Abstract
Inexpensive and simple non-invasive indexes for predicting liver inflammation are urgently required, but have been poorly studied in chronic hepatitis B (CHB) patients with alanine transaminase (ALT) ≤2 times the upper limit of normal (ULN). A total of 356 CHB patients with ALT ≤2 ULN who presented at Huashan Hospital (n=181) and the First Hospital of Quanzhou (n=175) were enrolled and randomly divided into an experimental assessment cohort (n=238) and validation cohort (n=118) at a ratio of 2:1. Histological analysis of liver tissue was performed to determine the pathological stage according to the Scheuer scoring system. For the experimental assessment cohort, univariate and multivariate analysis identified aspartate aminotransferase (AST) and albumin (ALB) as independent predictors of liver necroinflammation [liver necroinflammation grade (G)≥2] in patients with ALT ≤2 ULN. Therefore, a novel index, the AST-to-ALB ratio (ATAR), was proposed, which had a better diagnostic performance [area under receiver operating characteristic curve (AUC)=0.721] than that of ALB (AUC=0.632; P=0.039 vs. ATAR) and AST (AUC=0.682; P=0.082 vs. ATAR). In the validation cohort, the AUC of ATAR (0.728) to identify patients with a G≥2 was slightly greater than that of AST (0.660; P=0.149 vs. ATAR) and ALB (0.672; P=0.282 vs. ATAR). Furthermore, a similar diagnostic superiority was also demonstrated in patients with ALT ≤1 ULN. Thus, ATAR may be a promising non-invasive surrogate marker for liver necroinflammation CHB patients with ALT ≤2 ULN and thereby determine whether anti-viral treatment should be initiated.
Keywords: chronic hepatitis B, hepatic necroinflammation, anti-viral therapy, model, diagnosis
Introduction
Hepatitis B virus (HBV) infection is a worldwide health problem that affects >0.35 billion individuals globally (1). Patients with chronic hepatitis B infection (CHB) have an increased risk of progressive end-stage liver disease, including liver cirrhosis and hepatocellular carcinoma (HCC). Anti-viral therapy may effectively inhibit HBV replication, alleviate the disease and ultimately prevent disease progression (2).
The recommended treatment guidelines proposed by the American Association for the Study of Liver Diseases (2), the European Association for the Study of the Liver (3) and the Asian Pacific Association for the Study of the Liver (4) all indicate that CHB patients with a Knodell histology activity index of ≥4 or moderate/severe necroinflammation [Scheuer grade (G) ≥2] and fibrosis (grade S ≥2) require anti-viral therapy. Liver biopsy (LB), the golden standard for evaluating liver pathology (4), has certain limitations owing to inter- and intra-observer variability, its invasive nature, pain, sampling errors and non-dynamic assessment of liver histopathology. FibroScan (5,6) and serum non-invasive biomarkers, including aspartate aminotransferase (AST) to platelet (PLT) ratio index (7) and Fibrosis-4 [based on age, alanine transaminase (ALT), AST and PLT] (8), have been reported to have high diagnostic accuracy in identifying liver fibrosis and cirrhosis. However, these biomarkers were less useful in diagnosing liver necroinflammation, and no non-invasive biomarker for predicting liver necroinflammation has been recommended by recent guidelines. Thus, additional novel non-invasive biomarkers to assess liver necroinflammation and initiation of anti-viral therapy are urgently required.
Circulating microRNAs (miRs), including miR-122 (9,10), miR-124 (11) and miR-125b (12), as well as intracellular HBV covalently closed circular DNA (13), quantitative hepatitis B core antibody (14), the PIPS index (based on phosphatidylserine and phosphatidylinositol) (15) and inflammatory activity scoring models (16), were reported to be significant predictors for liver necroinflammation in chronic HBV patients. However, several of these biomarkers are difficult to determine in clinical practice, particularly in resource-limited settings, and it has remained elusive whether they are able to independently predict liver necroinflammation in CHB patients with ALT ≤2 times the upper limit of normal (ULN). The aim of the present study was to develop and validate a simple model based on routine blood indexes for predicting significant liver necroinflammation according to a modified Scheuer scoring system (17) in CHB patients with ALT ≤2 ULN. The indexes established may be used to determine whether anti-viral treatment should be initiated and may spare certain patients from unnecessary liver biopsy, particularly in developing countries.
Patients and methods
Patients
A retrospective study was performed on a set of CHB patients from Shanghai between January 2006 and September 2016 who presented at the Department of Infectious Diseases of Huashan Hospital, Fudan University (Shanghai, China). Another retrospective independent study of consecutive CHB patients from the First Hospital of Quanzhou, Fujian Medical University (Quanzhou, China) who underwent LB between October 2005 and August 2015 was selected with the same inclusion and exclusion criteria (18), which were as follows: (1) All CHB patients were positive for hepatitis B surface antigen (HBsAg) for >6 months (19); (2) Patients with any other types of viral hepatitis (HAV, HCV or HDV) or human immunodeficiency virus infection, drug-induced hepatitis, autoimmune hepatitis, decompensated cirrhosis, primary biliary cirrhosis, non-alcoholic steatohepatitis and Wilson's disease were excluded; (3) None of the patients had thyroid disease, heart disease or kidney disease. The demographics and laboratory parameters were recorded within 1 week prior to LB. Written informed consent was obtained from each patient prior to LB and all trials were approved by the Ethics Committees of Huashan Hospital, Fudan University (Shanghai, China) and the First Hospital of Quanzhou, Fujian Medical University (Quanzhou, China).
Data collection
The Patients' demographic data and results of laboratory tests were recorded within 7 days following LB, including age, gender, white blood cell count (WBC), granulocyte ratio (GR), red blood cell count (RBC), hemoglobin (Hgb), platelet count (PLT), prothrombin time (PT) and international normalized ratio (INR). Serum albumin (ALB), globulin (GLB), total bilirubin (TBil), ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT) were detected using an automatic biochemical analyzer (Hitachi 7600P; Hitachi, Tokyo, Japan) for the Shanghai set and an automatic biochemical analyzer (Beckman LX-20; Beckman Coulter, Brea, CA) for the Quanzhou set. HBsAg, anti-HBs, hepatitis B e antigen (HBeAg), anti-HBe, anti-HBc, anti-HAV and anti-HCV were determined by an Architect QT assay (Architect i2000 SR; Abbott Core Laboratory, Lake Forest, IL, USA) for the Shanghai and Quanzhou sets. Serum HBV DNA was determined using a commercial Real-time polymerase chain reaction (PCR) kit on a Light Cycler 480 Real-time PCR system (Roche Diagnostics, Basel, Switzerland) for the Shanghai set and on a PE 9700 Thermal Cycler (Perkin Elmer, Inc., Waltham, MA, USA) for the Quanzhou set in accordance with the manufacturer's protocols. An ALT level of ≤40 U/l or 80 U/l in the experimental assessment and validation cohort were defined as ALT ≤1 ULN or ≤2 ULN, respectively.
Liver biopsy
Percutaneous LB under ultrasound guidance was performed using 16 G needles (MAX-CORE® MC1616; BARD® Peripheral Vascular, Inc., Tempe, AZ, USA) for the Shanghai set and disposable needles (Manan Super-Core; Medical Device Technologies Co., Ltd, Gainesville, FL, USA) for the Quanzhou set. The mean number of portal tracts was 10 (range, 8–18) and samples with a length of >1.5 cm were obtained for diagnosis (range, 1.7–3.6 cm). The specimens were formalin-fixed, paraffin-embedded and H&E stained for histological analysis. The histological grading for liver necroinflammation (G0-G4) was performed according to Scheuer's scoring system (17) by specialized pathologists. Moderate/severe necroinflammation was considered if G≥2.
Statistical analysis
The data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as the median and interquartile range and compared using the Mann-Whitney U-test. Categorical variables were compared using the chi-square test. Correlations were analyzed by calculating Spearman's rank correlation coefficient. A multiple logistic regression analysis was performed for all the factors significantly associated with liver necroinflammatory activity on univariate analyses, and a new predictive model was selected using stepwise-forward logistic regression. The diagnostic efficacy was evaluated by drawing the receiver operating characteristic (ROC) curve and determining the area under the curve (AUC), and the diagnostic accuracy was evaluated by determining the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 740 patients with chronic HBV infection from Huashan Hospital (Shanghai, China; n=330) and the First Hospital of Quanzhou (Quanzhou, China; n=410) who had undergone LB were enrolled in the present study. As presented in Fig. 1, a total of 149 patients from Huashan Hospital were excluded owing to accompanying HCC (n=5), insufficient laboratory data (n=40) and elevated ALT levels (ALT ≥2 ULN; n=104). Similarly, 235 subjects from the First Hospital of Quanzhou were excluded due to HCC (n=16) and alcoholic liver disease (n=24), incomplete laboratory data (n=102) and elevated ALT levels (n=93). The final study population, consisting of 356 patients, was randomly divided into an experimental assessment cohort (n=238) and a validation cohort (n=118) at a ratio of 2:1.
Figure 1.
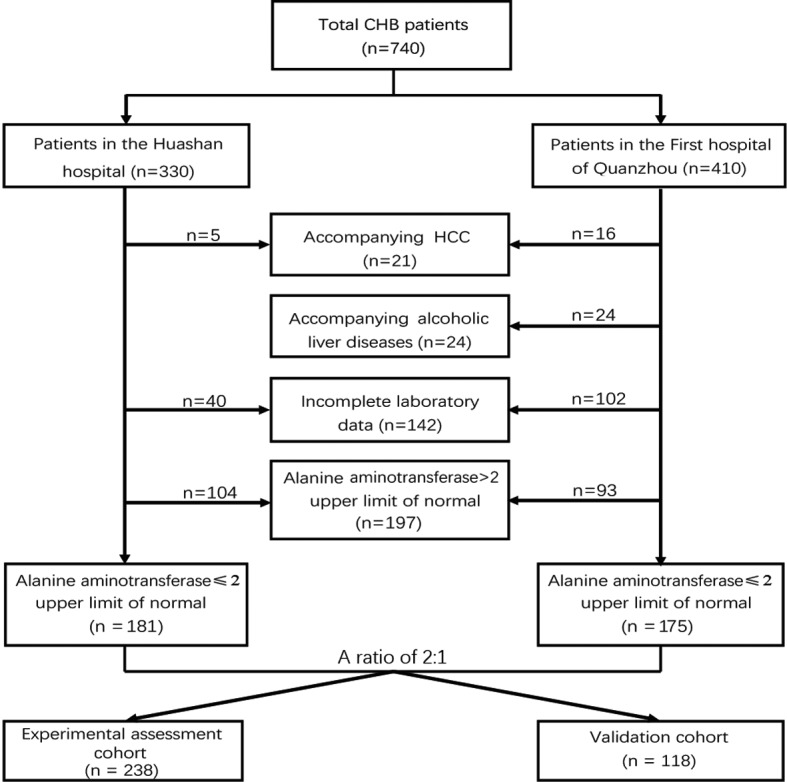
Flow chart of the study design and recruitment of study subjects. Patients with CHB (n=740) from Huashan Hospital affiliated to Fudan University (Shanghai, China; n=330) and the First Hospital of Quanzhou affiliated to Fujian Medical University (Quanzhou, China; n=410) were evaluated. A total of 356 patients with alanine aminotransferase ≤2 upper limit of normal were included in the present study and were randomly divided into an experimental assessment cohort (n=238) and a validation cohort (n=118) at a ratio of 2:1. Histological grading of liver necroinflammation was performed according to the Scheuer scoring system. HCC, hepatocellular carcinoma; CHB, chronic hepatitis B.
The baseline characteristics of all CHB patients in the experimental assessmentand validation cohorts are presented in Table I. The two cohorts were well matched in terms of their baseline characteristics. The stages of liver necroinflammation were also similar between the two cohorts (Table I).
Table I.
Baseline characteristics of the study population in the experimental assessment set and in validation set.
| Variable | Normal ranges | Experimental assessment cohort (n=238) | Validation cohort (n=118) | P-value |
|---|---|---|---|---|
| Male sex | – | 166 (69.70) | 60 (50.80) | <0.001 |
| Age (years) | – | 34.00 (26.00–41.25) | 32.50 (27.00–40.00) | 0.856 |
| ALB (g/l) | 40–55 | 42.90 (36.80–46.00) | 42.00 (34.25–45.05) | 0.312 |
| GLB (g/l) | 20–40 | 31.90 (28.08–36.38) | 31.60 (29.00–37.95) | 0.491 |
| TBil (µmol/l) | 3.4–39.7 | 13.90 (10.70–19.60) | 13.85 (9.48–18.80) | 0.448 |
| ALT (IU/l) | 9–40 | 37.00 (26.00–52.00) | 38.00 (26.75–3.25) | 0.701 |
| AST (IU/l) | 15–40 | 29.00 (23.25–37.75) | 28.00 (22.75–35.00) | 0.178 |
| GGT (IU/l) | 10–60 | 18.00 (13.00–34.50) | 19.50 (13.00–30.75) | 0.852 |
| ALP (IU/l) | 45–125 | 69.00 (56.00–85.00) | 73.00 (59.50–85.00) | 0.172 |
| PT (sec) | 10.7–13.1 | 11.80 (11.10–12.40) | 11.50 (10.90–12.10) | 0.052 |
| INR | 0.92–1.15 | 1.03 (0.99–1.08) | 1.04 (0.97–1.09) | 0.742 |
| WBC (109/l) | 3.5–9.5 | 5.70 (4.82–6.98) | 5.74 (4.90–6.90) | 0.736 |
| GR (%) | 40–75 | 58.71 (51.80–64.65) | 60.10 (52.35–66.15) | 0.313 |
| Lym (%) | 20–50 | 32.50 (26.45–38.40) | 32.20 (24.60–36.00) | 0.080 |
| RBC (1012/l) | 4.3–5.8 | 4.80 (4.44–5.17) | 4.92 (4.37–5.21) | 0.572 |
| Hgb (g/l) | 130–175 | 146.00 (134.00–157.00) | 151.00 (136.00–159.00) | 0.153 |
| PLT (109/l) | 125–350 | 196.00 (165.25–232.75) | 202.00 (164.50–238.00) | 0.613 |
| HBeAg+ | 0–1 | 150 (63.0) | 90 (76.3) | 0.012 |
| HBV DNA (lg IU/ml) | 2.74–9 | 4.72 (3.70–7.07) | 6.36 (3.76–8.07) | 0.055 |
| Necroinflammation stagea | ||||
| G0 | 44 (18.5) | 18 (15.3) | ||
| G1 | 75 (31.5) | 33 (28.0) | ||
| G2 | 70 (29.4) | 45 (38.1) | ||
| G3 | 27 (11.3) | 18 (15.3) | ||
| G4 | 22 (9.2) | 4 (3.4) | 0.120 | |
| ≥G2 | 118 (49.6) | 67 (56.8) | 0.201 |
Values are expressed as n (%) or the median (25th to 75th percentile).
Staging according to Scheuer (≥G2 is considered to indicate moderate/severe liver necroinflammation). HBsAg, hepatitis B surface antigen; Lym, lymphocytes; HBeAg, hepatitis B e antigen; GLB, globulin; ALB, albumin; TBil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; PT, prothrombin time; WBC, white blood cell count; GR, granulocyte ratio; RBC, red blood cell count; Hgb, hemoglobin; PLT, platelet count.
Predictors and regression models
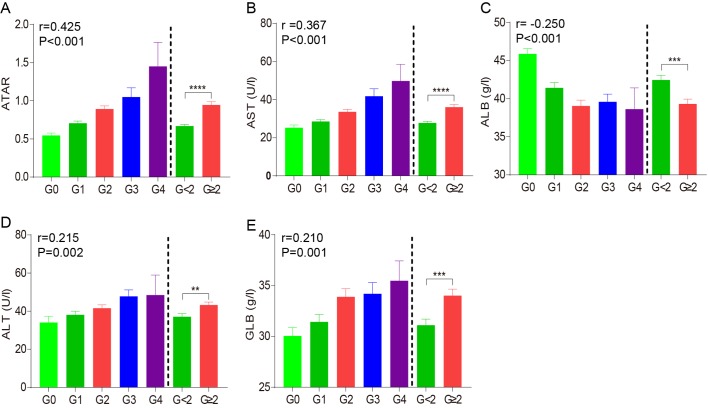
In the experimental assessment cohort, ALB, GLB, ALT and AST were significantly different between moderate/severe liver necroinflammation groups (G≥2) and mild liver necroinflammation groups (G<2) (Fig. 2), and univariate analysis revealed that ALB (P=0.005), GLB (P=0.002), ALT (P=0.010) and AST (P<0.001) were predictive factors of G≥2. (Table II). However, no differences were observed for the other indicators (P>0.05). The step-forward multiple regression analysis revealed that only AST (P<0.001) and ALB (P=0.002) were independently correlated with G≥2. AST (r=0.367, P<0.001) was positively associated with liver necroinflammation, while the ALB (r=−0.250, P<0.001) was negatively correlated with liver necroinflammation. To improve the prediction of significant liver necroinflammation, a final multiple regression model incorporating AST and ALB was developed: The AST (U/l)/ALB (g/l) ratio (ATAR). This proposed ATAR value progressively increased with ascending liver necroinflammation stage, with a higher correlation coefficient than AST or ALB alone (r=0.425, P<0.001; Fig. 2A-C). The median ATAR in the G≥2 group was obviously higher than that in the G<2 group (0.95 vs. 0.67, P<0.001; Fig. 2A). Therefore, the new ATAR model based on AST and ALB levels is a good independent indicator for reflecting the degree of liver necroinflammation.
Figure 2.
Histograms displaying the (A) ATAR ratio, as well as the levels of (B) AST, (C) ALB, (D) ALT and (E) GLB for patients with different Scheuer score of liver necroinflammation in the experimental assessment cohort. **P<0.01, ***P<0.001 and ****P<0.0001. AST, aspartate aminotransferase; ATAR, AST to ALB ratio; ALB, albumin; ALT, aminotransferase; GLN, globulin.
Table II.
Univariate and multivariate analyses of the association between biomarkers and liver inflammation.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
| ALB | 0.940 (0.901–0.981) | 0.005 | 0.098 (0.885–0.972) | 0.002 |
| GLB | 1.072 (1.025–1.121) | 0.002 | ||
| ALT | 1.020 (1.005–1.035) | 0.010 | ||
| AST | 1.068 (1.038–1.099) | <0.001 | 1.006 (1.033–1.101) | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; GLB, globulin; OR, odds ratio; CI, confidence interval.
ATAR has an improved predictive value for G≥2 over that of other markers in patients with CHB with ALT ≤2 ULN
In the experimental assessment cohort (n=238), the distribution of histopathological stages of liver necroinflammation was as follows: G0, 44 (18.5%); G1, 75 (31.5%); G2, 70 (29.4%); G3, 27 (11.3%); and G4, 22 (9.2%; Table I). Except for ALB, the ATAR and GLB, ALT and AST increased with increasing liver necroinflammation stage. For the prediction of G≥2, the AUC for ATAR (0.721, 95% CI: 0.656–0.780) was markedly larger than that for ALB (0.632, 95% CI: 0.563–0.696; P=0.039 vs. ATAR), GLB (0.633, 95% CI: 0.564–0.697; P=0.029 vs. ATAR) and ALT (0.602, 95% CI: 0.537–0.665; P=0.001 vs. ATAR), but only slightly larger than that for AST (0.682, 95% CI: 0.619–0.741; P=0.082 vs. ATAR; Table III). Furthermore, ATAR demonstrates the best sensitivity (71.19%) for predicting G≥2 than ALB (46.61%), GLB (41.18%), ALT (56.72%) and AST (60.61%). The best cut off values were as follows: 0.707 for ATAR, 39.9 for ALB, 35 for GLB, 38 for ALT and 29 for AST.
Table III.
Diagnostic accuracy of various indices in patients with ALT ≤2 ULN or ALT ≤1 ULN.
| ALT ≤2 ULN | ALT ≤1 ULN | |||
|---|---|---|---|---|
| Model | Experimental assessment cohort (n=238) | Validation cohort (n=118) | Experimental assessment cohort (n=130) | Validation cohort (n=63) |
| ATAR | ||||
| AUC (95% CI) | 0.721 (0.656–0.780) | 0.770 (0.683–0.843) | 0.728 (0.638–0.806) | 0.709 (0.581–0.817) |
| Cut-off value | 0.707 | 0.707 | 0.640 | 0.640 |
| Sensitivity/specificity (%) | 71.19/65.31 | 71.64/78.00 | 70.91/72.58 | 59.38/77.42 |
| PPV/NPV (%) | 67.24/69.39 | 76.51/73.34 | 72.11/70.69 | 72.45/65.59 |
| Positive/negative LR | 2.05/0.44 | 3.26/0.36 | 2.59/0.40 | 2.63/0.52 |
| Correctly classified (%) | 71.59 | 81.35 | 70.18 | 73.08 |
| ALB | ||||
| AUC (95% CI) | 0.632 (0.563–0.696) | 0.724 (0.633–0.802) | 0.672 (0.580–0.756) | 0.689 (0.560–0.800) |
| Cut-off value | 39.9 | 39.9 | 42.5 | 42.5 |
| Sensitivity/specificity (%) | 46.61/80.61 | 50.75/86.00 | 69.09/61.29 | 71.87/67.74 |
| PPV/NPV (%) | 70.62/60.16 | 78.38/63.59 | 63.50/66.48 | 69.02/70.66 |
| Positive/negative LR | 2.4/0.66 | 3.62/0.57 | 1.78/0.50 | 2.23/0.42 |
| Correctly classified (%) | 69.23 | 78.57 | 60.66 | 68.75 |
| GLB | ||||
| AUC (95% CI) | 0.633 (0.564–0.697) | 0.601 (0.506–0.690) | 0.629 (0.536–0.716) | 0.539 (0.409–0.665) |
| Cut-off value | 35 | 35 | 34.8 | 34.8 |
| Sensitivity/specificity (%) | 41.18/84.21 | 41.79/82.00 | 46.55/80.00 | 40.63/80.65 |
| PPV/NPV (%) | 72.28/58.88 | 69.89/58.48 | 69.95/59.95 | 67.74/57.60 |
| Positive/negative LR | 2.61/0.70 | 2.32/0.71 | 2.33/0.67 | 2.1/0.74 |
| Correctly classified (%) | 66.56 | 73.68 | 69.23 | 68.41 |
| ALT | ||||
| AUC (95% CI) | 0.602 (0.537–0.665) | 0.577 (0.482–0.667) | 0.550 (0.460–0.637) | 0.519 (0.389–0.646) |
| Cut-off value | 38 | 38 | 26 | 26 |
| Sensitivity/specificity (%) | 56.72/62.50 | 53.73/56.86 | 57.14/59.70 | 56.25/48.39 |
| PPV/NPV (%) | 60.85/59.08 | 55.47/55.13 | 58.64/58.21 | 52.15/52.52 |
| Positive/negative LR | 1.51/0.69 | 1.25/0.81 | 1.42/0.72 | 1.09/0.90 |
| Correctly classified (%) | 66.09 | 62.07 | 56.25 | 52.94 |
| AST | ||||
| AUC (95% CI) | 0.682 (0.619–0.741) | 0.697 (0.606–0.778) | 0.660 (0.572–0.741) | 0.677 (0.548–0.790) |
| Cut-off value | 29 | 29 | 27 | 27 |
| Sensitivity/specificity (%) | 60.61/68.27 | 55.22/74.51 | 46.03/82.09 | 37.50/80.65 |
| PPV/NPV (%) | 65.64/63.41 | 67.58/62.41 | 71.99/60.33 | 65.96/56.34 |
| Positive/negative LR | 1.91/0.58 | 2.17/0.60 | 2.57/0.66 | 1.94/0.86 |
| Correctly classified (%) | 70.80 | 74 | 69.05 | 66.67 |
| Comparison of AUC | ||||
| ATAR vs. ALB | 0.039 | 0.297 | 0.282 | 0.695 |
| ATAR vs. GLB | 0.029 | 0.005 | 0.043 | 0.054 |
| ATAR vs. ALT | 0.001 | <0.001 | 0.006 | 0.021 |
| ATAR vs. AST | 0.082 | 0.023 | 0.149 | 0.491 |
AST, aspartate aminotransferase; ATAR, AST to ALB ratio; ALB, albumin; ALT, aminotransferase; GLN, globulin; ULN, upper limit of normal; AUC, area under receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value; LR, Likelihood ratio; CI, confidence interval; ULN, upper limit of normal.
In the validation cohort (n=118), the distribution of histopathological staging of liver necroinflammation was as follows: G0, 18 (15.3%); G1, 33(28.0%); G2, 45 (38.1%); G3, 18 (15.3%); and G4, 4 (3.4%; Table I). For predicting G≥2, the AUC for ATAR (0.770, 95% CI: 0.683–0.843) was markedly higher than that for GLB (0.601, 95% CI: 0.506–0.690; P=0.005), ALT (0.577, 95% CI: 0.482–0.667; P<0.001) and AST (0.697, 95% CI: 0.606–0.778; P=0.023), but only slightly higher than that of ALB (0.724, 95% CI: 0.633–0.802; P=0.297 vs. ATAR; Table III). The best cut-off value for ATAR, GLB, ALB, ALT and AST were the same as in the experimental assessment cohort. In short, compared to other markers, the novel ATAR model had the highest AUC for predicting moderate/severe liver necroinflammation.
Predictive performance of ATAR in patients with ALT ≤1 ULN
In the clinical setting, numerous CHB patients with ALT ≤1 ULN have an existing severe liver necroinflammation, which eventually progresses to cirrhosis or HCC. Therefore, the present study determined whether ATAR may be used for predicting G≥2 in patients with ALT ≤1 ULN. The experimental assessment cohort contained 130 patients (54.62%) with ALT ≤1 ULN, of which 63 (48.46%) were staged as G≥2. In the validation cohort, 63 patients (53.39%) had normal ALT levels, of which 32 (50.79%) were staged as G≥2.
As in patients with slightly elevated ALT, the ATAR also performed well in patients with normal ALT from the experimental assessment cohort and displayed a higher AUC (0.728, 95% CI 0.638–0.806) in predicting G≥2 than ALB (0.672, 95% CI: 0.580–0.756; P=0.282), GLB (0.629, 95% CI: 0.536–0.716; P=0.043), ALT (0.550, 95% CI: 0.460–0.637; P=0.006) and AST (0.660, 95% CI 0.572–0.741, P=0.149; Table III). The similar phenomenon was also observed in the validation cohort (Table III). For predicting G≥2, the AUC for ATAR (0.709, 95% CI: 0.581–0.817) was markedly higher than that for ALT (0.519, 95% CI: 0.389–0.646; P=0.021), but only slightly higher than that of GLB (0.539, 95% CI: 0.409–0.665; P=0.054), ALB (0.689, 95% CI: 0.560–0.800; P=0.695) and AST (0.677, 95% CI: 0.548–0.790; P=0.491; Table III). For predicting G≥2, the best cut-off value was 0.640 for ATAR, 42.5 for ALB, 34.8 for GLB, 26 for ALT and 27 for AST in the experimental assessment and validation cohort. These results indicate that ATAR is more specific than the other clinical markers in predicting liver necroinflammation in patients with ALT ≤1 ULN.
Predictive performance of ATAR in HBeAg+ and HBeAg− CHB patients
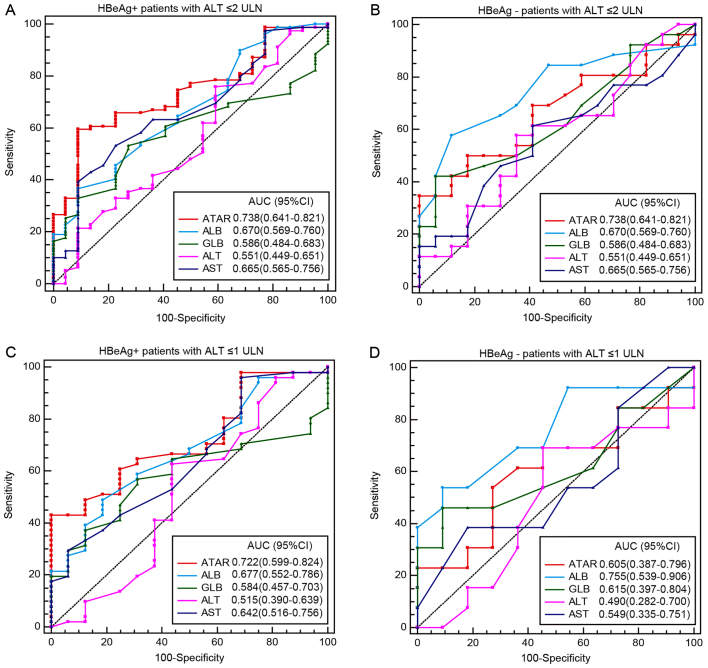
To validate whether ATAR may be used for predicting G≥2 in CHB patients with different HBeAg status, the diagnostic performance of ATAR compared with that of the other markers was assessed in HBeAg+ patients and HBeAg− patients separately (Fig. 3). In the population with ALT ≤2 ULN, 240 patients (67.41%) were positive for HBeAg, of which 134 (55.83%) were staged as G≥2. Furthermore, 116 patients (32.59%) were negative for HBeAg, of which 66 (56.90%) were staged as G≥2. Of the patients with ALT ≤1 ULN, 131 (66.88%) were positive for HBeAg and 62 (33.12%) were negative, of which 59 (45.04%) and 34 (54.83%) patients were staged as G≥2, respectively. For predicting G≥2, the ATAR displayed the highest AUC (0.738, 95% CI: 0.641–0.821) in HBeAg+ patients with ALT ≤2 ULN among the markers assessed, including ALB (0.670, 95% CI: 0.569–0.760; P=0.439), GLB (0.586, 95% CI: 0.484–0.716; P=0.073), ALT (0.551, 95% CI: 0.449–0.651; P<0.001) and AST (0.665, 95% CI: 0.565–0.756; P=0.006; Fig. 3A). Similarly, in HBeAg+ patients with ALT ≤1 ULN, the AUC of ATAR (0.722, 95% CI: 0.559–0.824) was significantly higher than that of ALT (0.515, 95% CI: 0.390–0.639; P=0.007) and AST (0.642, 95% CI: 0.516–0.756; P=0.035), but only slightly higher than that of GLB (0.584, 95% CI: 0.457–0.703; P=0.174) and ALB (0.677, 95% CI: 0.552–0.786; P=0.642; Fig. 3C). However, the ATAR did not display the highest AUC neither in HBeAg− patients with ALT ≤2 ULN or ALT ≤1 ULN (Fig. 3B and D).
Figure 3.
Receiver operating characteristic curves of non-invasive biomarkers, including the ATAR ratio and the levels of AST, ALB, ALT and GLB for prediction of significant liver necroinflammation in HBeAg+ patients with (A) ALT ≤2 ULN (the cut-off values were as follows: 0.617 for ATAR, 41.9 for ALB, 30 for GLB, 22 for ALT and 29 for AST), (B) HBeAg− patients with ALT ≤2 ULN (the cut-off values were as follows: 0.830 for ATAR, 43 for ALB, 34 for GLB, 37 for ALT and 25 for AST), (C) HBeAg+ patients with ALT ≤1 ULN (the cut-off values were as follows: 0.617 for ATAR, 42.9 for ALB, 30 for GLB, 32 for ALT and 16 for AST) and (D) HBeAg− patients with ALT ≤1 ULN (the cut-off values were as follows: 0.5 for ATAR, 42. for ALB, 34 for GLB, 26 for ALT and 18 for AST). AST, aspartate aminotransferase; ATAR, AST to ALB ratio; ALB, albumin; ALT, aminotransferase; GLN, globulin; ULN, upper limit of normal; AUC, area under curve; CI, confidence interval; HBeAg, hepatitis B e antigen.
Discussion
Accurate assessment of liver necroinflammation is essential for the determination of appropriate anti-viral treatment and the prognosis for patients with CHB. However, the lack of an accurate, easily applied and reproducible model for assessment of liver inflammation remains a major limitation in clinical practice. In the present study, a non-invasive model (named as ATAR) was constructed to predict liver necroinflammation in patients with ALT ≤2 ULN. It successfully predicted G≥2 for 71.59% of patients in the experimental assessment cohort, 81.35% in the validation cohort and 73.86% in the entire cohort. Therefore, the ATAR may be a potential efficient non-invasive index to predict liver necroinflammation and determine whether to initiate anti-viral treatment in patients with ALT ≤2 ULN.
At present, the degree of liver necroinflammation and therapeutic judgment for anti-viral therapy are mainly reflected by the levels of ALT, which are affected by numerous factors. Numerous studies have indicated that ALT levels are not in parallel to liver inflammation. For instance, as certain CHB patients with normal ALT levels have an existing liver inflammation, they fail to receive anti-viral therapy on time, thus gradually progressing to cirrhosis or HCC. In the experimental assessment cohort, >50% of patients with normal ALT levels had significant liver necroinflammation, which is similar to the result of a previous study (11). Thus, the early diagnosis of liver necroinflammation has an important role not only in the therapeutic assessment of HBV infection but also in the control of disease progression (20).
Apart from LB and FibroScan, numerous non-invasive models have been established to estimate liver fibrosis or cirrhosis with high accuracy. However, only a small number of them were further identified as good predictors for liver necroinflammation. Therefore, the present study was performed to develop a simple index derived from routine blood parameters to predict liver necroinflammation in Chinese CHB patients. AST and ALB were identified as independent predictors of liver necroinflammation and ATAR was proposed as the ratio of these two parameters. Serum AST had been widely used to evaluate liver necroinflammation due to its association with mitochondrial injury (7). Serum ALB is also associated with liver necroinflammation, as its production is affected by liver injury (21). In addition, ALT is commonly used to evaluate the severity of liver necroinflammation, with increases in ALT levels indicating histopathological changes in the liver, even in patients with ALT within the normal range (22). Serum GLB is also synthesized in the liver and the concentration of GLB changes after exposure to HBV (23). In the experimental assessment cohort, the ATAR displayed a better diagnostic value than AST, ALB, GLB and ALT. The sensitivity and specificity of the ATAR in predicting liver necroinflammation were 71.19 and 65.31%, respectively, at a cut-off value of 0.707. Of note, ATAR also performed well in patients with ALT ≤1 ULN in the experimental assessment as well as validation cohorts, where it displayed the highest AUC in predicting liver necroinflammation among all indexes assessed. However, ATAR only displayed a better diagnostic value than AST, ALB, GLB and ALT in HBeAg+ patients, but not in HBeAg− CHB patients, regardless of their ALT levels being ≤2 or ≤1 ULN.
Several limitations were noted in the present study. First, it is a retrospective study and the sample size is relatively small, and the cohorts are not representative of the entirety of CHB patients in China. Furthermore, the percentage of male patients and the ratio of patients positive for HBeAg differed between the two cohorts, which may lead to differences in results between the two groups. Lastly, we did not determine whether ATAR was an independent predictor for liver necroinflammation using multivariate regression.
In conclusion, the present study indicated that the ATAR is a novel and simple independent indicator for predicting moderate/severe liver necroinflammation in CHB patients with ALT ≤2 and ≤1 ULN. By applying the pre-defined cutoffs, most of the patients were correctly classified with regard to their requirement of anti-viral therapy. Thus, the new ATAR model comprising the common blood test parameters AST and ALB may be a promising non-invasive surrogate marker to determine whether anti-viral treatment should be initiated, particularly in developing countries.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81400625, 81670528 and 81672009), the Shanghai Pujiang Program (grant no. 17PJD005), the National Science and Technology Major Project of China (grant nos. 2017ZX10202202 and 2017ZX10202203-007), the Fujian province Natural Science Foundation of China (grant nos. 2015J01413 and 2016Y9065) and the Chinese foundation for hepatitis prevention and control (grant no. TQGB 20150092).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contribution
XY and XW searched, identified and reviewed the literature, collected the data and wrote the manuscript; RM, ZSu and JZ conceived the current study, identified and reviewed the literature, interpreted the data and wrote the manuscript; JLi, YZ, JLo and WZ collected the data; PJ, JW and BZ searched and identified the literature; QJ, FY and ZSh interpreted the data, gave critical comments and revised the manuscript. All authors have made an intellectual contribution to the manuscript and approved the final version.
Ethics approval and consent to participate
Written informed consent was obtained from each patient prior to LB and all trials were approved by the Ethics Committees of Huashan Hospital, Fudan University and the First Hospital of Quanzhou, Fujian Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 2.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. Electronic address, corp-author. easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302.e4. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Verveer C, Zondervan PE, Ten Kate FJ, Hansen BE, Janssen HL, de Knegt RJ. Evaluation of transient elastography for fibrosis assessment compared with large biopsies in chronic hepatitis B and C. Liver Int. 2012;32:622–628. doi: 10.1111/j.1478-3231.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 7.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 8.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski S M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 9.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arataki K, Hayes CN, Akamatsu S, Akiyama R, Abe H, Tsuge M, Miki D, Ochi H, Hiraga N, Imamura M, et al. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. J Med Virol. 2013;85:789–798. doi: 10.1002/jmv.23540. [DOI] [PubMed] [Google Scholar]
- 11.Wang JY, Mao RC, Zhang YM, Zhang YJ, Liu HY, Qin YL, Lu MJ, Zhang JM. Serum microRNA-124 is a novel biomarker for liver necroinflammation in patients with chronic hepatitis B virus infection. J Viral Hepat. 2015;22:128–136. doi: 10.1111/jvh.12284. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Zhou P, Deng W, Wang J, Mao R, Zhang Y, Li J, Yu J, Yang F, Huang Y, et al. Serum microRNA-125b correlates with hepatitis B viral replication and liver necroinflammation. Clin Microbiol Infect. 2016;22:384.e1–384.e10. doi: 10.1016/j.cmi.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Liang LB, Zhu X, Yan LB, Du LY, Liu C, Liao J, Tang H. Quantitative intrahepatic HBV cccDNA correlates with histological liver inflammation in chronic hepatitis B virus infection. Int J Infect Dis. 2016;52:77–82. doi: 10.1016/j.ijid.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhang TY, Song LW, Qi X, Yu XP, Li FH, Zhou P, Qin YL, Yang L, Zhao JH, et al. Role of quantitative hepatitis B core antibody levels in predicting significant liver inflammation in chronic hepatitis B patients with normal or near-normal alanine aminotransferase levels. Hepatol Res. 2018;48:E133–E145. doi: 10.1111/hepr.12937. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Sun Z, Pan H, Chen M, Tong Y, Zhang J, Chen D, Su X, Li L. Serum metabolomic signatures discriminate early liver inflammation and fibrosis stages in patients with chronic hepatitis B. Sci Rep. 2016;6:30853. doi: 10.1038/srep30853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong MZ, Ye L, Jin LX, Ren YD, Yu XF, Liu XB, Zhang RM, Fang K, Pan JS. Noninvasive scoring system for significant inflammation related to chronic hepatitis B. Sci Rep. 2017;7:43752. doi: 10.1038/srep43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. doi: 10.1002/hep.1840190629. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Society of Hepatology, Chinese Medical Association, corp-author; Chinese Society of Infectious Diseases, Chinese Medical Association, corp-author. Hou JL, Lai W. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;23:888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 19.European Association For The Study Of The Liver, corp-author. EASL clinical practice guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 21.Gao S, Li XY, Fan YC, Sun FK, Han LY, Li F, Ji XF, Wang K. A noninvasive model to predict liver histology in HBeAg-positive chronic hepatitis B with alanine aminotransferase ≤2 upper limit of normal. J Gastroenterol Hepatol. 2017;32:215–220. doi: 10.1111/jgh.13452. [DOI] [PubMed] [Google Scholar]
- 22.Li YP, Li CY, Chen YP. Independent predictive factors for significant liver histological changes in patients with HBeAg-positive high-viral-load chronic HBV infection and a normal alanine aminotransferase level. J Clin Hepatol. 2016;32:4. [Google Scholar]
- 23.Chen LY, Wang J, Wang WY, Tang H, Feng P. Correlation between serological indices and liver histological pathology in patients with HBV infection. Sichuan Da Xue Xue Bao. Yi Xue. 2015;46:641–644. (In Chinese) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.