Abstract
Single-nucleotide polymorphisms (SNPs) play an important role in our susceptibility to disease, the severity of illness and the way our body responds to treatment. This study evaluated the impact of three polymorphisms on the susceptibility and functional outcome of ischemic stroke (IS). Three hundred and eight patients and 300 healthy volunteers were enrolled. Polymorphisms of NOX4 rs11018628, MTHFR rs1801133 and NEIL3 rs12645561 were detected in both groups. Smoking (P<0.001), drinking (P<0.001), hypertension (P<0.001) and diabetes (P=0.006), as traditional vascular risk factors for IS, were confirmed in our study. Logistic regression analyses with adjustment for age, sex, smoking, drinking, diabetes, hypertension and total cholesterol showed that the variant genotypes of NOX4 rs11018628 were associated with a significantly decreased risk (Dominant model: OR=0.32, 95% CI=0.22–0.48, P<0.001) and a better short-term recovery of IS (Dominant model: OR=0.57, 95% CI=0.35–0.95, P=0.029). This study demonstrates that the NOX4 rs11018628 SNP is associated with decreased risk in developing IS and better short-term recovery of patients. This suggests that the genetic variant of NOX4 rs11018628 may contribute to the etiology of IS.
Keywords: ischemic stroke, SNPs, NOX4, reactive oxygen species, functional recovery
Introduction
Stroke is one of the most common causes of mortality and disability in the elderly population worldwide. In China, the annual stroke mortality rate is approximately 157 per 100,000, which is the leading cause of death and adult disability (1). In the clinic, stroke is presented in two major forms: ischemic stroke (IS) and hemorrhagic stroke (HS), of which the former accounts for 80% of stroke patients (2). The cost of initial care, rehabilitation, and follow-up care of lasting disabilities is a huge burden to the healthcare system. It is reported that each stroke patient costs the US healthcare system approximately US$140,048 (3).
There are various risk factors for IS, including hypertension, poor diet, smoking, lack of exercise, obesity, hypercholesterolemia, and diabetes (4). Besides, evidence from animal, clinical and epidemiological studies suggests that genetic factors also play an important role in stroke susceptibility and prognosis (5,6). There are several causes contributing to the genetic variations in humans, of which single-nucleotide polymorphisms (SNPs) play a vital role. An SNP could alter the DNA in the genome by exchanging one single base pair for another, thus influencing the expression of the gene (7). SNPs express differences in our susceptibility to disease, the severity of illness and the way our body responds to treatment (8). Evidence from previous studies has shown the association between several SNPs and IS (9–12).
Reactive oxygen species (ROS) are byproducts of cell metabolism, which results in significant damage to DNA structures. The brain needs a strong system to repair the DNA structure damaged by ROS. Our previous study reported that the DNA repair system pathway gene XRCC1 is associated with susceptibility and short-term recovery of IS (11). We assumed that genetic variants affecting the DNA damage and repair system may influence the clinical outcome following IS. NADPH oxidase 4 (NOX4) is a member of the family of NADPH oxidases, containing a catalytic NOX subunit that transfers electrons from NADPH to oxygen, thereby forming ROS. Methylene tetrahydrofolate reductase (MTHFR) is the key enzyme involved in plasma homocysteine (tHcy) metabolism by catalyzing the conversion of 5, 10-methyltetrahydrofolate to 5-methyltetrahydrofolate, a methyl donor during tHcy remethylation. tHcy is a crucial intermediate in methionine metabolism and causes excessive production of reactive oxygen species (ROS). Therefore, NOX4 and MTHFR are key enzymes involved in ROS producing. NEIL3 belongs to a class of DNA glycosylases that recognizes oxidized DNA bases, which is the first step of base excision repair pathway. NEIL3 plays an important role in repairing the ROS induced DNA damage. In this study, we further investigated the contribution of three SNPs referring to the damage and repair system, NOX4 rs11018628, MTHFR rs1801133 and NEIL3 rs12645561, to the development of stroke and outcome of ischemia.
Materials and methods
Study subjects
In this study, we enrolled 308 patients (180 males and 128 females) diagnosed with IS from the Department of Neurology, The Affiliated Jiangyin People's Hospital of Southeast University Medical College (Wuxi, China) and the Department of Neurology, Huai'an First People's Hospital, Nanjing Medical University (Huai'an, China). In addition, 300 healthy volunteers were recruited from The Affiliated Jiangyin People's Hospital of Southeast University Medical College as the case groups, during the same period, however, they were not age-matched samples. After consulting Pub-med, the frequency of gene mutations in the population was assumed to be 15%. The odds ratio OR was 0.4, the two-sided test α was 0.05, and the power of 1-β was 90%. The minimum sample size of the case and control groups was estimated to be 285 cases by PASS software. Taking into account factors such as loss of follow-up and single-gene weak effects, this study planned to prepare 300 samples per group. Diagnosis of patients with IS was established clinically and confirmed by using X-ray computed tomography and/or magnetic resonance imaging scans of the brain, according to the World Health Organization criteria (13). We also recruited 300 healthy volunteers from the Affiliated Jiangyin People's Hospital of Southeast University Medical College during the same period of time. Conventional clinical hematology, biochemistry and immunology examinations were conducted. Patients with atypical symptoms, including brain trauma, intracranial hemorrhage, post-seizure palsy, vascular malformations, metabolic disorders (except diabetes mellitus), infections, autoimmune diseases, blood diseases, cancer and severe chronic diseases (e.g., liver and kidney dysfunction) were excluded.
This study was approved by the Local Ethics Association and the Ethics Committee of The Affiliated Jiangyin People's Hospital of Southeast University Medical College and each participant signed a written informed consent (Project identification code: 2016-011).
Data collection
All case and control individuals filled out a study questionnaire, which included demographic information, medical history (hypertension or diabetes mellitus), alcohol consumption, daily cigarette smoking, obesity, and parameters of hypercholesterolemia. Hypertension was defined as blood pressure (BP) >140/90 mmHg (average of three independent measures) or the use of antihypertensive drugs. Specially, in the diabetic subjects hypertension was defined as BP>130/85 mmHg (average of three independent measures) or the use of antihypertensive drugs. Diabetes mellitus was defined as fasting glucose level ≥7.0 mmol/l and/or ≥11.1 mmol/l 2 h after oral glucose challenge, or receiving antidiabetic drugs. Subjects were considered as smokers if they smoked >10 cigarettes per day for five years, and as drinkers if they drank >50 ml alcoholic beverages per day for five years. Subjects with body mass index (BMI) ≥25 kg/m2 were considered as overweight. The National Institutes of Health Stroke Scale (NIHSS) score and FIM instrument score (14) were used to quantify stroke severity and functional independence of patients at the time of presentation and discharge. The FIM instrument is an 18-item scale, which measures independence involved in feeding, grooming, dressing, toileting, mobility, and cognition. Subjects are scored from 7 (totally independent) to 1 (totally dependent) on each item, with a score of 126 indicating total functional independence. The NIHSS scale is composed of 11 items, each of which scores a specific ability between 0 and 4. For each item, a score of 0 typically indicates normal function in that specific ability, while a higher score is indicative of some level of impairment.
SNP selection and DNA extraction and genotyping
Based on information in the NCBI SNP database and the International HapMap project data for the Han Chinese population, the polymorphisms of NOX4 rs11018628, MTHFR rs1801133 and NEIL3 rs12645561 were selected. Blood samples were collected after a 12 h overnight fasting period and then separated into serum, red blood cells, and buffy coat. Total DNA from leukocytes was extracted using the salt fractionation method according to a standard protocol (15). DNA samples were subjected to DNA genotyping using the TaqMan SNP Genotyping Assay (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Briefly, each genotyping assay contained one pair of primers and one pair of probes (Table I). The PCR conditions were 50°C for 2 min, 95°C for 10 min followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. The genotyping results were calculated by the allelic discrimination mode of the SDS 2.3 software package (Applied Biosystems; Thermo Fisher Scientific, Inc.), and a 100% concordance was achieved.
Table I.
Information of primers and probes for TaqMan allelic discrimination.
| Polymorphism | Sequence (5′-3′) |
|---|---|
| rs7124442 | |
| Primer | F: CCCTCAAAAGGAAGCTGCAT |
| R: ACAGTACCATCTGAATACCTCTTTGAAA | |
| Probe | FAM-AGTTGACATATAGCAGATAT-MGB |
| HEX-AAGTTGACATACAGCAGATA-MGB | |
| rs1801133 | |
| Primer | F: CACAAAGCGGAAGAATGTGTCA |
| R: GACCTGAAGCACTTGAAGGAGAA | |
| Probe | FAM-AAATCGGCTCCCGCA-MGB |
| HEX-TGAAATCGACTCCCG-MGB | |
| rs11018628 | |
| Primer | F: TGACTAGATAACTGAAACAGGCCTTGT |
| R: AGGCAACATTTTGGAATGATTGT | |
| Probe | FAM-TCTCATCTACTCCTCATC-MGB |
| HEX-CCTCTCATCTACTCCCCAT-MGB |
F, forward; R, reverse.
Statistical analysis
All statistical analyses were performed with Stata/SE (V.12.0 for Windows; StataCorp LP, College Station, TX, USA). Demographic data were compared using the two-sample t-test and Chi-square (χ2) test. The association of different genotypes with IS severity and short-term recovery and functional outcome were evaluated by calculation of the odds ratio (OR) and 95% confidence intervals (95% CI) using univariate and multivariate logistic regression analysis. All statistical analyses were two-sided and P<0.05 was considered statistically significant.
Results
Characteristics of participants
Characteristics of patients with IS and healthy controls are shown in Table II. Chi-square test showed that the patient group had higher proportion of males compared to the control group (58.6 vs. 46%, P=0.002). Compared with the healthy controls, IS patients were more likely to smoke cigarettes (P<0.001), drink alcoholic beverages (P<0.001), have diabetes (P=0.006) and hypertension (P<0.001). The IS patients also showed a trend toward having higher serum triglyceride concentrations. However, our study failed to identify serum total cholesterol, serum HDL-C, serum LDL-C concentration or BMI as risk factors for IS (Table II). These observations confirm the role of smoking, drinking, hypertension and diabetes as traditional vascular risk factors for IS.
Table II.
Demographic characteristics of patients and controls.
| Characteristics | Cases (n=308) | Control (n=300) | P-value |
|---|---|---|---|
| Age, year (mean ± SD) | 65.81±11.13 | 67.07±9.35 | 0.133 |
| Sex (male) (%) | 180 (58.6) | 138 (46) | 0.002 |
| Smoking (%) | 120 (38.9) | 74 (24.7) | <0.001 |
| Drinking (%) | 144 (46.8) | 63 (21.0) | <0.001 |
| Diabetes (%) | 65 (21.1) | 38 (12.7) | 0.006 |
| Hypertension (%) | 191 (62.1) | 118 (39.3) | <0.001 |
| BMI ≥25 kg/m2 (%) | 112 (36.4) | 101 (33.6) | 0.486 |
| Blood glucose | 5.86±2.34 | 5.69±1.53 | 0.287 |
| Total cholesterol (mmol/l) (mean ± SD) | 4.40±1.12 | 5.19±0.95 | <0.001 |
| Triglycerides (mmol/l) (mean ± SD) | 1.73±1.45 | 1.56±1.27 | 0.118 |
| HDL-C (mmol/l) (mean ± SD) | 1.25±0.52 | 1.18±0.26 | 0.065 |
| LDL-C (mmol/l) (mean ± SD) | 2.45±0.97 | 2.65±0.60 | 0.243 |
SD, standard deviation.
Association of SNP genotypes with IS susceptibility
The distributions of the three genotype frequencies in patients and controls are presented in Table III. A significantly different distribution of NOX4 rs11018628 was observed between the patient and control group in our study. The other two SNPs showed no significantly different distributions. Logistic regression analyses with adjustment for age, sex, smoking, drinking, diabetes, hypertension and total cholesterol revealed that the variant genotypes of NOX4 rs11018628 were associated with a significantly decreased risk of IS (Dominant model: OR=0.32, 95% CI=0.22–0.48, P<0.001). Next we compared the known allele frequency of each SNP in the general population and the control group of this study. After consulting the SNP database of NCBI (https://www.ncbi.nlm.nih.gov/snp/), we confirmed that the frequency of TT/TC/CC on rs1801133, among Hap Map-HCB population, were 0.30, 0.42 and 0.28, respectively, which is close to that of 0.37, 0.46 and 0.18 among our control group. The situation of rs12645561 and rs11018628 is similar to the above.
Table III.
Association of SNP genotypes with IS susceptibility.
| Genotype | Control (n=300) | Cases (n=308) | OR (95% CI) | P value |
|---|---|---|---|---|
| rs1801133 | ||||
| TT | 101 (36.7) | 106 (36.7) | 1 | |
| TC | 125 (45.5) | 127 (42.8) | 0.96 (0.62–1.47) | 0.841 |
| CC | 49 (17.8) | 64 (21.5) | 1.18 (0.69–2.00) | 0.546 |
| Dominant | 0.95 (0.64–1.40) | 0.782 | ||
| Additive | 1.07 (0.82–1.38) | 0.625 | ||
| rs11018628 | ||||
| TT | 134 (47.0) | 210 (68.2) | 1 | |
| TC | 87 (30.5) | 88 (28.6) | 0.56 (0.36–0.86) | 0.008 |
| CC | 64 (22.5) | 10 (3.2) | 0.08 (0.04–0.18) | <0.001 |
| Dominant | 0.32 (0.22–0.48) | <0.001 | ||
| Additive | 0.37 (0.28–0.50) | <0.001 | ||
| rs12645561 | ||||
| CC | 162 (53.4%) | 163 (50.9%) | 1.00 | |
| CT | 122 (40.3%) | 124 (38.8%) | 0.97 (0.66–1.44) | 0.898 |
| TT | 19 (6.3%) | 33 (10.3%) | 1.72 (0.85–3.49) | 0.132 |
| Dominant | 1.07 (0.74–1.55) | 0.707 | ||
| Additive | 1.15 (0.87–1.54) | 0.328 | ||
Logistic regression analyses adjusted for age, sex, smoking, drinking, diabetes, hypertension, and total cholesterol.
Association of NOX4 rs11018628 polymorphism with IS severity, short-term recovery and functional outcome
Considering the relationship of the NOX4 rs11018628 polymorphism to IS susceptibility, we next examined its contribution to IS severity and short-term recovery. IS severity was determined by admission NIHSS score and short-term recovery was defined as change of NIHSS score from admission to discharging (∆NIHSS). As NIHSS score and ∆NIHSS score were not skewed and could not be transformed to normal distribution, they were dichotomized for logistic regression analysis. According to previous studies (10,11,16), the cut-off of NIHSS score for mild and severe IS was set between 6 and 7. For clinical improvement and no change or deterioration, the cut-off of ∆NIHSS score was set at 0. Logistic regression analyses with adjustment for age, sex, smoking, drinking, diabetes, hypertension and total cholesterol indicated that the rs11018628 TC/CC genotype was associated with a better short-term recovery (Dominant model: OR=0.57, 95% CI=0.35–0.95, P=0.029). However, no significant association between rs11018628 and initial IS severity was observed. We also investigated associations of rs1801133 and rs12645561 with IS severity and short-term recovery. As shown in Table IV, rs1801133 and rs12645561 showed no significant associations with IS severity and short-term recovery. Finally, we investigated whether there was a difference in functional outcome between patients harboring the rs11018628 TT and TC/CC genotypes. The short-term functional outcome was defined as the change of FIM scores from admission to discharging (∆FIM). As presented in Fig. 1, there was no significant difference in terms of ∆FIM score between the two groups (P>0.05) (Table IV).
Table IV.
Associations of three polymorphisms with IS severity and short-term recovery.
| A, SNPs associated with IS severity | ||||
|---|---|---|---|---|
| Genotype | Mild (n=218) | Severe (n=90) | OR (95% CI) | P-value |
| rs11018628 | ||||
| TT | 150 (68.8) | 60 (66.7) | 1 | |
| TC | 61 (28.0) | 27 (30.0) | 1.22 (0.70–2.14) | 0.476 |
| CC | 7 (3.2) | 3 (3.3) | 1.13 (0.28–4.63) | 0.863 |
| Dominant | 1.21 (0.71–2.08) | 0.478 | ||
| Additive | 1.16 (0.73–1.83) | 0.523 | ||
| rs1801133 | ||||
| TT | 82 (37.6) | 28 (31.1) | 1 | |
| TC | 94 (43.1) | 38 (42.2) | 1.24 (0.69–2.22) | 0.480 |
| CC | 42 (19.3) | 24 (26.7) | 1.74 (0.88–3.41) | 0.109 |
| Dominant | 1.32 (0.78–2.27) | 0.300 | ||
| Additive | 1.31 (0.94–1.84) | 0.115 | ||
| rs12645561 | ||||
| CC | 115 (52.6) | 60 (46.7) | 1 | |
| CT | 81 (37.3) | 27 (42.4) | 1.43 (0.83–2.47) | 0.201 |
| TT | 22 (10.1) | 3 (10.9) | 1.13 (0.72–1.76) | 0.596 |
| Dominant | 1.39 (0.83–2.32) | 0.213 | ||
| Additive | 1.21 (0.83–1.77) | 0.316 | ||
| B, SNPs associated with IS short-term recovery | ||||
| Genotype | Improvement (n=173) | No change/deterioration (n=135) | OR (95% CI) | P-value |
| rs11018628 | ||||
| TT | 109 (63.0) | 101 (74.8) | 1 | |
| TC | 57 (33.0) | 31 (23.0) | 0.59 (0.35–0.98) | 0.043 |
| CC | 7 (4.0) | 3 (2.2) | 0.47 (0.12–1.89) | 0.290 |
| Dominant | 0.57 (0.35–0.95) | 0.029 | ||
| Additive | 0.62 (0.40–0.96) | 0.032 | ||
| rs1801133 | ||||
| TT | 66 (38.2) | 44 (32.6) | 1 | |
| TC | 73 (42.2) | 59 (43.7) | 1.24 (0.73–2.11) | 0.423 |
| CC | 34 (19.6) | 32 (23.7) | 2.43 (0.76–2.69) | 0.269 |
| Dominant | 1.28 (0.79–2.08) | 0.315 | ||
| Additive | 1.20 (0.88–1.64) | 0.253 | ||
| rs12645561 | ||||
| CC | 81 (46.8) | 75 (55.6) | 1 | |
| CT | 74 (42.8) | 46 (34.1) | 0.67 (0.41–1.10) | 0.111 |
| TT | 18 (10.4) | 14 (10.3) | 0.92 (0.63–1.35) | 0.674 |
| Dominant | 0.71 (0.45–1.12) | 0.137 | ||
| Additive | 0.83 (0.59–1.16) | 0.273 | ||
Logistic regression analyses adjusted for age, sex, smoking, drinking, diabetes, hypertension, and total cholesterol.
Figure 1.
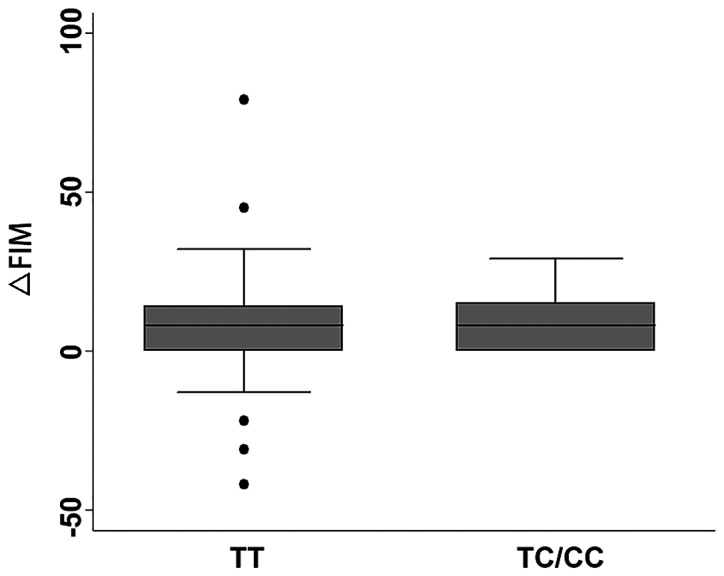
The relationship of the NOX4 rs11018628 genotypes to the short-term functional outcome.
Discussion
In the current study, we analyzed the polymorphism of NOX4 rs11018628, MTHFR rs1801133 and NEIL3 rs12645561 in blood samples obtained from IS patients vs. healthy controls and then associated them with IS risk and outcome in a Chinese Han population. We found that smoking, drinking, diabetes and hypertension were associated with an increased risk of IS. Moreover, we found that NOX4 rs11018628 TC and CC genotypes were associated with decreased risk of IS compared with the TT genotype. In addition, patients harboring rs11018628 TC/CC genotype also had a better short-term recovery. However, MTHFR rs1801133 and NEIL3 rs12645561 showed no significant association with IS susceptibility, severity and short-term recovery.
It is interesting that there is significant association of NOX4 rs11018628 with change of NIHSS score, but not with FIM score change and initial NIHSS score. The initial NIHSS score represents the level of initial neurogical impairment. However, patients with a milder initial neurogical impairment may not have a better short-term recovery, as determined by change of NIHSS score. Thus, NOX4 rs11018628 may be associated with the change of NIHSS score while not with the initial NIHSS score. The FIM and NIHSS scores evaluate different aspects of patients. The FIM instrument puts emphasis on the functional outcome while NIHSS score puts emphasis on the neurogical deficits. For example, an IS patient may have a certain degree of strength recovery of his hand. However, this extent of strength recovery may not be strong enough to make his hand grasp a cup. Thus, this patient would only have an NIHSS score change while FIM score would show no improvement. NADPH oxidase 4 (NOX4) is a member of the family of NADPH oxidases, which are multicomponent protein complexes containing a catalytic NOX subunit that transfers electrons from NADPH to oxygen, thereby forming ROS (17). Reactive oxygen species are byproducts of the normal cell metabolism of oxygen. The brain is particularly vulnerable to ROS owning to its high oxygen consumption and low capacity of oxidative clearance (18). The overload of ROS could cause oxidative stress, which has been suggested as a key underlying mechanism of IS (19).
Mounting evidence has demonstrated that the NOX family is deeply associated with IS (20–23). Of all the five NOX isoforms, NOX4 is expressed in the central nervous system, including intracranial vessels and neuronal tissues (24). Therefore, NOX4 is most likely relevant in IS. Several studies have reported that the mRNA level of NOX4 is increased after middle cerebral artery occlusion (MCAO) (25,26). Besides, a study conducted by Kleinschnitz et al observed that NOX4 knock-out mice had smaller infarcts, lower mortality rates and neurological deficit scores at seven days after MCAO (22). NOX4 has been suggested as a potential therapeutic target for IS (23). To the best of our knowledge, this study first showed that NOX4 rs11018628 TC and CC genotypes were associated with better short-term recovery of IS. Our study may provide information beneficial for further investigation of potential candidates for stroke therapy.
There are some limitations to this study. The relatively small sample size may limit our ability to detect the positive relationship between the SNPs and IS. Therefore, the negative findings of MTHFR rs1801133 and NEIL3 rs12645561 should be taken into consideration with caution. Further replication studies of the relation of these SNPs in independent and large subject panels should be performed. Besides, our findings also need to be further independently verified in different ethnic populations.
In summary, this study showed that smoking, drinking, hypertension and diabetes were associated with increased risk of IS. Furthermore, the genetic variant of NOX4 rs11018628 was associated with a decreased risk and better short-term recovery of IS. However, further studies are needed to elucidate the underlying mechanism.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WH and QW collected the data of patients and extracted DNA. LG and LZ performed PCR. DL helped with statistical analysis. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Local Ethics Association and the Ethics Committee of The Affiliated Jiangyin People's Hospital of Southeast University Medical College (Wuxi, China), and informed consents were signed by the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Akoudad S, Portegies ML, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, Vernooij MW. Cerebral microbleeds are associated with an increased risk of stroke: The Rotterdam Study. Circulation. 2015;132:509–516. doi: 10.1161/CIRCULATIONAHA.115.016261. [DOI] [PubMed] [Google Scholar]
- 2.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee, corp-author. Executive summary: Heart disease and stroke statistics - 2012 update: A report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research; Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research, corp-author. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 5.Jeffs B, Clark JS, Anderson NH, Gratton J, Brosnan MJ, Gauguier D, Reid JL, Macrae IM, Dominiczak AF. Sensitivity to cerebral ischaemic insult in a rat model of stroke is determined by a single genetic locus. Nat Genet. 1997;16:364–367. doi: 10.1038/ng0897-364. [DOI] [PubMed] [Google Scholar]
- 6.Marousi S, Ellul J, Antonacopoulou A, Gogos C, Papathanasopoulos P, Karakantza M. Functional polymorphisms of interleukin 4 and interleukin 10 may predict evolution and functional outcome of an ischaemic stroke. Eur J Neurol. 2011;18:637–643. doi: 10.1111/j.1468-1331.2010.03228.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren A. Stroke genetics: A review and update. J Stroke. 2014;16:114–123. doi: 10.5853/jos.2014.16.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf AB, Caselli RJ, Reiman EM, Valla J. APOE and neuroenergetics: An emerging paradigm in Alzheimer's disease. Neurobiol Aging. 2013;34:1007–1017. doi: 10.1016/j.neurobiolaging.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shyu HY, Shieh JC, Ji-Ho L, Wang HW, Cheng CW. Polymorphisms of DNA repair pathway genes and cigarette smoking in relation to susceptibility to large artery atherosclerotic stroke among ethnic Chinese in Taiwan. J Atheroscler Thromb. 2012;19:316–325. doi: 10.5551/jat.10967. [DOI] [PubMed] [Google Scholar]
- 10.Stanne TM, Tjärnlund-Wolf A, Olsson S, Jood K, Blomstrand C, Jern C. Genetic variation at the BDNF locus: Evidence for association with long-term outcome after ischemic stroke. PLoS One. 2014;9:e114156. doi: 10.1371/journal.pone.0114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Huang P, Liu D, Zhong L, Yu R, Li J. Polymorphism of the XRCC1 gene is associated with susceptibility and short-term recovery of ischemic stroke. Int J Environ Res Public Health. 2016;13:13. doi: 10.3390/ijerph13101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Yang H, ZhiPing T. Association studies of genetic polymorphism, environmental factors and their interaction in ischemic stroke. Neurosci Lett. 2006;398:172–177. doi: 10.1016/j.neulet.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 13.WHO MONICA Project Principal Investigators: The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 14.Kidd D, Stewart G, Baldry J, Johnson J, Rossiter D, Petruckevitch A, Thompson AJ. The functional independence measure: A comparative validity and reliability study. Disabil Rehabil. 1995;17:10–14. doi: 10.3109/09638289509166622. [DOI] [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Wu H, Zheng L, Weng Y, Mo Y. Brain-derived neurotrophic factor G196A polymorphism predicts 90-day outcome of ischemic stroke in Chinese: A novel finding. Brain Res. 2013;1537:312–318. doi: 10.1016/j.brainres.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9:445–447. doi: 10.1161/01.STR.9.5.445. [DOI] [PubMed] [Google Scholar]
- 19.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Radermacher KA, Wingler K, Kleikers P, Altenhöfer S, Jr Hermans J, Kleinschnitz C, Hhw Schmidt H. The 1027th target candidate in stroke: Will NADPH oxidase hold up? Exp Transl Stroke Med. 2012;4(11) doi: 10.1186/2040-7378-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:8. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radermacher KA, Wingler K, Langhauser F, Altenhöfer S, Kleikers P, Hermans JJ, Hrabě de Angelis M, Kleinschnitz C, Schmidt HH. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 25.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 26.McCann SK, Dusting GJ, Roulston CL. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J Neurosci Res. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


