Abstract
In this study, the yields and composition of essential oils obtained from the cones of Pinaceae family species natively grown in Turkey were investigated. Essential oils were obtained by hydrodistillation. Oil yields were 0.13-0.48 mL/100 g in pine cones, 0.42-0.59 mL/100g in fir, 0.36 mL/100g in spruce and 0.37 mL/100g in cedar. While α-pinene (47.1-14.8%) was the main constituent of P. slyvestris, P. nigra and P. halepensis, limonene (62.8%) in P. pinea and β-pinene (39.6%) in P. brutia were found in higher amounts. Like in P. pinea, limonene was the main compound in Cedrus libani (22.7%). In fir species the major compounds were α-pinene (70.6-53.0%) and β-pinene (10.9-8.2%). Contrary to other species β-pinene (32.7%) was found as a major compound in Picea orientalis.
Keywords: essential oil; terpenes; Pinaceae, fir, cones; GC/MS
1. Introduction
Essential oils are used in many industrial fields for the perfuming and flavouring of various products [1,2]. Essential oils are natural, complex, multi-component systems composed mainly of terpenes, in addition to some other non-terpene components. Several techniques can be used to extract essential oils from different parts of the aromatic plant, including water or steam distillation, solvent extraction, expression under pressure, supercritical fluid and subcritical water extractions [3].
Essential oils from aromatic and medicinal plants have been known since antiquity to possess biological activity, most notably antibacterial, antifungal and antioxidant properties. With growing interest in the use of essential oils in both the food and pharmaceutical industries, the systematic examination of plant extracts for these properties has become increasingly important. The terpene composition of seed cone oleoresin has been reported, and headspace techniques were developed to isolate volatile compounds from plant odour compounds in order to determine the composition of the host odour, which is attractive to insects [4,5,6,7,8].
Turkey, because of its geographical position at the crossing region of temperate continental and Mediterranean climates, is rich in coniferous woods that grow in different regions of the country, occupying about half of the county’s total forest area [9,10].
Five pine species are recorded in Turkey (Pinus brutia, Pinus nigra, Pinus sylvestris, Pinus pinea, Pinus halepensis) and three of them (P. brutia, P. nigra, P. sylvestris) are commercially utilized. Previous studies on Pinus species in Turkey were mainly focused on improving the yield of turpentine production. Pine oils are widely used as fragrances in cosmetics, as flavoring additives for food and beverages, as scenting agents in a variety of household products, and intermediates in the synthesis of other perfume chemicals [11,12].
Fir species exhibit parallel variation in indumentum characteristics and in the presence or absence of resinous buds. These features are well correlated with their geographical distribution [13]. Fir species are represented in Turkey by Abies nordmanniana (Stev.) Spach., Abies bornmulleriana Mattf., Abies equi-trojani (Asch.&Sint. ex Boiss.) Mattf. and Abies cilicica (Ant. et Kotschy) Carr. A. bornmulleriana, A. equi-trojani and A. cilicica subsp. isaurica are also endemic plants in Turkey [14,15]. A. nordmanniana, A. bornmulleriana, A. equi trojani are distributed in northern Turkey and A. cilicica (Ant. et Kotschy) Carr. is distributed in southern Turkey [13,16,17,18].
Cones of some coniferous species find uses in industry [19]. Essential oil constituents of the cones of the family Pinaceae are poorly known, although there have been some studies on the antioxidant activity, terpenoids, steroids, anti-HIV activity, procyanidins, etc. of all the Pinaceae cones [20,21,22].
2. Results and Discussion
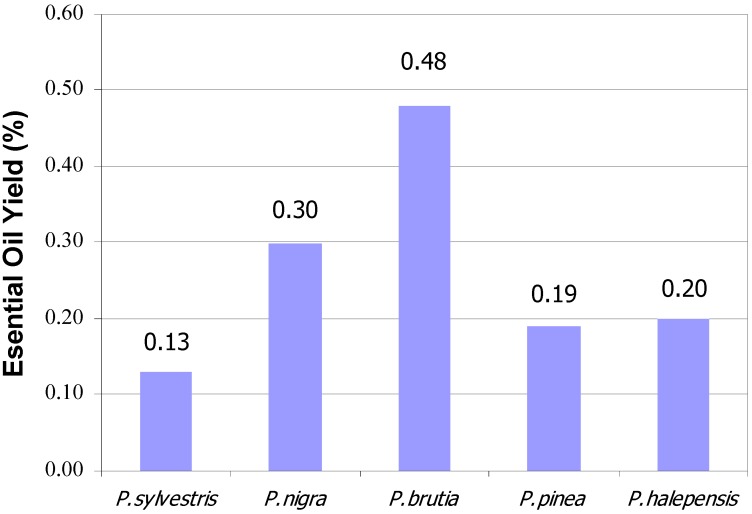
Oil yields of pine species are given in Figure 1 and those of fir, spruce and cedar species given in Figure 2. Evidently, the highest oil content (0.48%) was found in P. brutia and the lowest (0.13%) in P. sylvestris among the pine species. The essential oil compounds of pine cones are given in Table 1 and those of fir, spruce and cedar in Table 2. As can be seen from these tables, the main compounds were as follows: α-pinene, β-pinene, β-myrcene, Δ3-carene, limonene and β-caryophyllene. α-Pinene was the major compound in the cones of Pinaceae family. This compound was also found to account for more than 50% of the contents in the fir species too. α-Pinene was also identified as a major compound in P. nigra (45.36%) and P. halepensis (47.09%). Except for P. brutia and P. orientalis,β-pinene was found to be the second most important component in all cones. In the P. brutia (39.56%) and P. orientalis (32.67%) samples this compound was the most abundant compound. Limonene was the dominant component in P. pinea (69.54%, combined with β-phellandrene) and in C.libani (17.71%). This terpene is used as an antimicrobial inhibitor in the food industry. Although β-caryophyllene, an important sesquiterpene, was found to be less than 1% in the Abies species, the amount of this compound was more than 10% in P. halepensis (11.22%).
Figure 1.
Essential Oil Yields of Pine Species from Turkey (%).
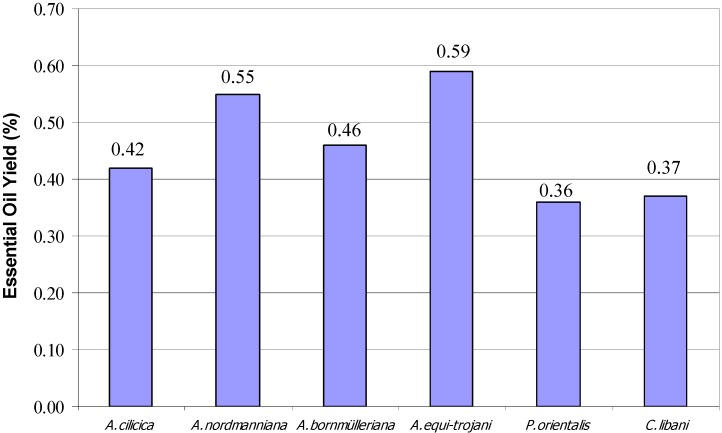
Figure 2.
Essential Oil Yields of Fir Spruce and Cedar species from Turkey (%).
Table 1.
Percent (w/w %) composition1) of components in Pines.
| Nr | RI | Compounds2) | P. pinea | P. brutia | P. sylvestris | P. nigra | P. halepensis |
|---|---|---|---|---|---|---|---|
| 1 | 925 | tricyclene | 0.02 | 0.04 | 0.05 | 0.08 | 0.16 |
| 2 | 936 | α-Pinene | 17.90 | 30.91 | 14.76 | 45.36 | 47.09 |
| 3 | 949 | camphene | 0.32 | 0.64 | 0.60 | 1.04 | 0.88 |
| 4 | 955 | 2,4 (10)-Thujadiene | 0.19 | 0.15 | 0.62 | 0.39 | 0.60 |
| 5 | 978 | β-Pinene | 1.70 | 39.56 | 1.78 | 1.50 | 2.75 |
| 6 | 989 | β-Myrcene | 0.79 | 1.13 | 0.17 | 0.16 | 6.25 |
| 7 | 1010 | Δ3-carene | - | 7.80 | - | - | 1.72 |
| 8 | 1021 | p-cymene | 0.29 | 0.37 | 0.47 | 0.30 | 0.42 |
| 9 | 1026 | limonene + beta-phellandrene | 69.54 | 2.05 | 0.48 | 1.88 | 0.79 |
| 10 | 1087 | α-terpinolene | 0.04 | 0.48 | 0.04 | 0.03 | 0.09 |
| 11 | 1088 | p-cymenene | 0.13 | 0.14 | 0.23 | 0.16 | 0.21 |
| 12 | 1123 | α-campholene aldehyde | 0.27 | 0.13 | 0.87 | 0.47 | 0.45 |
| 13 | 1135 | trans-pinocarveol | 0.47 | 1.38 | 0.91 | 0.49 | 0.88 |
| 14 | 1138 | cis-verbenol | 0.06 | 0.03 | 0.02 | 0.02 | 0.24 |
| 15 | 1141 | trans-verbenol | 0.25 | 0.07 | 0.05 | 0.07 | 0.71 |
| 16 | 1145 | p-mentha-1,5-dien-8-ol3) | 0.08 | 0.11 | 0.49 | 0.26 | 0.38 |
| 17 | 1156 | pinocarvone | 0.10 | 0.42 | 0.22 | 0.15 | 0.27 |
| 18 | 1162 | borneol | - | 0.10 | 0.03 | 0.07 | 0.11 |
| 19 | 1165 | p-mentha-1,5-dien-8-ol | 0.18 | 0.24 | 1.07 | 0.42 | 1.03 |
| 20 | 1177 | 4-terpineol | 0.08 | 0.26 | 0.01 | 0.03 | 0.08 |
| 21 | 1190 | α-terpineol | 0.14 | 0.93 | 0.41 | 0.77 | 0.30 |
| 22 | 1194 | myrtenal + myrtenol | 0.47 | 1.30 | 1.22 | 0.58 | 0.91 |
| 23 | 1206 | verbenone | 0.08 | 0.08 | 0.59 | 0.12 | 0.22 |
| 24 | 1218 | trans-carveol | 0.64 | 0.04 | 0.04 | 0.09 | 0.12 |
| 25 | 1286 | bornyl acetate | 0.26 | 0.22 | 0.02 | 0.69 | 0.64 |
| 26 | 1348 | α-longipinene | 0.18 | - | - | - | - |
| 27 | 1374 | α-copaene | - | 0.06 | 0.06 | 0.24 | 0.34 |
| 28 | 1403 | longifolene | 0.52 | 0.06 | 1.16 | 0.34 | - |
| 29 | 1420 | β-caryophyllene | 0.73 | 5.01 | 2.87 | 6.73 | 11.22 |
| 30 | 1453 | α-humulene | 0.10 | 1.25 | 0.61 | 1.48 | 2.65 |
| 31 | 1484 | germacrene-D | - | 0.38 | 0.01 | 0.06 | 0.02 |
| 32 | 1502 | α-muurolene | - | 0.06 | 0.02 | 0.11 | 0.31 |
| 33 | 1514 | gamma-cadinene | - | 0.04 | 0.01 | 0.09 | 0.25 |
| 34 | 1525 | trans-calamenene + Δ-cadinene | - | 0.13 | 0.01 | 0.28 | 0.32 |
| 35 | 1578 | caryophyllene oxide | 0.33 | 1.61 | 12.58 | 8.05 | 7.47 |
| 36 | 1607 | humulene epoxide | - | 0.23 | 1.48 | 1.07 | 1.11 |
| 37 | 1960 | 19-norabieta-8,11,13-triene3) | 0.02 | 0.03 | 4.75 | 0.70 | 0.37 |
| 38 | 1974 | isopimaradiene3) | - | - | - | 0.26 | 0.81 |
| 39 | 1974 | manoyl oxide | - | 0.33 | - | - | - |
| 40 | 1987 | norabieta-4(18),8,11,13-tetraene3) | 0.02 | 0.03 | 4.59 | 0.50 | 0.17 |
| 41 | 2002 | manoyl oxide | 0.25 | - | - | - | - |
| 42 | 2005 | palustradiene3) | - | 0.08 | 0.39 | 0.87 | 0.39 |
| 43 | 2007 | 18-norabieta-8,11,13-triene3) | 0.11 | 0.14 | 15.78 | 3.42 | 1.17 |
| 44 | 2055 | abieta-8,11,13-triene | 0.06 | 0.14 | 5.20 | 1.48 | 0.78 |
| 45 | 2083 | abieta-7,13-diene3) | - | 0.09 | 0.96 | 0.33 | 0.83 |
| 46 | 2158 | neoabietadiene3) + cis-abienol3) | 0.44 | 0.05 | 0.10 | 0.03 | 0.68 |
| 47 | 2174 | pimaral3) | 0.05 | - | 0.60 | 0.91 | 0.01 |
| 48 | 2230 | isopimaral3) | - | 0.05 | 0.31 | 0.62 | 0.41 |
| 49 | 2247 | palustral3) | 0.17 | 1.54 | 2.59 | 0.83 | |
| 50 | 2278 | dehydroabietal | 0.07 | 0.12 | 7.12 | 3.33 | 0.78 |
| 51 | 2313 | abietal | 0.06 | 0.06 | 0.83 | 0.61 | 0.60 |
| 52 | 2372 | neoabietal3) | 0.04 | 0.03 | 0.24 | 0.45 | 0.39 |
| Sum of minor and unidentified components | 2.97 | 1.27 | 13.63 | 10.32 | 0.79 | ||
| Total | 100 | 100 | 100 | 100 | 100 |
1) peak area percents from total eluted components on GC-MS; 2) identified by MS and retention index (RI) data from literature (R.P. Adams, 2007); 3) identification was based on MS-data only
Table 2.
Percent (w/w %) composition1) of components in fir, spruce and cedar.
| Nr | RI | Compounds2) | A. nordmanniana | A. cilicica | A. equi-trojani | A. bornmulleriana | P. orientalis | C. libani |
|---|---|---|---|---|---|---|---|---|
| 1 | 925 | tricyclene | 0.08 | 0.04 | 0.11 | 0.17 | 0.47 | 0.03 |
| 2 | 936 | α-pinene | 65.74 | 53.03 | 64.21 | 70.58 | 23.41 | 12.30 |
| 3 | 949 | camphene | 0.89 | 0.60 | 0.78 | 0.93 | 1.13 | 0.24 |
| 4 | 955 | 2,4 (10)-thujadiene | 0.79 | 0.07 | 0.97 | 0.44 | 0.41 | 0.04 |
| 5 | 978 | β-pinene | 9.62 | 10.88 | 8.17 | 8.60 | 32.67 | 8.25 |
| 6 | 989 | β-myrcene | 0.49 | 21.33 | 0.26 | 2.54 | 2.50 | 4.94 |
| 7 | 1003 | 1,5,8-p-menthatriene3) | 0.23 | 0.03 | 0.23 | 0.06 | 0.20 | 0.05 |
| 8 | 1010 | 3-carene | 0.88 | 1.12 | - | 0.03 | 0.13 | 0.11 |
| 9 | 1021 | p-cymene | 0.36 | 0.15 | 0.54 | 0.34 | 0.45 | 0.46 |
| 10 | 1026 | limonene | 7.24 | 5.43 | 1.79 | 1.16 | 14.99 | 17.71 |
| 11 | 1087 | α-terpinolene | 0.10 | 0.24 | 0.13 | 0.10 | 0.19 | 0.29 |
| 12 | 1088 | p-cymenene | 0.21 | 0.07 | 0.50 | 0.22 | 0.16 | 0.10 |
| 13 | 1101 | perillene | - | 0.17 | - | 0.05 | 0.15 | 0.08 |
| 14 | 1123 | α-campholene aldehyde | 0.51 | 0.04 | 0.76 | 0.20 | 0.43 | 0.01 |
| 15 | 1130 | 4-acetyl-1-methylcyclohexene3) | 0.05 | 0.01 | - | - | 0.04 | 0.18 |
| 16 | 1135 | trans-pinocarveol | 1.18 | 0.16 | 2.17 | 0.57 | 2.62 | 0.18 |
| 17 | 1138 | cis-verbenol | 0.20 | 0.01 | 0.13 | - | 0.07 | 0.01 |
| 18 | 1141 | trans-verbenol | 0.79 | 0.04 | 0.58 | 0.24 | 0.16 | 0.02 |
| 19 | 1145 | p-mentha-1,5-dien-8-ol3) | 0.45 | 0.04 | 0.75 | 0.18 | 0.28 | 0.01 |
| 20 | 1156 | pinocarvone | 0.21 | 0.06 | 0.35 | 0.07 | 0.75 | 0.04 |
| 21 | 1162 | borneol | 0.11 | 0.05 | 0.17 | 0.30 | 0.40 | 0.02 |
| 22 | 1165 | p-mentha-1,5-dien-8-ol | 0.99 | 0.03 | 1.23 | 0.25 | 0.72 | 0.03 |
| 23 | 1177 | 4-terpineol | 0.07 | 0.08 | 0.14 | 0.11 | 0.29 | 0.14 |
| 24 | 1190 | α-terpineol | 0.47 | 1.17 | 1.52 | 0.75 | 0.77 | 0.33 |
| 25 | 1194 | myrtenal + myrtenol | 1.19 | 0.21 | 2.42 | 0.64 | 3.02 | 0.18 |
| 26 | 1206 | verbenone | 0.84 | 0.06 | 4.12 | - | 0.27 | 0.01 |
| 27 | 1218 | trans-carveol | 0.20 | 0.02 | 0.31 | 0.05 | 0.30 | 0.08 |
| 28 | 1235 | thymol methyl ether | - | - | - | - | 0.04 | 0.17 |
| 29 | 1242 | carvone | 0.08 | 0.02 | 0.11 | 0.02 | 0.24 | 0.08 |
| 30 | 1286 | bornyl acetate | - | - | 0.32 | - | 1.94 | 0.18 |
| 31 | 1374 | α-copaene | - | 0.02 | 0.10 | 0.13 | 0.91 | 1.17 |
| 32 | 1420 | β-caryophyllene | 0.95 | 0.04 | 0.25 | 0.42 | 1.35 | 0.44 |
| 33 | 1453 | α-humulene | 0.70 | 0.02 | 0.13 | 0.25 | 0.42 | 0.10 |
| 34 | 1464 | β-farnesene3) | 0.01 | 0.02 | - | - | - | 0.30 |
| 35 | 1478 | gamma-muurolene | 0.01 | 0.09 | 0.08 | 0.24 | 0.03 | 0.08 |
| 36 | 1484 | germacrene-D | 0.02 | 0.88 | 1.01 | 1.85 | 0.12 | 0.01 |
| 37 | 1502 | α-muurolene | 0.02 | 0.04 | 0.19 | 0.46 | 0.29 | 0.13 |
| 38 | 1514 | gamma-cadinene | 0.01 | 0.06 | 0.09 | 0.25 | - | 0.02 |
| 39 | 1525 | trans-calamenene + Δ-cadinene | 0.04 | 0.12 | 0.40 | 0.65 | 0.12 | 0.20 |
| 40 | 1546 | cis-α-bisabolene3) | 0.64 | 0.62 | 0.03 | 0.06 | 0.07 | 4.66 |
| 41 | 1578 | caryophyllene oxide | 0.67 | - | 0.21 | 0.26 | 2.16 | 0.18 |
| 42 | 1607 | humulene epoxide | 0.28 | - | - | 0.14 | 0.36 | 0.03 |
| 43 | 1642 | α-muurolol | 0.03 | 0.06 | 0.34 | 0.63 | 0.02 | 0.01 |
| 44 | 1974 | manoyl oxide | 0.07 | 0.13 | 0.08 | 0.06 | 0.12 | 0.26 |
| 45 | 2005 | palustradiene3) | - | - | - | - | 0.10 | 7.05 |
| 46 | 2007 | 18-norabieta-8,11,13-triene3) | 0.09 | 0.05 | 0.17 | 0.03 | 0.28 | - |
| 47 | 2055 | abieta-8,11,13-triene | 0.02 | 0.16 | 0.07 | 0.02 | 0.27 | 17.00 |
| 48 | 2083 | abieta-7,13-diene3) | 0.07 | 0.09 | 0.12 | 0.02 | 1.11 | 8.32 |
| 49 | 2158 | neoabietadiene 3) | 0.03 | 0.03 | 0.04 | - | 0.10 | 0.87 |
| 50 | 2247 | palustral3) | 0.01 | 0.13 | 0.04 | - | 0.05 | 0.33 |
| 51 | 2278 | dehydroabietal | 0.03 | 0.16 | 0.10 | 0.01 | 0.06 | 0.36 |
| 52 | 2304 | 7-oxo-abieta-8,11,13-triene3) | - | - | - | - | - | 1.07 |
| 53 | 2313 | abietal | 0.15 | 0.20 | 0.35 | 0.05 | 0.55 | 0.34 |
| 54 | 2372 | neoabietal3) | 0.03 | 0.05 | 0.07 | 0.01 | 0.55 | 0.08 |
| Sum of minor and unidentified components | 2.15 | 1.87 | 3.36 | 5.86 | 2.26 | 10.75 | ||
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
1) peak area percents from total eluted components on GC-MS; 2) identified by MS and retention index (RI) data from literature (R.P. Adams, 2007); 3) identification was based on MS-data only.
As it can be seen in Figure 2, the highest essential oil yield of cones was found in A. equ-i trojani with 0.59% and the lowest was in P. orientalis at 0.36%. Among the Pinaceae family the highest essential oil yield was observed in A. equi trojani and the lowest was determined in P. sylvestris.
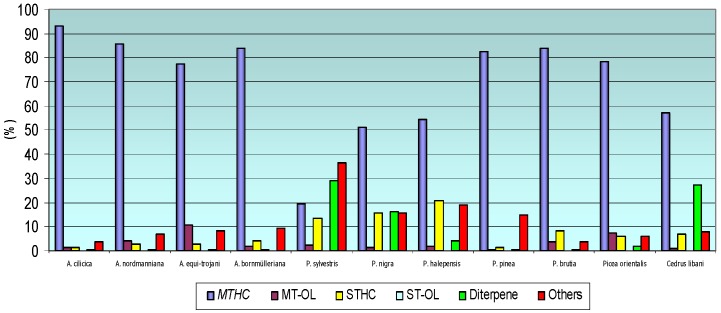
Terpene groups in the essential oils of Pinaceae cones were investigated. The terpenes were grouped into monoterpene hydrocarbons, monoterpene alcohols, sesquiterpene hydrocarbons, sesquiterpene alcohols and diterpenes. Those terpene groups and their amounts in different cones are given in Table 3 and Figure 3. Monoterpene hydrocarbons was found to be at the highest level in A. cilicica (93.14%), the highest level of monoterpene alcohols was found in A. equi-trojani (10.70%), sesquiterpene hydrocarbons were highest in P. halepensis (20.82%), diterpenes were the highest in P. sylvestris at 28.94%.
Table 3.
Terpene groups in Pinaceae family cones (%).
| Species | MTHK | MT-OL | STHK | ST-OL | Diterpene | Others |
|---|---|---|---|---|---|---|
| A.cilicica | 93.14 | 1.20 | 1.31 | - | 0.66 | 3.69 |
| A.nordmanniana | 85.72 | 4.10 | 2.80 | - | 0.39 | 6.99 |
| A.equi-trojani | 77.22 | 10.70 | 2.85 | 0.10 | 0.65 | 8.48 |
| A.bornmülleriana | 84.00 | 1.80 | 4.17 | 0.41 | 0.18 | 9.44 |
| P. orientalis | 78.40 | 7.55 | 6.16 | - | 1.83 | 6.06 |
| C. libani | 57.30 | 0.90 | 6.78 | - | 27.01 | 8.01 |
| P.sylvestris | 19.31 | 2.28 | 13.14 | - | 28.94 | 36.33 |
| P.nigra | 51.08 | 1.44 | 15.70 | - | 16.08 | 15.70 |
| P.halepensis | 54.50 | 1.70 | 20.82 | - | 4.10 | 18.88 |
| P.pinea | 82.62 | 0.64 | 1.34 | - | 0.67 | 14.73 |
| P.brutia | 83.94 | 3.53 | 8.11 | - | 0.62 | 3.80 |
MTHK: Monoterpene Hydrocarbons; MT-OL: Monoterpene alcohol; STHK: Sesquiterpene Hydrocarbons; ST-OL: Sesquiterpene alcohols.
Figure 3.
Terpene groups of Pinaceae Family Native Grown in Turkey (%).
3. Experimental
3.1. Plant Material
Eleven different coniferous cones were used in this study. Four different fir species (A.equi-trojani, A. cilicica, A. nordmannia, A. bormülleriana) and five different pine species (P. sylvestris, P. nigra, P. halepensis, P. pinea, P. brutia) and also C. libani and P. orientalis were collected directly from different parts of the trees. Approximately 5 kg of cones were collected for each species from their growth sites just at the time of maturity and stored in -24 ºC until the laboratory studies. Species names, sampling site, collection date, climate zone, and altitude of all specimens are listed in Table 4.
Table 4.
Names, collection place, climate zone, date and altitude of the analysed tree species.
| Species | Sampling Site | Climate Zone | Collection Date | Altitude |
|---|---|---|---|---|
| A. equi-trojani | Edremit-West Turkey | Mediterranean | October, 2007 | 800 m |
| A. cilicica | Adana-South Turkey | Mediterranean | May, 2007 | 700 m |
| A. nordmanniana | Trabzon-Northeast Turkey | Temperate | October, 2007 | 1,000 m |
| A. bornmülleriana | Bartin-Northwest Turkey | Temperate | October, 2007 | 1,100 m |
| P. orientalis | Trabzon-Northeast Turkey | Temperate | October, 2007 | 1,200 m |
| C. libani | Adana-South Turkey | Mediterranean | May, 2007 | 1,300 m |
| P. halepensis | Gokova, Mugla-West Turkey | Mediterranean | November, 2007 | 900 m |
| P. pinea | Bartin-Northwest Turkey | Temperate | March, 2007 | 600 m |
| P. sylvestris | Bartin-Northwest Turkey | Temperate | September, 2007 | 700 m |
| P. nigra | Bartin-Northwest Turkey | Temperate | September, 2007 | 750 m |
| P. brutia | Izmir-West Turkey | Mediterranean | May, 2007 | 850 m |
3.2. Isolation of the Essential Oil
The essential oils of each sample were obtained by hydrodistillation with a Clevenger apparatus (ILDAM CAM Ltd. Ankara-Turkey) using 100 g of fresh cones. The oils were collected for 3 h. and dried over anhydrous sodium sulphate in a sealed vial until used [23]. The results calculated as freeze dried samples were given in mL/100 g per dry raw material and given in Figure 1 and Figure 2 [24,25].
3.3. Essential Oil Analysis
The GC-MS analyses for the hydrodistilled samples were performed using an HP 6890-5973 GC-MSD instrument (Agilent Technologies Canada Inc., Mississauga, ON, Canada) equipped with an HP-5 capillary column (25 m/0.25 mm i.d., 0.11 μm film thickness). Helium was used as the carrier gas at 1.0 mL/min flow rate. The column oven was temperature programmed starting from 50 ºC (0.5 min) to 250 ºC , at 4 ºC/min heating rate. After 10 min of hold time at 250 ºC the temperature program was continued at 10 ºC/min to 290 ºC for 15 min. The split-injector and MS-transfer line were held at 260 ºC and 280 ºC, respectively. The MSD was operated in electron impact ionisation mode at 70 eV electron energy [26]. Compound identifications was based on mass spectra, referring to NIST98 and WILEY275 mass spectral libraries, and also comparing measured retention index (RI) values of components with literature data [27]. The quantitative area-percent measurements were based on peak-areas from the GC-MS data. Although, some researchers [28,29] have used cluster analysis to evaluate statistical data, the preliminary studies showed that there was no statistically significant differences between extraction and injections since the material was collected at one time [30,31,32,33]. Therefore, no statistical analysis was applied in this study.
4. Conclusions
Comparing the essential oil yields of Pinacea family tree cones, pine species yielded less than fir species. However, on a volatile compound basis, pine species yielded more than fir species except for α-pinene and β-pinene. On the other hand, monoterpene hydrocarbon compounds, an important group of terpenes, were more abundant in fir species rather than in pine species.
Acknowledgements
The authors wish to thank Bjarne Holmbom, Åbo Akademi Turku-Finland for scientific support and laboratory facilities. This work is a part of a project supported by Scientific and Technical Research Council of Turkey (TÜBITAK). A part of the study was presented orally at the Forest, Wildlife and Wood Sciences for Society Development International Scientific Conference of the 90th anniversary of the Forestry Faculty in Prague, April 2009.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Kıvanc A., Akgul A. Antibacterial activities of essential oils from Turkish spices and citrus. Flavour Fragr. J. 1986;1:75–179. [Google Scholar]
- 2.Bagcı E., Dıgrak M. Antibacterial activities of essential oils from Turkish spices and citrus. Flavour Fragr. J. 1996;11:251–256. doi: 10.1002/(SICI)1099-1026(199607)11:4<251::AID-FFJ577>3.0.CO;2-K. [DOI] [Google Scholar]
- 3.Edris E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 4.Baratta M.T., Damien Dorman H.J., Deans S.G., Cristina Figueiredo A., Barroso J.G., Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998;13:235–244. [Google Scholar]
- 5.Gabriela M., Lucia B., Giuseppe S. Antimicrobial properties of the linalol-rich essential oil of Hyssopus officinalis L. var decumbens (Lamiaceae) Flavour Fragr. J. 1998;13:289–294. doi: 10.1002/(SICI)1099-1026(1998090)13:5<289::AID-FFJ750>3.0.CO;2-A. [DOI] [Google Scholar]
- 6.Vila R., Valenzuela L., Bello H., Canigueral S., Montes M., Adzet T. Composition and antimicrobial activity of the essential oil of Peumus boldus leaves. Planta Med. 1999;65:178–179. doi: 10.1055/s-2006-960461. [DOI] [PubMed] [Google Scholar]
- 7.Teissedre P.L., Waterhouse A.L. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J. Agric. Food Chem. 2000;48:3801–3805. doi: 10.1021/jf990921x. [DOI] [PubMed] [Google Scholar]
- 8.Kurose K., Okamura D., Yataga M. Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. Flavour Fragr. J. 2007;22:10–20. doi: 10.1002/ffj.1609. [DOI] [Google Scholar]
- 9.Muthoo M.K. Forests and Forestry in Turkey. Food and Agriculture Organization of the United Nations (FAO); Ankara, Turkey: 1997. [Google Scholar]
- 10.Hafızoglu H., Usta M. Chemical composition of coniferous wood species occurring in Turkey. Holz Roh-Werkstoff. 2005;63:83–85. doi: 10.1007/s00107-004-0539-1. [DOI] [Google Scholar]
- 11.Ustun O., Sezik E., Kurkcuoglu M., Baser K.H.C. Study Of The Essential Oil Composition Of Pinus sylvestris From Turkey. Chem. Nat. Compd. 2006;42:26–31. doi: 10.1007/s10600-006-0029-2. [DOI] [Google Scholar]
- 12.Sezik E., Osman U., Demirci B., Baser K.H.C. Composition of the essential oils of Pinus nigra Arnold from Turkey. Turk. J. Chem. 2010;34:313–325. [Google Scholar]
- 13.Bagci E., Baser K.H.C., Kurkcuoglu M., Baabac T., Celik S. Study of the essential oil composition of two subspecies of Abies cilicica (Ant. et Kotschy) Carr. from Turkey. Flavour Fragr. J. 1999;14:47–49. doi: 10.1002/(SICI)1099-1026(199901/02)14:1<47::AID-FFJ782>3.0.CO;2-R. [DOI] [Google Scholar]
- 14.Yaltırık F. Dendrology, Gymnospermae. 2nd. University Press; Istanbul, Turkey: 1993. pp. 386–3443. [Google Scholar]
- 15.Sarıbas M. Dendrology, Gymnospermae, I. Donmez Press; Bartin, Turkey: 2008. pp. 192–198. [Google Scholar]
- 16.Aytug B. The Morphological and Anatomical Researches on Abies Species (Abies Tourn.) of Turkey. J. For. Fac. Istanbul. 1959;A9:165–217. [Google Scholar]
- 17.Davis P.H. Flora of Turkey and the East Aegean Islands. Vol. 1. University Press; Edinburgh, UK: 1965. pp. 67–69. [Google Scholar]
- 18.Dayisoylu K.S., Alma M.H. Chemical analysis of essential oils from cone’s rosin of Cilician fir (Abies cilicica subsp. cilicica) Afr. J. Biotechnol. 2009;8:3502–3505. [Google Scholar]
- 19.Villagomez H.Z., Peterson D.M., Herrin L., Young R.A. Villagomez, H.Z., Peterson, D.M., Herrin, L., Young, R.A. Holzforschung. 2005;59:156–162. [Google Scholar]
- 20.Sakagami H., Kawazoe N., Komatsu N., Simpson A., Nonoyama M., Konno K., Yoshida T., Kuroiwa Y., Tanuma S. Antitumor, antiviral and immunopotentiating activities of pine cone extracts:potential medicinal efficacy of natural and synthetic lignin-related materials. Anti-Cancer Res. 1991;11:881–888. [PubMed] [Google Scholar]
- 21.Tanaka R., Matsunaga S., Zasshi Y. Terpenoids and steroids from several euphorbiaceae and pinaceae plants. J. Pharm. Soc. Jpn. 1999;119:319–39. doi: 10.1248/yakushi1947.119.5_319. [DOI] [PubMed] [Google Scholar]
- 22.Sakar M.K., Ercil D., Engelshowe R. Procyanidins in cones of Pinus halepensis. Int. J. Pharmacog. 1991;29:221–224. doi: 10.3109/13880209109082882. [DOI] [Google Scholar]
- 23.Shahmir F., Ahmadi L., Mirza M., Korori S.A.A. Secretory elements of needles and berries of Juniperus communis L. ssp. communis and its volatile constituents. Flavour Fragr. J. 2003;18:425–428. doi: 10.1002/ffj.1243. [DOI] [Google Scholar]
- 24.Kurose K., Okamura D., Yatagai M. Composition of the essential oils from the leaves of nine Pinus species and the cones of three of Pinus species. Flavour Fragr. J. 2007;22:10–20. doi: 10.1002/ffj.1609. [DOI] [Google Scholar]
- 25.Tumen I., Hafizoglu H., Kollmannsberger H., Zimmermann B., Keskiner A., Gultekin H.C. Essential Oil Of Leaves Juniperus Ssp. Natively Grown in Turkey. Pharmacog. Mag. 2010;6:137. [Google Scholar]
- 26.Wajs A., Pranovich A., Reunanen M., Willför S., Holmbom B. Headspace-SPME Analysis of the Sapwood and Heartwood of Picea Abies, Pinus Sylvestris and Larix Dedicua. J. Essent. Oil Res. 2007;19:125–133. doi: 10.1080/10412905.2007.9699244. [DOI] [Google Scholar]
- 27.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 28.Jin M.Z.., He J.J., Bi H.Q, Cui Y.X., Duan C.Q. Phenolic Compound Profiles in Berry Skins from Nine Red Wine Grape Cultivars in Northwest China. Molecules. 2009;14:4922–4935. doi: 10.3390/molecules14124922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu Q., Xu J., Gu L. Selecting Diversified Compounds to Build a Tangible Library for Biological and Biochemical Assays. Molecules. 2010;15:5031–5044. doi: 10.3390/molecules15075031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anačkov G., Božin B., Zorić L., Vukov D., Dukić N.M., Merkulov L., Igić R., Jovanović M., Boža P. Chemical Composition of Essential Oil and Leaf Anatomy of Salvia bertolonii Vis. and Salvia pratensis L. (Sect. Plethiosphace, Lamiaceae) Molecules. 2009;14:1–9. doi: 10.3390/molecules14010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino L., Bruno M., Formisano C., Feo V., Napolitano F., Rosselli S., Senatore F. Chemical Composition and Antimicrobial Activity of the Essential Oils from Two Species of Thymus Growing Wild in Southern Italy. Molecules. 2009;14:4614–4624. doi: 10.3390/molecules14114614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dayısoylu K.S., Alma M.H. Chemical analysis of essential oils from cone’s rosin of Cilician fir (Abies cilicica subsp. cilicica) Afr. J. Biotechnol. 2009;8:3502–3505. [Google Scholar]
- 33.Sezik E., Ustun O., Demirci B., Baser K.H.C. Composition of the essential oils of Pinus nigra Arnold. From Turkey. Turk. J. Chem. 2010;34:313–325. [Google Scholar]