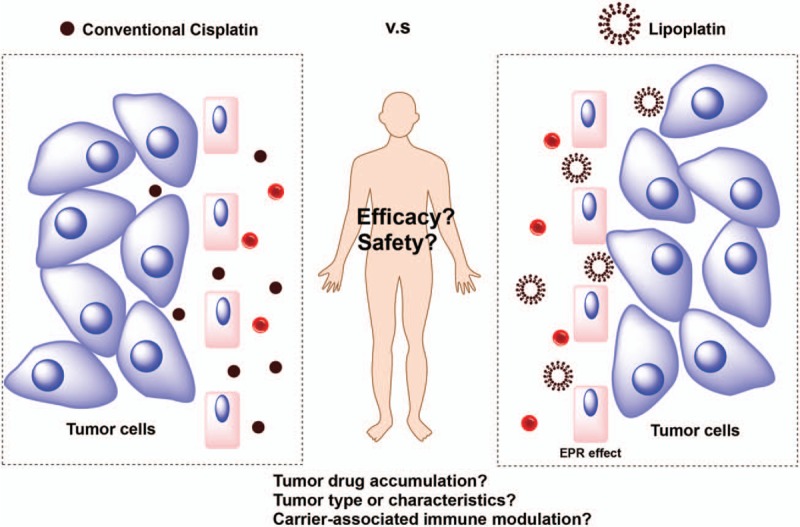
Is lipoplatin superior to conventional cisplatin in efficacy and safety?
2. Methods
We planned and performed this meta-analysis in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Intervention. This study did not require the ethic approval and informed consent as all analyses were carried out based on the data extracted from previous published trials.
2.1. Literature search
A systematic literature search was conducted in PubMed, Embase, The Cochrane Library, and ClinicalTrials.gov from inception to February 10, 2018. The search included the following terms: “liposomal cisplatin,” “cisplatin liposome,” “lipoplatin,” or “SPI-77.” The search was limited to clinical trials without restrictions on publication language. Any potentially relevant meeting abstracts and articles found in their reference lists were reviewed and considered for inclusion.
2.2. Inclusion and exclusion criteria
The following criteria were used for inclusion in the meta-analysis: randomized control trials (RCTs), containing both a cohort treated with the liposomal cisplatin and a cohort treated with the conventional formulation; at least 1 objective type of data reported, such as progressive disease (PD), partial response (PR), stable disease (SD), and adverse events (AEs). All cancer types, pretreatment status, and concurrent treatment were allowed. Studies were excluded in the following conditions: case reports; reviews; retrospective or prospective observational cohort studies and single-arm RCTs. Two investigators independently reviewed the articles for eligibility.
2.3. Data extraction
Extraction of study characteristics, efficacy and safety data from text, tables, and figures of included studies was done independently by 2 investigators. For each clinical trial, the following details were extracted and presented: study characteristics (first author, journal, year of publication), trial design characteristics (study design, outcome measurement, type of cancer, therapy regimen for each arm), study population (median age, number of patients evaluated for efficacy and safety endpoints in each arm), efficacy results (PD, PR, and SD) and AEs.
2.4. Statistical analysis
All pooled data on the PD, PR and SD, and AEs were analyzed with Review Manage (version 5.3, The Cochrane Collaboration, Oxford, UK). For response rates, the crude OR with the corresponding 95% CI was calculated in the meta-analysis. Heterogeneity was assessed by I2 index. When I2 value was < 50%, the fixed-effects model was employed for analysis, and if I2 value was ≥ 50%, random-effects model was used.[15] Considered of possible significant heterogeneity or inconsistency, subgroup analysis would be performed to find the possible sources. Results were considered statistically significant for a 2 tailed P value < .05.
2.5. Risk of bias assessment
The risk of bias of RCT studies was evaluated using the criteria described in the Cochrane handbook for systematic reviews of interventions.[16] These parameters included details of sequence generation, allocation concealment, treatment blinding, completeness of outcomes data, and presence of selective outcome reporting. The judgment was marked as “high risk,” “unclear risk,” or “low risk.” Trials that met all the criteria were categorized as low risk of bias, whereas those that met none were high risk of bias. The others were classified as unclear risk of bias if the information was insufficient to make a judgment.
3. Results
3.1. Search results
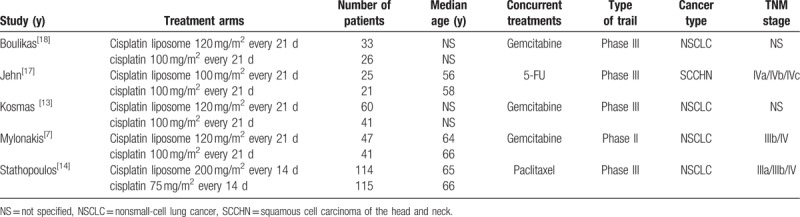
Based on the search criteria, a total of 361 records were retrieved, among which only 5 RCTs met criteria for inclusion in meta-analysis as summarized in Fig. 2. These studies were published between 2007 and 2010. A total of 523 patients were enrolled in trials and randomly assigned to receive chemotherapy with liposomal or conventional cisplatin (279 and 244 patients respectively). Among the 5 studies, 4 studies had data from NSCLC patients, while only 1 had data from SCCHN patients. In addition, the median age in 3 studies was similar, ranging from 56 to 66 years. The detailed characteristics of these studies, including treatment regimens and patient population, are summarized in Table 1.
Figure 2.
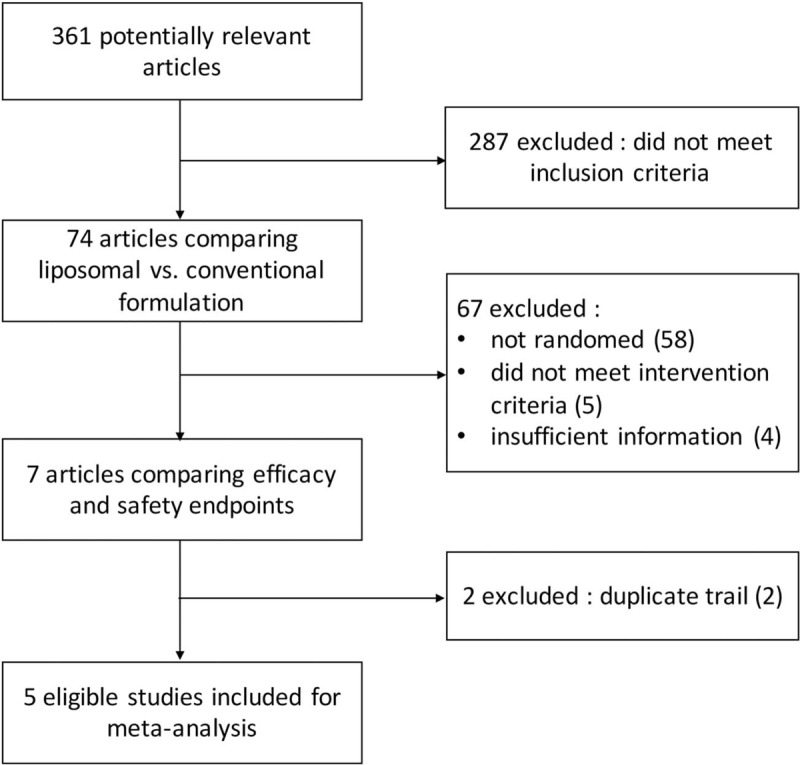
Flow diagram of systematic literature search for clinical trials comparing anticancer efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin.
Table 1.
Characteristics of selected clinical trials.
3.2. Efficacy results analysis
Response results including PD, PR, SD were all evaluated in all 5 trials. The statistical analysis revealed that lipoplatin significantly decreased the PD rate of cancer patients compared with the conventional cisplatin (OR, 0.46; 95% CI, 0.28–0.74; P = .002) (Fig. 3). Unfortunately, there was no significant difference between liposomal versus conventional formulations in the rates of PR (OR, 1.37; 95% CI, 0.95–1.98; P = .09) (Fig. 4) and SD (OR, 1.03; 95% CI, 0.72–1.48; P = .86) (Fig. 5). No considerable heterogeneities were found in all the terms of PD, PR, and SD. Moreover, among all the 5 trials, 4 studies as mentioned were related to NSCLC, thus subgroup analysis to explore the lipoplatin's efficiency in the treatment of NSCLC patients was carried out. The results revealed that lipid-based cisplatin significantly decreased the PD rate (OR, 0.38; 95% CI, 0.22–0.64; P = .0003), meanwhile improved the PR rate (OR, 1.53; 95% CI, 1.04–2.25; P = .03) in NSCLC populations. Nevertheless, lipoplatin regimen did not yield superior SD rate compared with the conventional cisplatin (OR, 0.97; 95% CI, 0.66–1.41; P = .86).
Figure 3.
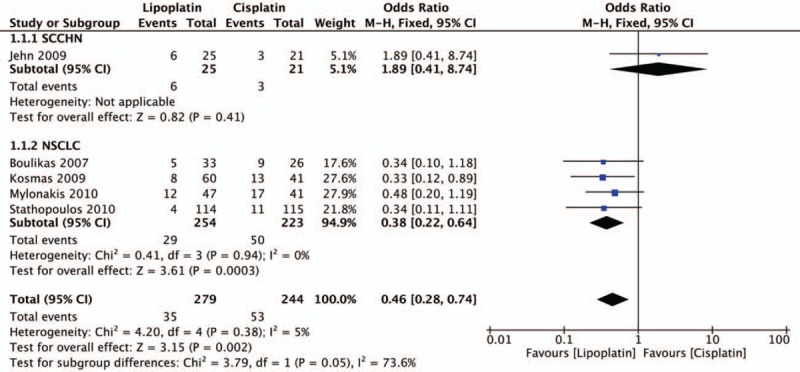
Forest plot of progressive disease (PD) rate.
Figure 4.
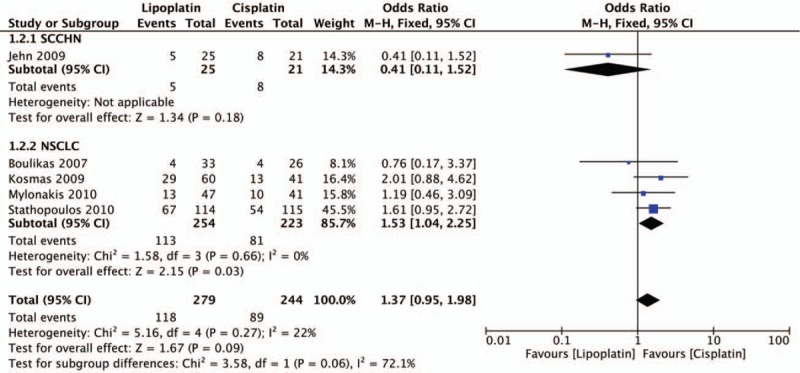
Forest plot of partial response (PR) rate.
Figure 5.
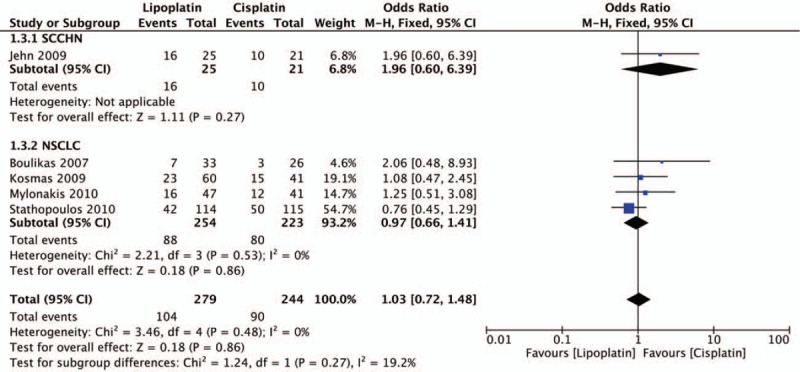
Forest plot of stable disease (SD) rate.
3.3. Adverse events analysis
The reporting of the side effects was heterogeneous among the 5 trials. Neurotoxicity, nephrotoxicity, anemia, leukopenia, thrombocytopenia, neutropenia, nausea/vomiting, asthenia, alopecia, diarrhea, and anorexia were evaluated in 2 or 3 trials (see Fig. S1–S21, Supplemental Content which presented the forest plots of comparison of toxicities between lipoplatin and conventional nonliposomal cisplatin). Overall, the risk of nephrotoxicity, anemia, thrombocytopenia, alopecia, diarrhea, and anorexia which were both in grade 1 to 2 and grade 3 to 4 between the 2 modalities was comparable, revealing that patients in the liposomal cisplatin group did not experience a greater incidence rate of the above-mentioned side effects. Nevertheless, the meta-analysis results showed significant differences in the incidence of grade 3 to 4 neurotoxicity (OR, 0.18; 95% CI, 0.04–0.74; P = .02), grade 3 to 4 leukopenia (OR, 0.47; 95% CI, 0.26–0.85; P = .01), grade 3 to 4 neutropenia (OR, 0.26; 95% CI, 0.09–0.71; P = .009), grade 1 to 2 nausea/vomiting (OR, 0.50; 95% CI, 0.32–0.77; P = .002), and grade 3 to 4 asthenia (OR, 0.11; 95% CI, 0.03–0.42; P = .001), favoring the use of the cisplatin lipid-based formulation when compared with nonliposomal cisplatin. A summary of toxicity meta-analysis is presented in Table 2 (detailed information see Fig. S1–S21, Supplemental Content).
Table 2.
Comparison of toxicities between liposomal cisplatin and conventional nonliposomal cisplatin.
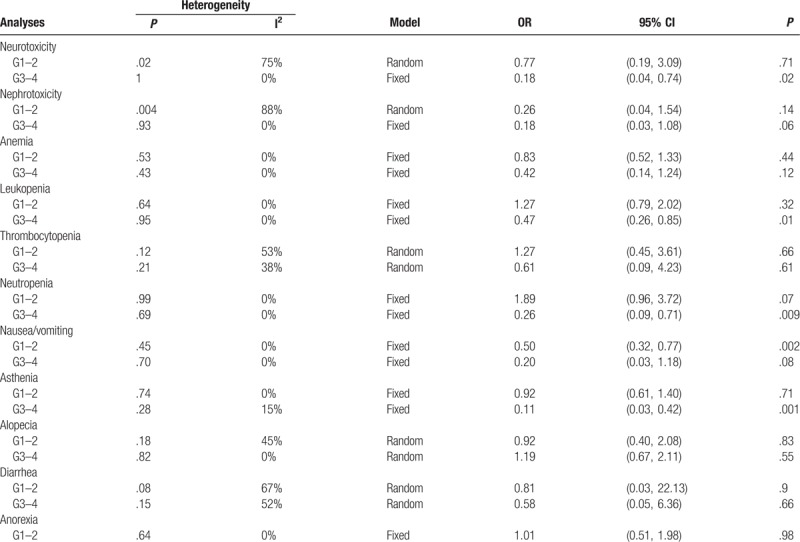
3.4. Quality assessment
The potential for study design bias is summarized in Fig. 6. All the 5 trials allocated patients to treatment arms randomly, but most of them did not report how the sequences were generated except that Stathopoulos et al[14] clarified their randomization was carried out according to the method of random permuted blocks within strata. Otherwise, 2 of the 5 trials were open-label[7,17] and 2 did not publish information on blinding,[13,18] while only one was conducted with evaluators blinded to treatment assignment.[14] Three trials showed evidence of incomplete outcomes’ data[13,17,18] and 2 trials with reporting biases had no information for key outcome data for interventions.[13,18] Together, these trial characteristic suggested a moderate risk of study design bias.
Figure 6.
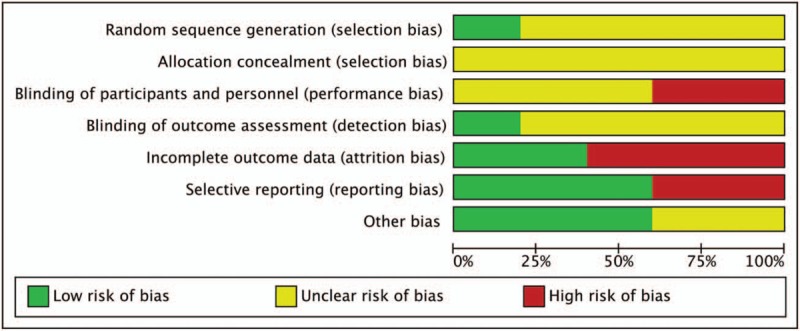
Risk of bias graph of the included clinical trials.
4. Discussion
Liposomes have been used to improve the therapeutic index of cytotoxic agents by modifying drug absorption, reducing metabolism, prolonging biological half-life, or reducing toxicity. Over the last 2 decades, a few anticancer nanoparticles based on liposomal platforms have been met efficacy criteria for regulatory approval, such as Caelyx and Myocet.[19] Although the liposomes have presented numerous advantages compared with their conventional chemotherapy, especially in the preclinical trials, there are concerns about their potential for their antitumor efficacy and toxicity to patients in clinic. For instance, Petersen et al[20] in a meta-analysis had previously found that liposomal doxorubicin did not show superior antitumor efficacy compared with the conventional doxorubicin in clinical trial settings, which indicated that the purported advantages of liposomal doxorubicin formulation in preclinical studies did not translate to the clinical trials successfully.
Lipoplatin is a liposomal formulation of cisplatin that is widely used in chemotherapy regimes.[21] Yet, the fundamental question also remains whether lipid-based cisplatin could significantly improve the therapeutic index of nonliposomal cisplatin in clinic. Our study is the first to systematically and objectively quantify the efficacy of liposomal cisplatin in comparison with conventional nonliposomal cisplatin. The liposome encapsulated cisplatin proved to be more clinical efficacious than cisplatin in the response rate of PD in cancer patients in our current study. Interestingly, further subgroup analysis of only the NSCLC patients showed higher response rates of PR and PD simultaneously. The improvement of PR might be related to the cancer type, whose specific parameters such as vascularity and immunogenicity can influence the pharmacokinetics and pharmacodynamics of liposomal drugs.[20] Analysis of overall survival (OS) and progression-free survival (PFS) were not performed in our study for the reason only 1 trial reported these endpoints, showing an average of 51 weeks versus 43 weeks (lipoplatin vs cisplatin) on OS, meanwhile an average of 30 weeks versus 26 weeks (lipoplatin vs cisplatin) concerning PFS. Although Stathopoulos et al[14] in a phase III trial had also observed the OS endpoint, the detailed results of the survival time were not reported and no significant difference was found between the 2 arms. Therefore, whether lipid-based cisplatin could contribute to enhanced OS and PFS remains to be determined and more clinical data are needed. Otherwise, almost no clinical trials explored the effect of empty liposomes on the clinical efficacy since recent preclinical studies[22,23] had observed that nondrug loaded nanoparticles including liposomes enhanced tumor growth in comparison with vehicle control in tumor-bearing mice unfortunately. This might be contributed to the polarization of tumor-associated macrophages, as well as the enhanced tumor angiogenesis by the empty nanocarriers.[22] Patients treated with nano-formulations might have the same immune responses, and whether the antitumor efficacy of nanodrug is offset by the tumor immunogenicity is still doubtful. Additional prospective clinical trials and preclinical mechanistic studies are needed to elucidate the role of drug vehicles in the antitumor efficacy.
All the 5 clinical studies had compared the adverse effects of lipoplatin with nonliposomal cisplatin, and our analysis is the first meta-analysis to our knowledge that combined the results of existing clinical trials and offered more practical results. Our analysis revealed cisplatin liposomal formulation was much less toxic than the original cisplatin. This minimized toxicity might be attributed to the tumor targeting profile of the lipid-based cisplatin, which reduced the drug accumulation in normal tissues.[10] In contrast, it should be clearly noticed that both liposomal and nonliposomal cisplatin formulations in the 5 clinical trials were in combination with one another cytotoxic agent, such as gemcitabine, 5-FU, or paclitaxel. So the toxicities in the patients were induced by lipoplatin/cisplatin and one of those chemotherapeutic agents together, not reflecting the real side effects of the cisplatin formulations themselves. What's more, the safety of the empty nano-vehicles also remains unclear. Therefore, more clinical trials should be encouraged to carry on to explore the real side effects of the lipoplatin itself, as well as the empty nanocarriers.
The current meta-analysis is limited by insufficient quantity of randomized clinical trials. Several trials in our initial search results were excluded because they compared liposomal formulation to standard of care regimens that did not contain the equivalent conventional formulation. Clearly, more clinical trials directly comparing carrier-mediated and conventional chemotherapy formulations are needed. Additionally, despite having covered several data based in our electronic search, with no limits used for publication date and language, some relevant clinical trials might not have been identified. Besides, our analysis centered on extracted data but not original data. Since an original data-based meta-analysis may produce more reliable estimation for association,[24] investigators should carefully study our results, especially for positive association in subgroup analysis. Moreover, the 2 regiments in each group were not the same, which may influence our results. Additionally, we included 2 studies[13,18] which were only published as abstracts, leading to insufficient details of patient characteristics for NSCLC patients. Overall, the intensity of its argumentation shall be improved through further development of additional large samples rigorously designed and high-quality RCTs.
5. Conclusion
In conclusion, liposomal cisplatin-based chemotherapy offers significant advantages regarding the PD and reduced toxicities of neurotoxicity, leukopenia, neutropenia, nausea/vomiting, and asthenia relative to conventional cisplatin in NSCLC and SCCHN patients. But there was no significant difference between liposomal versus conventional formulations in the rates of PR and SD. Additionally, after subgroup analysis of NSCLC populations, our study revealed that lipoplatin regimen yielded both superior PD and PR rates compared with the conventional cisplatin. But for OS and PFS, further studies are still needed to confirm the benefit. More importantly, the contribution of the EPR effect and the microenvironment to clinical efficacy is currently unclear and remains to be fully elucidated.
Author contributions
Data curation: Bei Xu, Min Zeng.
Funding acquisition: Lin Yu.
Methodology: Jiafu Feng.
Software: Jiawei Zeng.
Visualization: Jiafu Feng.
Writing – original draft: Bei Xu.
Writing – review & editing: Lin Yu.
Supplementary Material
Footnotes
Abbreviations: AE = adverse events, CI = confidence interval, NSCLC = nonsmall cell lung cancer, OR = odds ratio, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, RCT = randomized control trial, SCCHN = squamous cell carcinoma of the head and neck, SD = stable disease.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Xu B, Xia S, Wang FZ, et al. Polymeric nanomedicine for combined gene/chemotherapy elicits enhanced tumor suppression. Mol Pharm 2016;13:663–76. [DOI] [PubMed] [Google Scholar]
- [2].Maeda H, Fang J, Inutsuka T, et al. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol 2003 2003;3:319–28. [DOI] [PubMed] [Google Scholar]
- [3].Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A 1988;85:6949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu ZH, Liu DH, Wang LL, et al. Docetaxel-loaded pluronic p123 polymeric micelles: in vitro and in vivo evaluation. Int J Mol Sci 2011;12:1684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fantini M, Gianni L, Santelmo C, et al. Lipoplatin treatment in lung and breast cancer. Chemother Res Pract 2011;2011:125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boulikas T. Clinical overview on Lipoplatin™: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs 2009;18:1197–218. [DOI] [PubMed] [Google Scholar]
- [7].Mylonakis N, Athanasiou A, Ziras N, et al. Phase II study of liposomal cisplatin (LipoplatinTM) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer 2010;68:240–7. [DOI] [PubMed] [Google Scholar]
- [8].Boulikas T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep 2004;12:3–12. [PubMed] [Google Scholar]
- [9].Stathopoulos GP, Boulikas T, Vougiouka M, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep 2005;13:589–95. [PubMed] [Google Scholar]
- [10].Ravaioli A, Papi M, Pasquini E, et al. Lipoplatin monotherapy: a phase II trial of secondline treatment of metastatic non-small-cell lung cancer. J Chemother 2009;21:86–90. [DOI] [PubMed] [Google Scholar]
- [11].Devarajan P, Tarabishi R, Mishra J, et al. Low renal toxicity of lipoplatin compared to cisplatin in animals. Anticancer Res 2004;24:2193–200. [PubMed] [Google Scholar]
- [12].Jehn CF, Boulikas T, Kourvetaris A, et al. Pharmacokinetics of liposomal cisplatin (lipoplatin) in combination with 5-FU in patients with advanced head and neck cancer: first results of a phase III study. Anticancer Res 2007;27:471–5. [PubMed] [Google Scholar]
- [13].Kosmas C, Angel J, Athanasiou A, et al. Phase III study of lipoplatin plus gemcitabine versus cisplatin plus gemcitabine in advanced NSCLC; interim analysis. Eur J Cancer Suppl 2009;7:531. [Google Scholar]
- [14].Stathopoulos GP, Antoniou D, Dimitroulis J, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol 2010;21:2227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chiramel J, Backen AC, Pihlak R, et al. Targeting the epidermal growth factor receptor in addition to chemotherapy in patients with advanced pancreatic cancer: a systematic review and meta-analysis. Int J Mol Sci 2017;18:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins J, Green SR. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration 2011;http://handbook-5-1.cochrane.org [Google Scholar]
- [17].Boulikas T, Mylonakis N, Sarikos G, et al. Lipoplatin plus gemcitabine versus cisplatin plus gemcitabine in NSCLC: Preliminary results of a phase III trial. Asco Meeting Abstracts 2007;25. [Google Scholar]
- [18].Jehn CF, Boulikas T, Kourvetaris A, et al. First safety and response results of a randomized phase III study with liposomal platin in the treatment of advanced squamous cell carcinoma of the head and neck (SCCHN). Anticancer Res 2008;28:3961–4. [PubMed] [Google Scholar]
- [19].Pillai G. Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharmacy & Pharmaceutical Sciences 2014;doi:10.15226/2374-6866/1/2/0010. [Google Scholar]
- [20].Petersen GH, Alzghari SK, Chee W, et al. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Release 2016;28:255–64. [DOI] [PubMed] [Google Scholar]
- [21].Terkola R. Liposomal cisplatin: Lipoplatin. Euro J Oncol Pharmacy 2007;1: [Google Scholar]
- [22].Sabnani MK, Rajan R, Rowland B, et al. Liposome promotion of tumor growth is associated with angiogenesis and inhibition of antitumor immune responses. Nanomedicine 2015;11:259–62. [DOI] [PubMed] [Google Scholar]
- [23].Moghimi SM. Cancer nanomedicine and the complement system activation paradigm: anaphylaxis and tumour growth. J Control Release 2014;190:556–62. [DOI] [PubMed] [Google Scholar]
- [24].Liu Y, Ye G, Yan D, et al. Role of nab-paclitaxel in metastatic breast cancer: a meta-analysis of randomized clinical trials. Oncotarget 2017;8:72950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


