Supplemental Digital Content is available in the text
Keywords: nephrotic syndrome, rituximab, children
Abstract
Background:
The anti-CD20 monoclonal antibody rituximab (RTX) has been proposed as a rescue therapy for difficult-to-treat nephrotic syndrome (NS). We conducted a clinical trial to evaluate the efficacy and safety of RTX in children with difficult-to-treat NS dependent on or resistant to steroids and calcineurin inhibitors (CNIs).
Methods:
A multicenter open-label trial was performed at 8 major pediatric nephrology centers in Korea. The investigation consisted of a randomized controlled trial for steroid- and CNI-dependent NS (DDNS; randomization into the RTX group and the control group, at a ratio of 2:1) and a single-arm study of steroid and CNI-resistant NS (DRNS). DDNS patients in the RTX group and DRNS patients received a single dose of intravenous RTX (375 mg/m2 of body surface area) for B-cell depletion. A second RTX dose was administered at week 2 if the first dose failed to achieve depletion of CD19(+) cells. The primary endpoint was rate of maintaining remission at 6 months after treatment for DDNS and rate of remission achievement for DRNS.
Results:
Sixty-one children with DDNS were enrolled while in remission and randomized to the control group (21 patients) or the RTX group (40 patients). At 6 months after treatment, the remission rates were 74.3% in the RTX group and 31.3% in the control group (P = .003). The mean duration of remission maintenance was significantly higher in the RTX group than in the control group (9.0 vs 2.9 months, P = .004). Of the 23 patients with DRNS enrolled in the single-arm study and treated with RTX, 9 (39.1%) achieved partial or complete remission within 6 months. Depletion of B cells occurred in all patients with RTX therapy. Thirty patients (50.8% of 59 patients analyzed) experienced mild and transient infusion reaction during RTX administration, and most adverse events were mild.
Conclusions:
RTX administration was safe and effective in patients with difficult-to-treat NS. One or 2 doses of RTX may be sufficient to deplete B cells and achieve better control of pediatric NS.
1. Introduction
In children, idiopathic nephrotic syndrome (NS) generally responds well to corticosteroid treatment. However, most patients become dependent on steroid treatment, with frequent relapse. As long-term use of steroids in children is accompanied by adverse effects such as growth impairment, obesity, osteopenia, hypertension, and cataract, calcineurin inhibitors (CNIs) are recommended as steroid-sparing agents.[1–4] Unfortunately, CNIs remain effective only while the patient continues to take the treatment, providing no long-lasting benefits. Therefore, many patients become dependent on CNIs, which also have side effects such as nephrotoxicity, hypertension, and diabetes mellitus.[3,5,6] On the contrary, 10% to 20% of NS patients do not respond to steroid treatment; in such patients, remission is sometimes achieved with CNI treatment, but resistance to both steroids and CNIs is also noted. Patients who fail to achieve remission with steroid and CNI therapy are known to have a poor prognosis, and rapid progression to end-stage renal disease occurs in >50% of cases.[7]
Rituximab (RTX), a monoclonal antibody targeting the CD20 antigen of B lymphocytes, has recently been introduced as rescue therapy for difficult-to-treat NS, on the basis of favorable clinical observations.[8–13] Two randomized controlled trials (RCTs) have proved the efficacy and safety of RTX in patients with steroid-dependent NS,[14,15] whereas another RCT reported no benefit of RTX in patients with NS-resistant to steroids and CNIs.[16] However, some observational studies reported that RTX induced remission in patients resistant to conventional treatment.[17–19] The dosing regimen of RTX in NS patients varied widely in the previous publications. In most studies, RTX was administered at a dose of 375 mg/m2 once weekly for 4 weeks, which is similar to the protocol for the treatment of B-cell lymphoma.[10,13,15,17,18] Other studies have used single infusion of RTX.[9,14,16] The optimal dosing schedule of RTX in NS has not been determined.
In this study, we aimed to evaluate the efficacy and safety of single-dose RTX in childhood-onset, difficult-to-treat NS dependent on or resistant to steroids and CNIs.
2. Methods
2.1. Study design
A multicenter open-label trial was conducted in Korean patients with childhood-onset NS to evaluate the efficacy and safety of single-dose RTX. This investigation was designed as an RCT for steroid- and CNI-dependent NS (drug-dependent NS, DDNS), and as a single-arm study for steroid- and CNI-resistant NS (drug-resistant NS, DRNS). Eight major centers in Korea participated in this trial. This study was conducted in compliance with the current version of the Declaration of Helsinki and with the Korean Good Clinical Practice guidelines in effect at the time of the study. The study was approved by the ethics review committee of each participating hospital. Each participant entered the study only after informed consent was obtained from the legal guardians and/or the participant, as appropriate; assent was obtained from all pediatric patients. The trial was registered with ClinicalTrials.gov under trial registration number NCT01716442.
The inclusion criteria were as follows: age <24 years, and diagnosis of idiopathic NS established before the age of 18 years. The RCT of DDNS enrolled patients with steroid dependence and CNI dependence for >2 years, who had achieved remission with conventional treatment consisting of oral corticosteroids (60 mg/m2/d; maximum daily dose of 60 mg), with or without CNIs. Patients not taking CNIs because of intolerance to the medication were also considered eligible. Steroid and CNI dependence was defined as 2 consecutive relapses during steroid or CNI therapy or within 2 weeks of discontinuation of the respective medication. The single-arm study of DRNS enrolled patients who did not achieve remission of NS despite continuous use of steroids and CNIs for >3 months. The exclusion criteria were as follows: estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2 of body surface area; active or chronic infection; live-attenuated vaccination within 1 month leading up to the study; underlying cardiovascular or pulmonary disease; uncontrolled hypertension; neutropenia or thrombocytopenia; pregnancy or potential pregnancy; patients with known genetic causes such as NPHS1, NPHS2, WT1, or LAMB2; and previous use of RTX.
In the RCT of DDNS, participants were randomly assigned to the RTX group or the control group (conventional therapy) in a ratio of 2:1, using stratified block randomization, according to sex and age (Fig. 1A). Randomization was conducted using a computer-generated allocation sequence on the randomization website of Medical Research Collaborating Center of Seoul National University Hospital. In the single-arm study of DRNS, all patients received RTX in addition to their preenrollment treatment with steroids and/or CNIs (Fig. 1B). All participants were evaluated at baseline, week 2, week 4, and every 4 weeks thereafter for 1 year. Remission and relapse of NS were defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline.[1]
Figure 1.

Study design. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
2.2. Treatment protocol
In the RCT of DDNS, patients in the RTX group received a single dose of intravenous RTX (375 mg/m2; maximum of 500 mg) in addition to steroids and/or CNIs, whereas patients in the control group were treated conventionally, only with steroids and/or CNIs. As long as remission was maintained, oral corticosteroids were reduced to 40 mg/m2 administered every other day for 4 weeks and then tapered by 25% every 4 weeks for 3 months, followed by CNI tapering by 25% every 4 weeks. In the single-arm study of DRNS, patients continued their preenrollment treatment with steroids and CNIs during and after RTX treatment (single dose of intravenous RTX; 375 mg/m2; maximum of 500 mg); once remission was achieved, the steroid dose was reduced by 25% every 4 weeks, followed by CNI tapering by 25% every 4 weeks.
B-cell depletion by RTX treatment was monitored by counting the CD19(+) cells in peripheral blood samples obtained at week 2. This strategy was chosen because the CD19(+) cell count is recognized as a useful B-cell marker after RTX treatment.[20] A second RTX dose was administered if the first dose failed to achieve depletion of CD19(+) cells.
2.3. Study outcomes and safety
The primary endpoints were the rate of maintaining remission at 6 months after enrollment for the RCT of DDNS, and the rate of remission within 6 months after RTX administration for the single-arm study of DRNS. The secondary endpoints of the RCT of DDNS were the duration of remission, relapse rate per year, duration of steroid- and CNI-free period, steroid and CNI dosage during the study, and steroid and CNI toxicity. For the DRNS study, the secondary endpoints were the remission rate at 3, 6, 9, and 12 months, as well as the change in the following variables from enrollment to 1 year after treatment: renal function, dosage of steroids and/or CNIs, and steroid and/or CNI toxicity. The height Z-score, body mass index (BMI) Z-score, estimated GFR, and prevalence of hypertension were used in the assessment of steroid and CNI toxicity. Safety was assessed using the Common Terminology Criteria for Adverse Events, version 4.0.
2.4. Statistical analysis
In the RCT of DDNS, the required sample size was calculated considering an expected remission rate of 60% in the RTX group and 20% in the control group at 6 months from study entry, according to the literature reports on RTX available at the time of study initiation.[14] To attain 80% power at the nominal level of 2-sided alpha of 0.05, the required sample sizes were estimated as 40 participants for the RTX group and 20 participants for the control group, considering a dropout rate of 10% in the RTX group and 30% in the control group. To compare the 2 groups in the RCT of DDNS, the χ2 test or Fisher exact test was used for categorical variables, whereas the 2-sample t test or Wilcoxon rank-sum test was used for continuous values, as appropriate. The duration of remission was analyzed using the log-rank test, whereas time-to-event data were examined using Kaplan–Meier analysis. The incidences of relapse and infection were calculated as the number of events per person-years.
A similar approach was followed for estimating the required sample size for the single-arm study of DRNS. Specifically, the required sample size was 27 participants, considering an expected remission rate of 40% in the study group, based on the outcomes of standardized therapeutic studies, which reported 6-month remission rates of <5% for steroid-resistant NS.[21,22] The remission rates were analyzed using a binomial test.
Values recorded before and after the study were compared using the paired t test or Wilcoxon signed rank-sum test. Data regarding hypertension and estimated GFR were analyzed using mixed models or generalized estimating equations adjusted for the clustering effect.
3. Results
3.1. Efficacy of RTX in DDNS
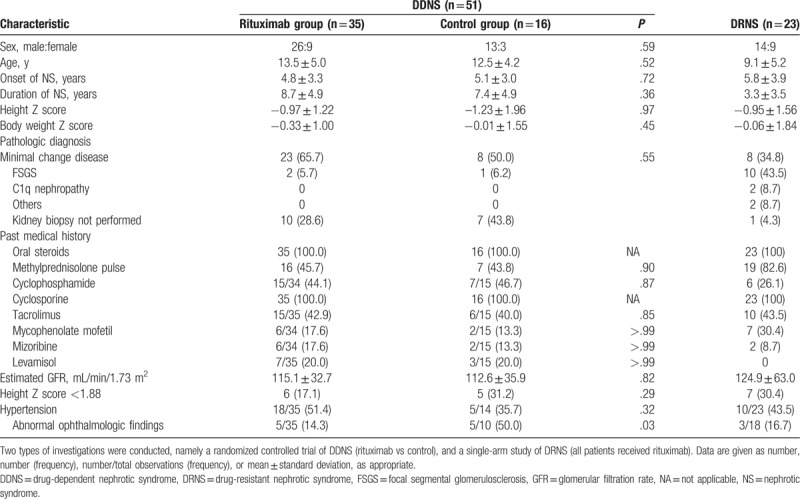
Sixty-one patients with DDNS were enrolled and randomly assigned to either the RTX group (n = 40) or to the control group (n = 21) (Fig. 2A). Of these, 51 participants were followed up for >6 months and thus included in the final analysis (RTX group, n = 35; control group, n = 16). The mean age at the first NS diagnosed was 4.8 years. At the time of enrollment in the study, the mean duration of treatment for NS was 8.3 years. Before enrollment, most participants (67%) had experienced one or more adverse effects of steroid or CNI treatment. The baseline characteristics of the participants did not differ significantly between the 2 groups (Table 1).
Figure 2.
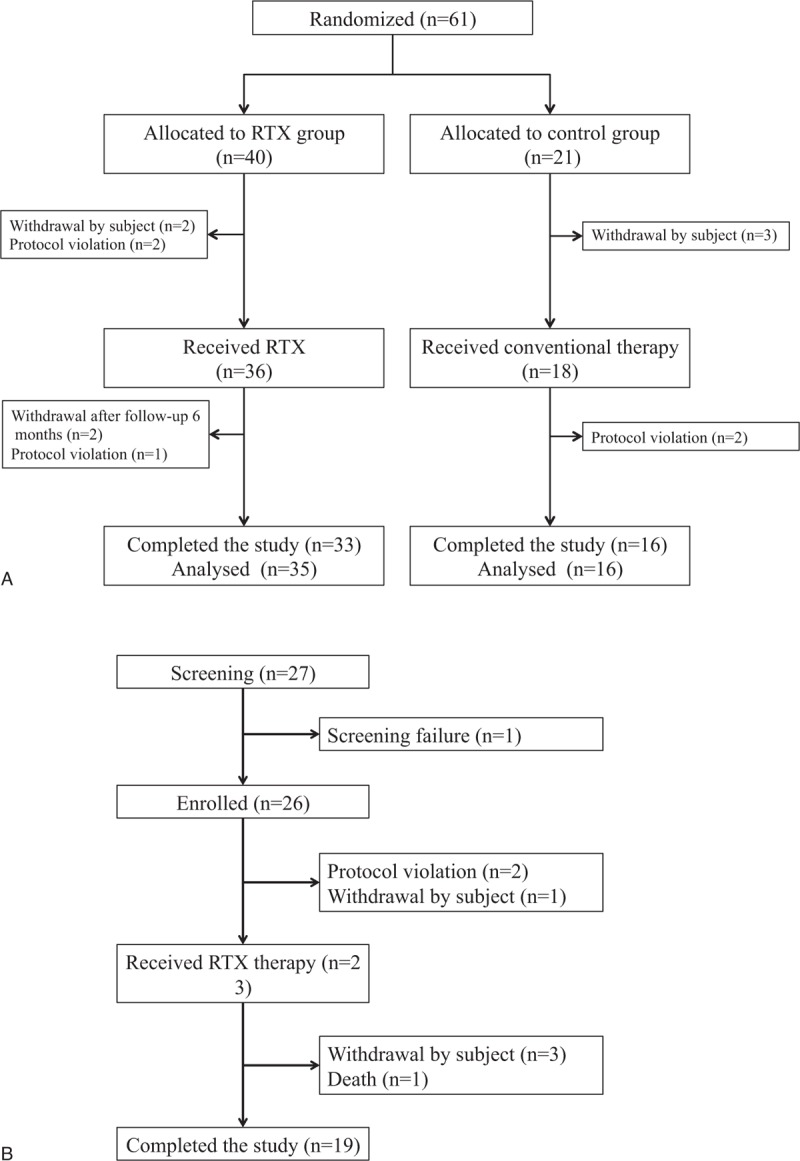
Flowchart of patient enrollment, evaluation, and follow-up. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
Table 1.
Baseline characteristics of patients with childhood-onset, difficult-to-treat nephrotic syndrome.
At 6 months after enrolment, 74.3% (26/35) of patients in the RTX group were at remission, whereas 68.7% (11/16) of patients in the control group were in relapse (P = .003). The median duration of remission throughout the study was 9.0 months in the RTX group and 2.9 months in the control group (Kaplan–Meier analysis, P = .004; Fig. 3A). Relapse rate was significantly lower in the RTX group than in the control group (3.4/person-year vs 9.4/person-year, P = .006).
Figure 3.
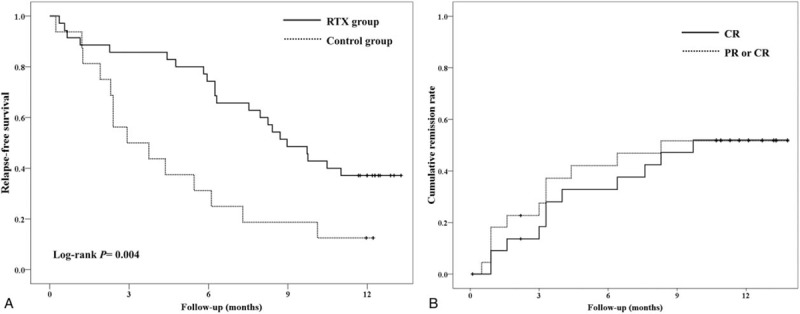
Survival analysis. (A) Kaplan–Meier analysis for relapse-free survival in the randomized controlled trial of drug-dependent nephrotic syndrome. (B) Cumulative remission rate in single-arm study of drug-resistant nephrotic syndrome.
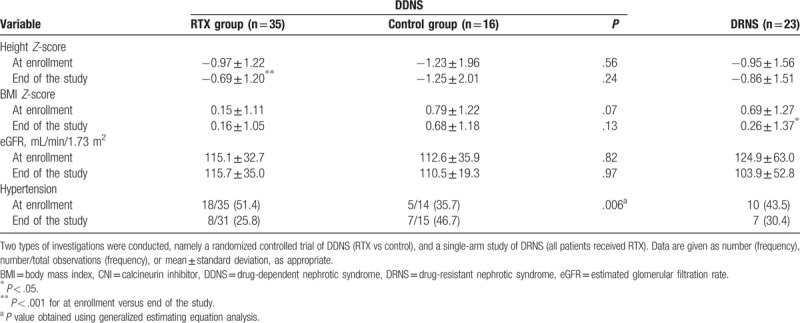
Corticosteroid dosage during the study period was significantly lower in the RTX group than in the control group, and lower than that noted during the year leading up to study enrollment among patients in the RTX group; the duration of the steroid-free period was significantly longer in the RTX group than in the control group (Table 2). CNIs dosage was significantly lower, and the duration of the CNI-free period was significantly longer during the study period than during the year leading up to the study among patients in the RTX group; nevertheless, the differences between the RTX group and the control group were not statistically significant (Table 2). The beneficial effect of RTX in terms of reducing the use of steroids and/or CNIs was clinically evident as well; specifically, the height Z-scores increased and prevalence of hypertension decreased from baseline to the end of the study in the RTX group, whereas no such changes were noted in the control group (Table 3). BMI and estimated GFR did not change significantly in either group.
Table 2.
Comparison of relapse rate, drug dose, and drug-free period in patients with childhood-onset, difficult-to-treat nephrotic syndrome.
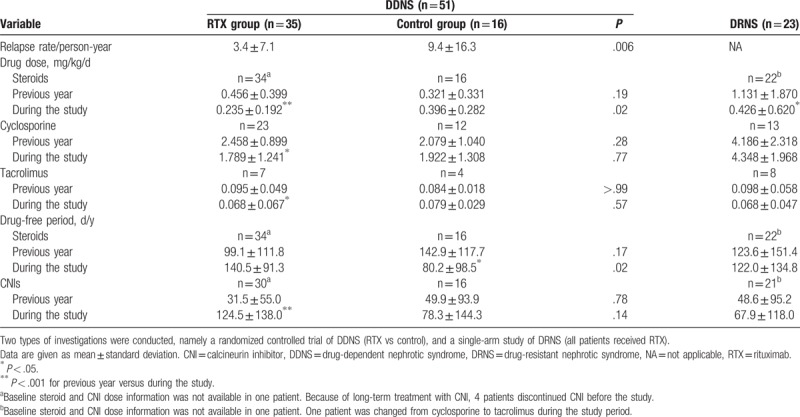
Table 3.
Steroid and calcineurin inhibitor toxicity in patients with childhood-onset, difficult-to-treat nephrotic syndrome.
3.2. Efficacy of RTX in DRNS
The single-arm study of DRNS enrolled 26 patients, of whom 23 were eventually treated with RTX (Fig. 2B). The mean age at the first diagnosis of NS was 5.8 years. At the time of enrollment, the duration of treatment for NS was 3.3 years (Table 1). The baseline characteristics of the participants are summarized in Table 1. Before enrollment, 70% had experienced one or more adverse effects of steroid or CNI treatment. Within 6 months after administration of RTX, 9 of 23 patients (39.1%) achieved partial (n = 2) or complete (n = 7, 30.4%) remission of proteinuria (binomial test, P < .001, Fig. 3B) The rates of partial or complete remission at 3, 6, 9, and 12 months were 34.8%, 39.1%, 43.5%, and 34.8%, respectively (binominal test, P < .001). The characteristics of patients who achieved complete remission (n = 7) differed from those of patients who did not (n = 16), especially regarding proteinuria and serum albumin levels at the time of RTX infusion (see Table S1, Supplemental Digital Content, which provides an overview of the characteristics of DRNS patients stratified according to whether complete remission was achieved within 6 months after RTX infusion); patients with complete remission had lower proteinuria and higher level of serum albumin (urine protein/creatinine ratio: 2.5 ± 2.5 vs 11.0 ± 14.3 mg/mg, P = .03; serum albumin: 3.4 ± 0.7 vs 2.1 ± 0.8 mg/dL, P = .002).
Steroid dosage decreased significantly after RTX treatment, but differences in CNI dosage and duration of drug-free period were not evident (Table 2). Following RTX treatment, the participants became slimmer, as BMI decreased but height remained unchanged; the estimated GFR and prevalence of hypertension did not change significantly after RTX treatment (Table 3).
3.3. Other effects of RTX
In all patients treated with RTX, CD19(+) B cells were depleted within 2 weeks after 1 or 2 (n = 9) doses of RTX. In DDNS patients, the proportion of B cells expressing CD19 was 0.86% at 3 months and 6.08% at 6 months after treatment (Fig. 4A). The mean duration of B-cell depletion in DDNS patients was 103.0 ± 63.7 days. Relapse after RTX treatment was not always related to recovery of B cells; specifically, 4 patients had <1% CD19(+) B cells at the time of NS relapse. On the contrary, the duration of B-cell depletion after RTX treatment in DRNS patients was 40.9 ± 70.0 days, which is significantly shorter than that noted among DDNS patients (P = .004) (Fig. 4B).
Figure 4.

CD19 B-cell counts after rituximab therapy. (A) Randomized controlled trial of drug-dependent nephrotic syndrome. (B) Single-arm study of drug-resistant nephrotic syndrome.
3.4. Safety of RTX treatment
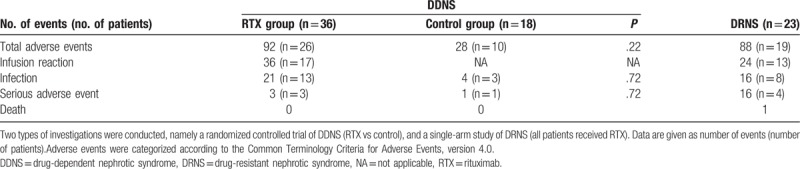
Regarding adverse events, 30 of 59 participants (50.8%) treated with RTX experienced infusion reactions such as chest discomfort, fever, vomiting, or skin rash, which responded to antihistamine management and reduction in the rate of infusion. Discontinuation of infusion was necessary in 5 patients, all of whom were able to tolerate subsequent RTX administration. Twenty-one patients (35.6% of RTX-treated participants) experienced infection (mostly mild) after RTX treatment. Overall, 45 patients (76.3% of RTX-treated participants) experienced adverse effects; however, among DDNS patients, there were no significant differences between the RTX group and the control group regarding the overall incidence of adverse events and infection (Table 4). Reported severe adverse events included chest discomfort, epigastric pain, periumbilical pain, urticaria, influenza, and otitis media. One patient died of hypertensive encephalopathy at 10 months after RTX treatment, but the event was judged to be due to complications associated with intractable NS, and not to RTX treatment.
Table 4.
Adverse events in patients with childhood-onset, difficult-to-treat nephrotic syndrome.
4. Discussion
In this prospective clinical trial, RTX was effective for childhood-onset, difficult-to-treat NS. A single dose of RTX treatment increased the duration of remission, reduced the need for steroids and CNIs (in terms of dosage and frequency of use), and induced remission in more than one-third of DRNS patients. Adverse events were mostly mild and transient.
In this study, the efficacy of single-dose RTX in DDNS was comparable to the efficacy previously reported for treatment involving multiple doses of RTX. Specifically, we found remission rates of 85.7% at 3 months, whereas previous RCTs reported 3-month remission rates of 81.5% with 1 or 2 doses of RTX and 83.3% with 4 doses (1 per week) of RTX; at 6 months, we found a remission rate of 74% for single-dose RTX, compared with 75% previously reported for multiple doses of RTX. Several other studies have also reported that single-dose or low-dose RTX therapy is effective in nonmalignant conditions including NS.[9,23–28] In this study, very few patients did not achieve complete eradication of CD19(+) B cells at week 2 after RTX infusion, and thus were given a second dose of RTX. Complete B-lymphocyte depletion was also observed after the first of 4 RTX doses administered in a previous study,[15] which reported a longer duration of B-cell depletion (B cells <1% for a mean of 148 days, 21 weeks vs 102 days, 14.6 weeks in the present study)[15]; however, considering that RTX treatment in the previous study took 4 weeks (with one RTX dose per week), whereas the treatment in our study consisted of a single dose of RTX, the difference regarding duration of B-cell depletion would actually be <3 weeks. Although the optimal dosage and interval of RTX administration in NS are yet to be established, single-dose RTX seems as effective as multiple-dose treatment provided that B-cell eradication is successful.
On the contrary, the efficacy of RTX for DRNS has been questionable. Some observational studies suggested that RTX was effective for inducing remission of NS,[17–19,29,30] and a multicenter cohort study reported that 48.5% (16/33) of patients with steroid-resistant NS achieved complete or partial remission at 6 months after RTX treatment.[18] RTX in combination with high-dose steroid and CNI treatment has been suggested as a possible therapeutic option for patients with DRNS.[17,18] However, an RCT reported that RTX failed to improve remission rates over those noted for conventional therapy in DRNS.[16] In our study, 39.1% of patients achieved partial or complete remission within 6 months of RTX treatment, which is similar to the findings of the previous cohort study. Such inconsistent results might be caused by the heterogeneity of DRNS manifestations. An international cohort study involving genetic analysis of 27 genes known to be associated with NS found that 29.5% of steroid-resistant NS cases involved a single-gene cause.[31] Although we excluded patients with known genetic causes such as NPHS1, NPHS2, WT1, or LAMB2, some of our participants might be carrying a yet unknown genetic factor of NS. In fact, among 13 patients who did not show any response to RTX, 3 patients were later found to harbor NUP107 gene mutations.[32]
Compared with RTX responders, RTX nonresponders had more severe nephrotic features at baseline. It is possible that, in DRNS, severe proteinuria causes urinary loss of immunoglobulin and thus of RTX, which is a monoclonal immunoglobulin G antibody, resulting in a severely shorter half-life of RTX; indeed, the half-life of RTX was previously reported to be <1 day.[33] Furthermore, in our study, the duration of B-cell depletion was much shorter in patients with DRNS than in those with DDNS. In addition, 30.4% (7/23) of patients with DRNS compared with only 5.7% (2/35) of patients with DDNS required a second dose of RTX for B-cell depletion. These findings suggest that multiple-dose RTX treatment might be more effective for inducing remission in patients with DRNS, who would benefit from monitoring of RTX concentration and more frequent monitoring of B-cell counts.
In patients with difficult-to-treat NS, long-term use of steroids and CNIs is associated with considerable adverse effects. Indeed, previous studies reported steroid or CNI toxicity in 44% to 75% of NS patients on long-term treatment,[14,15] which is consistent with our present findings, namely, that two-thirds of patients had experienced adverse effects of steroid or CNI treatment at the time of enrollment in this study. RTX treatment did ameliorate drug toxicity, especially in DDNS patients, and facilitated significant reduction in steroid and CNI exposure, which is in agreement with previous observations.[15,24,34,35]
Regarding the safety of RTX, we found an infection rate of 36.1% and an in incidence of serious adverse events of 8.3% in DDNS patients who underwent RTX treatment; these rates are lower than those reported in a previous RCT that employed 4 doses of RTX, with one dose per week (96% and 42%, respectively).[15] The discrepancy might stem from differences in the use of concomitant immunosuppressive medications, or, more likely, from the difference in cumulative dose of RTX, which was lower in our study. Most adverse events noted in our study, including infection, were mild and treatable. Progressive multifocal leukoencephalopathy and pulmonary fibrosis, which represent serious adverse effects of RTX treatment,[36,37] did not occur during the observation period of our study. However, longer-term observation is mandatory for these patients because such complications might develop as late adverse events.[37]
This study had some limitations. Our RCT for DDNS was not placebo-controlled and not blinded; therefore, some bias might be introduced. However, the authors had expected that the bias would be minimal because our endpoints were rather objective. On the contrary, the DRNS study had no control group. The authors had reckoned that it was not ethical to continue ineffective therapy in the control group during the observation period for the purpose of the study. In addition, the observation period of this study was one year, which was not long enough to assess the long-term effects and safety of RTX. We need to observe the patients enrolled in our study for a longer time to assess the long-term outcomes.
In conclusion, our study laid one more piece of evidence that RTX can be safe and effective in patients with childhood-onset, medication-dependent NS. In addition, more than one-third of patients with the medication-resistant NS may achieve remission with RTX treatment. Finally, one dose of RTX is likely sufficient to achieve B-cell depletion in patients with NS and is moreover associated with a lower incidence of adverse events; if necessary, a second dose may be administered. Controlled studies are warranted to determine the optimal dosing and interval for administration of RTX in patients with difficult-to-treat NS.
Author contributions
Conceptualization: Yo Han Ahn, Kyoung Hee Han, Hyun Jin Choi, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Data curation: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Heeyeon Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Hee Gyung Kang.
Formal analysis: Yo Han Ahn, Hyun Jin Choi, Heeyeon Cho, Jung Won Lee, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Il-Soo Ha, Seung Joo Lee, Hee Gyung Kang.
Funding acquisition: Hyun Jin Choi, Il-Soo Ha, Hee Gyung Kang.
Investigation: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Heeyeon Cho, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Methodology: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Heeyeon Cho, Jung Won Lee, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Project administration: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Heeyeon Cho, Jung Won Lee, Joo Hoon Lee, Il-Soo Ha, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Resources: Hyun Jin Choi, Il-Soo Ha, Hee Gyung Kang.
Software: Hyun Jin Choi, Il-Soo Ha, Hee Gyung Kang.
Supervision: Yo Han Ahn, Seong Heon Kim, Hyun Jin Choi, Heeyeon Cho, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Validation: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Hee Gyung Kang.
Visualization: Hyun Jin Choi, Il-Soo Ha, Hee Gyung Kang.
Writing—original draft: Yo Han Ahn, Seong Heon Kim, Il-Soo Ha, Hee Gyung Kang.
Writing—review and editing: Yo Han Ahn, Seong Heon Kim, Kyoung Hee Han, Hyun Jin Choi, Heeyeon Cho, Jung Won Lee, Jae Il Shin, Min Hyun Cho, Joo Hoon Lee, Young Seo Park, Il-Soo Ha, Hae Il Cheong, Su Young Kim, Seung Joo Lee, Hee Gyung Kang.
Yo Han Ahn orcid: 0000-0002-8185-4408.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CNI = calcineurin inhibitor, DDNS = drug-dependent nephrotic syndrome, DRNS = drug-resistant nephrotic syndrome, FSGS = focal segmental glomerulosclerosis, GFR = glomerular filtration rate, NA = not applicable, NS = nephrotic syndrome, RCT = randomized controlled trial, RTX = rituximab.
This research was supported by a grant (11172MFDS298) from Ministry of Food and Drug Safety in 2011-2012.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The authors of this work have nothing to disclose.
References
- [1].Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012;2:139–274. [Google Scholar]
- [2].Kyrieleis HA, Lowik MM, Pronk I, et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 2009;4:1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].El-Husseini A, El-Basuony F, Mahmoud I, et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant 2005;20:2433–8. [DOI] [PubMed] [Google Scholar]
- [4].Foster BJ, Shults J, Zemel BS, et al. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr 2004;80:1334–41. [DOI] [PubMed] [Google Scholar]
- [5].Iijima K, Hamahira K, Tanaka R, et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 2002;61:1801–5. [DOI] [PubMed] [Google Scholar]
- [6].Inoue Y, Iijima K, Nakamura H, et al. Two-year cyclosporin treatment in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 1999;13:33–8. [DOI] [PubMed] [Google Scholar]
- [7].Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 2006;21:344–9. [DOI] [PubMed] [Google Scholar]
- [8].Kemper MJ, Gellermann J, Habbig S, et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 2012;27:1910–5. [DOI] [PubMed] [Google Scholar]
- [9].Kamei K, Ito S, Nozu K, et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 2009;24:1321–8. [DOI] [PubMed] [Google Scholar]
- [10].Guigonis V, Dallocchio A, Baudouin V, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 2008;23:1269–79. [DOI] [PubMed] [Google Scholar]
- [11].Gilbert RD, Hulse E, Rigden S. Rituximab therapy for steroid-dependent minimal change nephrotic syndrome. Pediatr Nephrol 2006;21:1698–700. [DOI] [PubMed] [Google Scholar]
- [12].Nozu K, Iijima K, Fujisawa M, et al. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol 2005;20:1660–3. [DOI] [PubMed] [Google Scholar]
- [13].Benz K, Dotsch J, Rascher W, et al. Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 2004;19:794–7. [DOI] [PubMed] [Google Scholar]
- [14].Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011;6:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014;384:1273–81. [DOI] [PubMed] [Google Scholar]
- [16].Magnasco A, Ravani P, Edefonti A, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 2012;23:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nakagawa T, Shiratori A, Kawaba Y, et al. Efficacy of rituximab therapy against intractable steroid-resistant nephrotic syndrome. Pediatr Int 2016;58:1003–8. [DOI] [PubMed] [Google Scholar]
- [18].Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 2010;5:2207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ito S, Kamei K, Ogura M, et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 2013;28:257–64. [DOI] [PubMed] [Google Scholar]
- [20].Kamburova EG, Koenen HJ, Joosten I, et al. CD19 is a useful B cell marker after treatment with rituximab: comment on the article by Jones et al. Arthritis Rheum 2013;65:1130–1. [DOI] [PubMed] [Google Scholar]
- [21].Lieberman KV, Tejani A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J Am Soc Nephrol 1996;7:56–63. [DOI] [PubMed] [Google Scholar]
- [22].Ponticelli C, Rizzoni G, Edefonti A, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 1993;43:1377–84. [DOI] [PubMed] [Google Scholar]
- [23].Fujinaga S, Hirano D, Nishizaki N, et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 2010;25:539–44. [DOI] [PubMed] [Google Scholar]
- [24].Takei T, Itabashi M, Moriyama T, et al. Effect of single-dose rituximab on steroid-dependent minimal-change nephrotic syndrome in adults. Nephrol Dial Transplant 2013;28:1225–32. [DOI] [PubMed] [Google Scholar]
- [25].Visentini M, Tinelli C, Colantuono S, et al. Efficacy of low-dose rituximab for the treatment of mixed cryoglobulinemia vasculitis: phase II clinical trial and systematic review. Autoimmun Rev 2015;14:889–96. [DOI] [PubMed] [Google Scholar]
- [26].Mariette X, Rouanet S, Sibilia J, et al. Evaluation of low-dose rituximab for the retreatment of patients with active rheumatoid arthritis: a non-inferiority randomised controlled trial. Ann Rheum Dis 2014;73:1508–14. [DOI] [PubMed] [Google Scholar]
- [27].Bredemeier M, de Oliveira FK, Rocha CM. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:228–35. [DOI] [PubMed] [Google Scholar]
- [28].Ruggenenti P, Cravedi P, Remuzzi G. Rituximab for membranous nephropathy and immune disease: less might be enough. Nat Clin Pract Nephrol 2009;5:76–7. [DOI] [PubMed] [Google Scholar]
- [29].Nakayama M, Kamei K, Nozu K, et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol 2008;23:481–5. [DOI] [PubMed] [Google Scholar]
- [30].Bagga A, Sinha A, Moudgil A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med 2007;356:2751–2. [DOI] [PubMed] [Google Scholar]
- [31].Sadowski CE, Lovric S, Ashraf S, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 2015;26:1279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Park E, Ahn YH, Kang HG, et al. NUP107 mutations in children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplant 2017;32:1013–7. [DOI] [PubMed] [Google Scholar]
- [33].Counsilman CE, Jol-van der Zijde CM, Stevens J, et al. Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatr Nephrol 2015;30:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sinha A, Bhatia D, Gulati A, et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant 2015;30:96–106. [DOI] [PubMed] [Google Scholar]
- [35].Sato M, Ito S, Ogura M, et al. Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol 2014;29:1373–9. [DOI] [PubMed] [Google Scholar]
- [36].Chaumais MC, Garnier A, Chalard F, et al. Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 2009;24:1753–5. [DOI] [PubMed] [Google Scholar]
- [37].Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009;113:4834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


