Abstract
A series of twelve 2,5-bis(alkylamino)-1,4-benzoquinones were prepared in yields ranging from 9–58% via the reaction between p-benzoquinone and various amines. The structures of the synthesized compounds were confirmed by IR, 1H- and 13C-NMR and MS analyses. The phytotoxicity of the 2,5-bis(alkylamino)-1,4-benzoquinones was evaluated against two crop species, Cucumis sativus and Sorgum bicolor, at 1.0 × 10-3 mol/L. In general, the quinones displayed inhibitory effects on the dicotyledonous species C. sativus (7–74%). On the other hand stimulatory effects were observed on S. bicolor (monocotyledonous). Similar results were observed in the biological assays carried out with the weed species Ipomoea grandifolia (dicotyledonous) and Brachiaria decumbens (monocotyledonous). In addition, the cytotoxicity of the 2,5-bis(alkylamino)-1,4-benzoquinones was assayed against HL-60 (leukemia), MDA-MB-435 (melanoma), SF-295 (brain) and HCT-8 (colon) human cancer cell lines and human peripheral blood mononuclear cells (PBMC), as representatives of healthy cells, using a MTT and an Alamar Blue assay. Compound 12 was the most active, displaying cytotoxicity against all cancer cell lines tested.
Keywords: quinones, herbicide, cytotoxicity
1. Introduction
Plant roots perform a variety of functions such as mechanical support, water/nitrogen uptake, and production of exudates. It is known that roots are capable of producing and secreting compounds (exudates) into the rhizosphere. Root exudation includes release of ions, free oxygen and water, enzymes, mucilage, and a range of organic compounds (primary and secondary metabolites) [1,2,3,4].
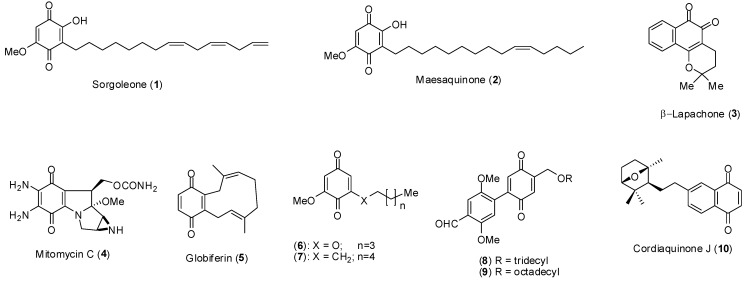
Various organic compounds present in root exudates are involved in chemical-mediated plant-plant or allelopathy interactions. Allelopathy is concerned with the chemical interactions that occur among plants and is mediated by the release of allelochemicals into the rhizosphere [5,6]. The study of such interactions has led to the discovery of several phytotoxic substances that have potential use as herbicides [7], or could be used as lead structures for the development of more active compounds [8]. Among such substances is a compound called sorgoleone (1, Figure 1), isolated from root exudates of Sorghum bicolor [9] and characterized as 2-hydroxy-5-methoxy-3-[(8Z,11Z)-pentadeca-8,11,14-trienyl]- cyclohexa-2,5-dien-1,4-dione.
Figure 1.
Structures of several benzoquinones.
Since its discovery, it has been demonstrated that 1 is a potent inhibitor of chlorophyll formation in Lemma minor L., and it also inhibits the growth of several grass and broadleaf weeds at concentrations as low as 10 μmol/L [10]. Further investigations revealed that 1 is an effective inhibitor of electron transfer between QA- to QB at the reducing side of photosystem II [11] and also of the mitochondrial electron transport [12,13]. During in vitro assays, 1 has been shown to be more active than the commercial herbicide atrazine in inhibiting photosystem II [14]. This quinone also causes disturbance of plasma H+-ATPase activity in root cells [15].
It has also been demonstrated that several benzoquinones displayed cytotoxic effects. For example, the naturally occurring maesaquinone (2, Figure 1) exhibited in vitro cytotoxicity against several solid tumor cells [16]. β-Lapachone (3, Figure 1), has a diversity of useful biological activities against various cancer cell lines such as human ovarian and prostate tumors and, at lower doses, is a radiosensitizer of several human cancer cell lines [17]. The quinone mitomycin C (4, Figure 1) has been used in chemotherapy against certain solid tumors [18,19]. More recently, the biological profile of globiferin (5, Figure 1) a terpenoid benzoquinone isolated from root extracts of Cordia globifera was investigated [20]. It was found that this compound displays cytotoxic activity against NCI-H187 cell line. The biological profile of cordiaquinone J (10, Figure 1), a 1,4-naphthoquinone isolated from the roots of Cordia leucocephala, has been shown to present antiproliferative effects related to reactive oxygen species (ROS) generation [21].
In the last few years, we have targeted various natural products as lead structures towards the development of plant growth regulators [22,23,24,25,26,27,28,29], including sorgolene (1) [30,31]. In this context, several sorgoleone derivatives, such as compounds 6-9 (Figure 1), were prepared and subsequently evaluated against crop and weed species. It has been found that the synthetic analogues 6 and 7 are more effective than 1. Moreover, while compound 6 inhibited the root development of the weed species Euphorbia heterophylla and Brachiaria decumbens, compound 7 was effective in inhibiting the development of the aerial parts and roots of B. decumbens, an aggressive weed commonly found in several crop plantations in Brazil. When tested against E. heterophylla, the aryl-p-benzoquinone 8 caused 34.2 and 76.5% inhibition of the aerial part and roots, respectively. The quinone 9 significantly inhibited the aerial parts (5l.7%) and roots (85.2%) of the weed species Ipomoea grandifolia.
In continuation to our studies in this area we report in this paper the preparation and investigation of the phytotoxic effects of twelve 2,5-bis(alkylamino)-1,4-benzoquinone analogues of sorgoleone (1). Considering the cytotoxic effects reported in the literature for several benzoquinones, we also evaluated the cytotoxicity of these analogues against several human cancer cell lines and peripheral blood mononuclear cells (PMBC). The results of the cytotoxicity screening are also discussed.
2. Results and Discussion
2.1. Synthesis of 2,5-diamino-p-benzoquinones
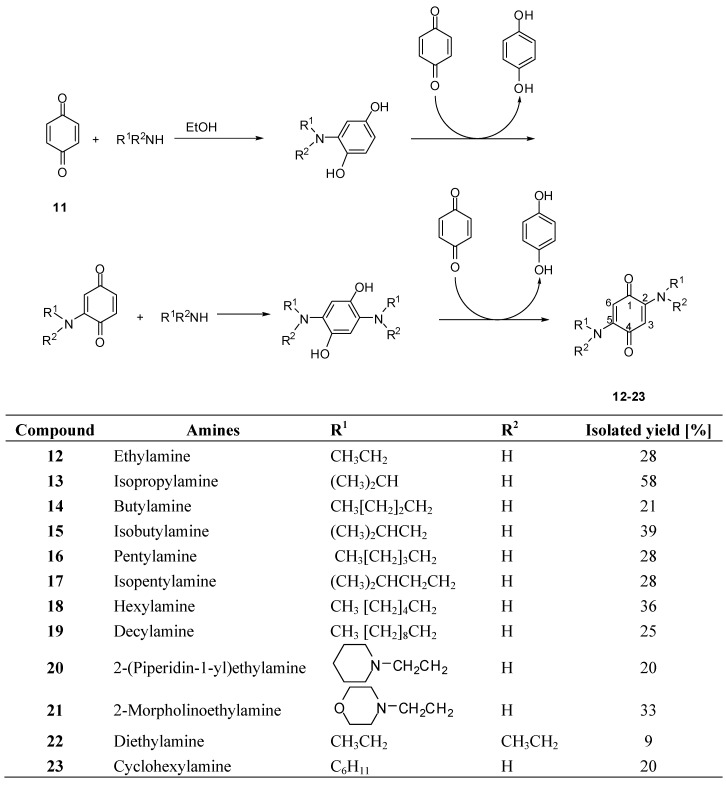
Compounds 12-23 were synthesized by the reaction between p-benzoquinone 11 and various amines [32,33,34]. A 3:2 molar ratio of benzoquinone/amine was required due to successive reductions and oxidations involved in the formation of the products as shown in Scheme 1. The reaction was carried out first in dry EtOH and later it was found that slightly better yields were obtained when wet EtOH containing 2% (v/v) of H2O was used. Although no detailed investigation on the mechanism was carried out, we believe that H2O might enhance the reactivity and polarizability of the quinone carbonyl group facilitating the nucleophilic addition step [33].
Scheme 1.
Synthesis of 2,5-bis(alkylamino)-1,4-benzoquinones 12-23.
After column chromatography purification, the 2,5-bis(alkylamino)-1,4-benzoquinones were obtained as red compounds. Bayen and co-workers [32] reported the preparation of several 2,5-bis(alkylamino)-1,4-benzoquinones in good yields employing similar conditions used in the present work. In our hands, however, benzoquinones 12-23 were obtained in only low to fair yields (Scheme 1). This fact can possibly be attributed to polymerization side reactions as previously reported [35]. These results are also consistent with a great quantity of base line material observed during the column chromatographic purification.
The IR spectra of compounds 12-23 exhibited strong absorptions in the 1,613 to 1,550 cm-1 range due to carbonyl stretchings. The vinylic H-atoms in these quinones were characterized in the NMR spectrum by signals observed at 5.3–5.4 ppm, while resonance signals for the C=O groups were observed at 176-180 ppm. The expected molecular formulae were confirmed by high resolution ESI-MS.
2.2. Phytotoxic activity
In a preliminary screen carried out on Petri dishes the effect of 2,5-bis(alkylamino)-1,4-benzoquinones 12-23 on the radicle growth of S. bicolor and C. sativus was evaluated at a concentration of 1.0 × 10-3 mol/L (Table 1).
Table 1.
Effect of 2,5-bis(alkylamino)-1,4-benzoquinones on the radicle growth of C. sativus and S. bicolor at 1.0 × 10-3 mol/L.
| Compound | Cucumis sativus | Sorghum bicolor | ||||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |||||
| Radiclelength [cm]a | Inhibition [%] | Radiclelength [cm]a | Inhibition [%] | Radiclelenght [cm]a | Inhibition [%] | Radiclelength [cm]a | Inhibition [%] | |
| 12 | 1.40 def | 42 | 1.94 fg | 59 | 0.87 cd | 36 | 1.04 fg | 30 |
| 13 | 1.75 cde | 28 | 3.91 bcd | 18 | 1.90 ab | -40 | 3.50 abc | -135 |
| 14 | 2.17 abc | 10 | 4.87 a | -2 | 1.69 ab | -24 | 3.70 ab | -148 |
| 15 | 2.02 abc | 17 | 3.43 de | 28 | 1.14 bcd | 17 | 1.87 defg | -26 |
| 16 | 2.02 abc | 17 | 4.24 abcd | 11 | 1.52 abc | -12 | 1.91 defg | -28 |
| 17 | 1.89 bcd | 22 | 2.73 ef | 43 | 1.17 bcd | 14 | 2.25 bcdef | -51 |
| 18 | 1.25 ef | 48 | 1.25 g | 74 | 0.49 d | 64 | 0.51 g | 66 |
| 19 | 2.26 abc | 7 | 3.73 ef | 22 | 1.81 ab | -33 | 3.81 a | -156 |
| 20 | 1.85 cd | 24 | 2.33 f | 51 | 1.23 bcd | 10 | 2.07 cdef | -39 |
| 21 | 2.46 a | -2 | 4.41 abc | 7 | 2.09 a | -54 | 3.21 abcd | -115 |
| 22 | 1.16 f | 52 | 1.79 fg | 62 | 0.85 cd | 38 | 0.92 fg | 38 |
| 23 | 2.10 abc | 13 | 4.41 abc | 7 | 1.47 abc | -8 | 2.82 abcde | -89 |
| Control | 2.42 ab | 4.76 ab | 1.36 abc | 1.49 efg | ||||
a Means, in the same column, with the same letter are not significantly different at P = 0.05% by Tukey’s test.
This type of biological assay is commonly used as a general screening for identifying potential phytotoxic substances [36]. With the exception of compound 14, all of the remaining substances inhibited the radicle growth of the dicotyledonous species C. sativus after 48 h. Compound 18 displayed the highest effectiveness against this species, causing 74% inhibition. A simple correlation could not be found between the length of the side chain and the observed biological activity of compounds 12, 13, 14, 16, 18, and 19. Comparing quinones 14 (-2%)and 15 (28%) as well as 16 (11%) and 17 (43%) it can be observed that the compounds with an unbranched side chain exhibited major inhibitory activity in relation to the branched side chain. Considering compounds 20, 21, and 23, which present cyclic groups in their side chain portions, the highest inhibitory effect was associated with compound 20. From the results presented in Table 1 it is clear that while inhibitory effects were observed for C. sativus, after 48 hours radicle promotion was noticed with the compounds for the monocotyledonous species S. bicolor. Exceptions to this generalization were the substances 12, 18, and 22.
The effect of the compounds 12-23 on the radicle growth of the weed species Ipomoea grandifolia (dicotiledonous) and Brachiaria decumbens (monocotiledonous) was subsequently investigated. As presented in Table 2, differential effects were observed between the two weed species. As a general trend, inhibitory effects were observed for I. grandifolia while stimulation was noticed for B. decumbens.
Table 2.
Effect of 2,5-bis(alkylamino)-1,4-benzoquinones on the radicle growth of I. grandifolia and B. decumbens at 1.0 × 10-3 mol/L.
| Compound | Ipomoea grandifolia | Brachiaria decumbens | ||||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |||||
| Radiclelength [cm]a | Inhibition [%] | Radiclelength [cm]a | Inhibition [%] | Radiclelenght [cm]a | Inhibition [%] | Radiclelength [cm]a | Inhibition [%] | |
| 12 | 0.91 de | 48 | 0.91 d | 67 | 0.87 cd | 47 | 1.06 bcd | 43 |
| 13 | 1.19 cd | 32 | 2.47 ab | 10 | 1.82 a | -11 | 2.90 a | -56 |
| 14 | 1.41 abc | 20 | 2.74 a | 0 | 1.69 ab | -3 | 3.07 a | -65 |
| 15 | 1.43 abc | 19 | 2.39 ab | 13 | 1.48 abc | 10 | 2.35 ab | -26 |
| 16 | 1.48 abc | 16 | 2.41 ab | 12 | 1.10 bcd | 33 | 1.50 bcd | 19 |
| 17 | 1.23 cd | 30 | 1.49 bcd | 46 | 1.59 abc | 3 | 2.21 ab | -19 |
| 18 | 0.94 de | 47 | 0.94 d | 66 | 0.45 d | 73 | 0.45 d | 76 |
| 19 | 1.69 ab | 4 | 2.25 abc | 18 | 1.67 ab | -2 | 2.98 a | -60 |
| 20 | 1.22 cd | 31 | 1.57 bcd | 43 | 1.51 abc | 8 | 1.93 abc | -4 |
| 21 | 1.32 bcd | 26 | 2.40 ab | 12 | 1.80 ab | -10 | 2.82 a | -51 |
| 22 | 0.77 e | 56 | 1.35 cd | 51 | 0.65 d | 60 | 0.86 cd | 54 |
| 23 | 1.35 bc | 23 | 2.72 a | 1 | 1.73 ab | -5 | 3.02 a | -62 |
| Control | 1.76 a | 2.72 a | 1.64 ab | 1.86 abc | ||||
a Means, in the same column, with the same letter are not significantly different at P = 0.05% by Tukey’s test
2.3. ATP assay
Considering a previous report on the literature concerning the influence of 2,5-diaminobenzo-quinones on ATP synthesis [37], we assessed the ability of compounds 12, 13, and 23 to interfere with this process. The choice of these substances was made based on the higher availability of them in our laboratories. These three compounds displayed inhibitory activity on the ATP synthesis in isolated spinach chloroplasts. The best inhibitory activity was observed for compound 13 which presented an IC50 equal to 150 µM (data not shown).
2.4. Anti-tumor activity
The anti-tumor activity of the 2,5-bis(alkylamino)-1,4-benzoquinone sorgoleone analogues 12-14, 16-23 was evaluated against four human cancer cell lines: HL-60 (promyelocytic leukemia), HCT-8 (colon), SF-295 (central nervous systems) and MDA-MB-435 (melanoma), obtained from the National Cancer Institute (Bethesda, MD, USA), using the MTT assay as previously described [38]. To investigate the selectivity of different compounds toward normal proliferating cells, an Alamar Blue assay was performed with PBMC after 72 h of drug exposure. The results of the IC50 data [μg/mL] for the antitumor activities are presented in Table 3. Doxorubicin was used as positive control. After 72 h, compounds 12 and 20 exhibited cytotoxicity against all tumor cell lines tested. Compound 12 was a more potent proliferation inhibitor (IC50 values in the range 2.0–6.0 µg/mL) than compound 20. The other compounds investigated were not able to significantly inhibit cell growth under the assay conditions, presenting IC50 values higher than 25 µg/mL.
Table 3.
Cytotoxic activity of 2,5-bis(alkylamino)-1,4-benzoquinone analogues to sorgoleone.
| Compound | Cellsa IC50b [µg/mL]; Confident interval | |||||
|---|---|---|---|---|---|---|
| HL-60 | SF-295 | HCT-8 | MDA-MB-435 | PBMC | ||
| 12 | 2.3(1.3-3.9) | 6.0 (4.6-7.9) | 5.2(2.4-11.2) | 5.6(3.8-8.4) | >25 | |
| 13 | >25 | >25 | >25 | >25 | Nd | |
| 14 | >25 | >25 | >25 | >25 | Nd | |
| 16 | >25 | >25 | >25 | >25 | Nd | |
| 17 | >25 | >25 | >25 | >25 | Nd | |
| 18 | >25 | >25 | >25 | >25 | Nd | |
| 19 | >25 | >25 | >25 | >25 | Nd | |
| 20 | 20.3(17.6-23.4) | 11.3(8.6-14.9) | 13.5(9.1-20.1) | 21.5(18.7-24.8) | 21,8 (15,6-30,1) | |
| 21 | >25 | >25 | >25 | >25 | Nd | |
| 22 | >25 | >25 | >25 | >25 | Nd | |
| 23 | >25 | >25 | >25 | >25 | Nd | |
| Doxorrubicin | 0.02(0.01-0.02) | 0.23(0.19-0.25) | 0.01(0.01-0.02) | 0.48(0.34-0.66) | 0,96 (0,51-1,71) | |
a Cells were plated in 96-well plates incubated under a 5% CO2 atmosphere at 37 °C for 72 h in the presence of pure compounds (0.39-25 μg/mL). Each concentration was tested in triplicate and the analyses were performed in duplicate; b Data are presented as IC50 [μg/mL] values and 95% confidence interval (given in parentheses) obtained from at least three independent experiments. Compound 15 was not evaluated due to limited amount. Nd: not determined.
The cytotoxic activity of the compounds 12 and 20 against normal cells (PBMC) was assessed by Alamar Blue assay [39]. This assay was chosen because of its low toxicity to normal cells [40]. It was found that compound 20 was cytotoxic, with an IC50 value 21.8 µg/mL. Compound 12 showed no activity against PBMC at the highest concentration tested (>25 µg/mL). This compound exhibited the most selective cytotoxicity against the tumor lines and is not toxic to normal cells. It is important to emphasize that selectivity is one important feature towards the development of new antitumoral drugs [41].
The quinone structural motif is present in many anticancer drugs such as anthracyclines (daunorubicin, doxorubicin), mitomycin and mitoxantrone, which are used clinically in the therapy of solid tumors [42]. The mechanisms by which quinones cause these effects can be quite complex. Quinones are Michael acceptors, and cellular damage can occur through alkylation of crucial cellular proteins and/or DNA. Alternatively, quinones are highly redox active molecules which can, by intermediacy of their semiquinone radicals, lead to formation of reactive oxygen species (ROS), which can cause severe oxidative stress within cells through the formation of oxidized cellular macromolecules, including lipids, proteins, and DNA. ROS can also activate a number of signaling pathways. Additionally, quinones, including azaquinones, can work as DNA intercalators, inhibitors of topoisomerases and of some enzymes of the mitochondrial electron transfer chain.
3. Experimental
3.1. General
Hydroquinone and amines were purchased from Aldrich (Milwaukee, WI, USA) Reagents and solvents were purified, when necessary, according to procedures described by Perrin and Armarego [43]. p-Benzoquinone 11 was synthesized from hydroquinone employing a previously described procedure [44]. Analytical thin layer chromatography analyses were conducted on aluminum backed pre-coated silica gel plates. Column chromatography was performed on silica gel (60–230 mesh) and eluting with hexane:diethyl ether mixtures. Melting points are uncorrected and were obtained on a MQAPF-301 apparatus (Microquimica, Brazil). IR spectra were recorded on a Perkin Elmer Paragon 1000 FTIR spectrophotometer using KBr discs (1% w/w) and scanning from 400 to 4,000 cm-1. The 1H- and 13C-NMR spectra were recorded on a Varian Mercury 300 instrument (at 300 MHz and 75 MHz, respectively), using deuterated CHCl3 as solvent and tetramethylsilane (TMS) as internal standard (δ = 0); coupling constants (J) in Hz. MS were recorded on a Shimadzu GCMS-QP5050A instrument under electron impact (70 eV) conditions. HRMS data were recorded under ESI conditions on a Bruker MicroToF spectrometer (resolution = 10,000 FWHM) using a lock-spray source. The lock-mass used for calibration was tetraoctylammonium bromide in positive ion mode.
3.2. Synthesis of compounds 12-16, 18-23, exemplified by the synthesis of 2,5-bis(isopentylamino)-1,4-benzoquinone (17)
To a round-bottomed flask (125 mL) were added p-benzoquinone 11 (500 mg, 4.63 mmol) and EtOH 98% (v/v) (10 mL). After complete dissolution of 11, isopentylamine (268 mg, 3.08 mmol) dissolved in EtOH 98% (v/v) (6 mL) was added slowly. The mixture was stirred at r.t. until complete consumption of the starting material (TLC analysis). The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography eluting with 1:3 (v/v) hexane/CH2Cl2 to give compound 17 as red crystals in 28% yield (122 mg, 0.44 mmol). Mp 167.3–169.7 ºC; IR: 3,264 (NH), 2,956, 2,870, 1,642, 1,549, 1,495, 1,459, 1,364, 1,283, 1,231, 1,071, and 812 cm-1; 1H-NMR δ: 6.59 (bs, NH); 5.30 [s, H-C(3), H-C(6)]; 3.16 [q, J = 6.9 Hz, 2 H-C(1′), 2 H-C(1′′)]; 1.61-1.72 [m, H-C(3′), H-C(3′′)]; 1.54 [q, J = 6.9 Hz, 2 H-C(2′), 2 H-C(2′′)]; 0.93 [d, J = 6.9 Hz, 3 H-C(4′), 3 H-C(5′), 3 H-C(4′′), 3 H-C(5′′)]; 13C-NMR δ: 176.1 [C(1), C(4)]; 149.4 [C(2), C(5)]; 90.6 [C(3), C(6)]; 38.9 [C(1′), C(1′′)]; 35.0 [C(2′), C(2′′)]; 23.9 [C(3′), C(3′′)]; 20.4 [C(4′), C(5′), C(4′′), C(5′′)]; MS, m/z (%): 278 (57) [M+], 235 (87), 222 (100), 221 (51), 208 (15), 194 (14), 179 (27), 163 (23), 152 (15), 138 (14), 125 (18), 82 (17), 68 (27), 55 (24); HRMS (ESI TOF-MS): Calcd. for C16H27N2O2 279.2067; found: 279.2069.
The other compounds 12-16, 18-23 were prepared employing a procedure similar to that described for 17, and yields are presented in Scheme 1. All the compounds were fully characterized by IR, 1H- and 13C-NMR and mass spectrometry. The structural characterization of compounds 12, 13, 14, 18, 22, and 23 has already been described [34,45,46]. Compounds 15, 16, 17, 20, and 21 have already been synthesized, although their complete spectroscopic data were not given [47,48,49,50,51]. Structures for 15, 16, 17, 20, and 21 and the remaining compounds are supported by the following spectroscopic data:
2,5-Bis(isobutylamino)-1,4-benzoquinone (15). Red crystals. Purified by column chromatography, eluent hexane/diethylether 1:1 (v/v). Mp 188.4–191.5 ºC; IR: 3,274 (NH), 2,953, 2,869, 1,645, 1,554, 1,491, 1,443, 1,366, 1,255, 1,065, 815, and 723 cm-1; 1H-NMR δ: 6.71 (br. s, NH); 5.37 [s, H-C(3), H-C(6)]; 2.97 [t, J = 6.6 Hz, 2 H-C(1′), 2 H-C(1′′)]; 1.90-2.04 [m, H-C(2′), H-C(2′′)]; 0.98 [d, J = 6.6 Hz, 3 H-C(3′), 3 H-C(4′), 3 H-C(3′′), 3 H-C(4′′)]; 13C-NMR δ: 178.3 [C(1), C(4)]; 151.8 [C(2), C(5)] 93.0 [C(3), C(6)]; 50.3 [C(1′), C(1′′)]; 28.0 [C(2′), C(2′′)]; 20.5 [C(3′), C(4′), C(3′′), C(4′′)]; MS, m/z (%): 250 (84) [M+], 235 (13), 207 (100), 193 (40), 165 (28), 164 (31), 151 (60), 138 (23), 123 (20), 67 (21), 53 (46); HRMS (ESI TOF-MS): Calcd. for C14H23N2O2 251.1754; found: 251.1756.
2,5-Bis-(pentylamino)-1,4-benzoquinone (16). Red crystals. Purified by column chromatography, eluent hexane/dichloromethane 1:2 (v/v). Mp 134.6–137.1 ºC; IR: 3,263 (NH), 2,957, 2,927, 2,857, 1,644, 1,553, 1,497, 1,463, 1,369, 1271, and 812 cm-1; 1H-NMR (* indicates assignments that could be reversed) δ: 6.62 (br. s, NH), 5.28 [s, H-C(3), H-C(6)]; 3.12 [q, J = 6.8 Hz, 2 H-C(1′); 2 H-C(1′′)]; 1.59-1.68 [m, 2 H-C(2′), 2 H-C(2′′)]; 1.21-1.38 [m, 2 H-C(3′), 2 H-C(4′), 2 H-C(3′′), 2 H-C(4′′)]; 0.86-0.91 [m, 3 H-C(5′), 3 H-C(5′′)]; 13C-NMR δ: 177.1 [C(1), C(4)]; 150.4 [C(2), C(5)]; 91.6 [C(3), C(6)]; 41.6 [C(1′), C(1′′)]; 28.1 [C(3′), C(3′′)]*; 27.0 [C(2′), C(2′′)]; 21.3 [C(4′), C(4′′)]*; 13.0 [C(5′), C(5′′)]; MS, m/z (%): 278 (100) [M+], 235 (73), 223 (30), 207 (40), 193 (46), 179 (28), 165 (62), 164 (19), 151 (45), 138 (23), 137 (23), 110 (23), 67 (54), 54 (56); HRMS (ESI TOF-MS): Calcd. for C16H27N2O2 279.2067; found: 279.2070.
2,5-Bis-(decylamino)-1,4-benzoquinone (19): Red crystals. Purified by column chromatography, eluent hexane/diethylether 2:1 (v/v). Mp 126.4–127.3 ºC; IR: 3,256 (NH), 2,954, 2,917, 2,848, 1,644, 1,554, 1,503, 1,456, 1,365, 1,291, and 683 cm-1; 1H-NMR δ: 6.62 (br. s, NH); 5.30 [s, H-C(3), H-C(6)]; 3.13 [q, J = 6.6 Hz, 2 H-C(1′), 2 H-C(1′′)]; 1.50-1.70 [m, 2 H-C(2′), 2 H-C(2′′)]; 1.15-1.40 [m, 14 H-C(3′-9′), 14 H-C(3′′-9′′)]; 0.88 [t, J = 6.6 Hz, 3 H-C(10′), 3 H-C(10′′)]; 13C-NMR (* indicates assignments that could be reversed) δ: 178.3 [C(1), C(4)]; 151.6 (C(2), C(5)]; 92.8 [C(3), C(6)]; 42.8 (C(1′), C(1′′)]; 32.1 [C(2′), C(2′′)]*; 29.7 [C(8′), C(8′′)]*; 29.7 [C(3′), C(3′′)]*; 29.5 [C(4′), C(4′′)]*; 29.4 [C(5′), C(5′′)]*; 28.5 [C(6′), C(6′′)]*; 27.2 [C(7′), C(7′′)]*; 22.9 [C(9′), C(9′′)]*; 14.4 [C(10′), C(10′′)]; MS, m/z (%): 418 (100) [M+], 362 (35), 333 (11), 305 (31), 165 (25), 162 (27), 151 (17), 138 (17), 68 (20), 54 (30). HRMS (ESI TOF-MS): Calcd. for C26H47N2O2 419.3632; found: 419.3637.
2,5-Bis-(1-(2-aminoethyl)piperidino)-1,4-benzoquinone (20). Red crystals. Purified by column chromatography, eluent hexane/methanol 1:3 (v/v). Mp 164.2–165.5 ºC; IR: 3,291 (NH), 3,251 (NH), 2,935, 2,849, 1,641, 1,551, 1,493, 1,462, 1,364, 1,289, 1,223, 1,126, 993, and 693 cm-1; 1H-NMR δ: 7.04 (br. s, NH); 5.30 [s, H-C(3), H-C(6)]; 3.19 [q, J = 6.0 Hz, 2 H-C(1′), 2 H-C(1′′)]; 2.58 [t, J = 6.0 Hz, 2 H-C(2′), 2 H-C(2′′)]; 2.34-2.42 [m, 2 H-C(3′), 2 H-C(7′), 2 H-C(3′′), 2 H-C(7′′)]; 1.59 [quint., J = 5.5 Hz, 2 H-C(4′), 2 H-C(6′), 2 H-C(4′′), 2 H-C(6′′)]; 1.40-1.44 [m, 2 H-C(5′), 2 H-C(5′′)]; 13C-NMR δ: 178.5 [C(1), C(4)]; 151.6 [C(2), C(5)]; 93.3 [C(3), C(6)]; 56.11 [C(2′), C(2′′)]; 54.5 [C(3′), C(7′), C(3′′), C(7′′)]; 39.3 (C(1′), C(1′′)]; 26.1 [C(4′), C(6′), C(4′′), C(6′′)]; 24.5 [C(5′), C(5′′)]; MS, m/z (%): 360 (2) [M+], 99 (11), 98 (100), 70 (6), 55 (14). HRMS (ESI TOF-MS): Calcd. for C20H33N4O2 361.2598; found: 361.2599.
2,5-Bis-(4-(2-aminoethyl)morfoline)-1,4-benzoquinone (21). Red crystals. Purified by column chromatography, eluent hexane/ethanol 1:4 (v/v). Mp 186.8–188.0 ºC; IR: 3,354 (NH), 2,869, 2,811, 1,645, 1,613, 1,495, 1,455, 1,354, 1,293, 1,115, 1,026, and 809 cm-1; 1H-NMR δ: 7.00 (br. s, NH); 5.28 [s, H-C(3), H-C(6)]; 3.72 [t, J = 4.5 Hz, 2 H-C(4′), 2 H-C(5′), 2 H-C(4′′), 2 H-C(5′′)]; 3.20 [q,J = 6.0 Hz, 2 H-C(1′), 2 H-C(1′′)]; 2.64 [t, J = 6.0 Hz, 2 H-C(2′), 2 H-C(2′′)]; 2.47 [t, J = 4.5 Hz, 2 H-C(3′), 2 H-C(6′), 2 H-C(3′′), 2 H-(6′′)]; 13C-NMR (CDCl3) δ: 178.3 [C(1), C(4)]; 151.0 [C(2), C(5)]; 93.1 [C(3), C(6)]; 66.8 [C(4′), C(5′), C(4′′), C(5′′)]; 55.5 [C(2′), C(2′′)]; 53.2 [C(3′), C(6′), C(3′′), C(6′′)]; 38.5 [C(1′), C(1′′)]; MS, m/z (%): 364 (3) [M+], 101 (10), 100 (100), 70 (8), 56 (24); HRMS (ESI TOF-MS): Calcd. for C18H29N4O4 365.2183; found: 365.2186.
3.3. Biological assays
3.3.1. Phytotoxic activity
The phytotoxic activity of the 2,5-bis(alkylamino)-1,4-benzoquinones 12-23 was preliminarily evaluated as the ability of these compounds to interfere with the radicle growth of the cultivars Sorghum bicolor and Cucumis sativus. For these biological assays, stock solutions at 1.0 × 10-3 mol/L were prepared as follows: each compound 12-23 was dissolved in xylene (84 μL), Tween 80 surfactant (127 μL) and pentan-3-one (42 μL). The resultant suspension was shaken for 1 min and then transferred to a volumetric flask and the volume supplemented with water to 88 mL. The resultant suspension was sonicated for 5 min.
3.3.2. Assay of radicle elongation on Petri dishes
This biological assay was carried out as previously described [52,53]. Groups of seven pregerminated plants of S. bicolor (purchased from Geneze Company, Paracatu, Minas Gerais State, Brazil) were placed in Petri dishes (i.d. = 9 cm) containing washed sand (660 g) and the solution (88 mL) containing the compound to be evaluated. The Petri dishes were sealed with Parafilm, incubated at 28 °C, and inclinated at 75°. After 24 h and 48 h, the radicle length was measured to the nearest millimeter. All treatments were replicated four times in a completely randomized design. The percentage of radicle growth inhibition was calculated in relation to the root length of the control. Positive values represent inhibition and negative values correspond to stimulation. The data were analyzed using Tukey′s test at 0.05 probability level [54]. This biological assay was also conducted with C. sativus L. (purchased from ISLA Company, Porto Alegre, Rio Grande do Sul State, Brazil), B. decumbens (Marangatú Company, Ribeirão Preto, São Paulo State) and I. grandifolia (Agro Cosmos, Engenheiro Coelho, São Paulo State).
3.3.3. Measurement of the ATP synthesis
Intact chloroplasts were isolated from spinach leaves (Spinacea oleracea L.) obtained from local market as previously described [55]. Chloroplasts were suspended in the following medium: 400 mmol/L sucrose, 5 mmol/L MgCl2, 10 mmol/L KCl, and buffered with 0.03 mol/L Na+-tricine at pH 8.0. They were stored as a concentrated suspension in the dark for 1 h at 0 °C. Intact chloroplasts were efficiently lysed to yield free thylakoids prior to each experiment by incubating them in the following electron transport medium: 100 mmol/l sorbitol, 10 mmol/L KCl, 5 mmol/L KCN, and 30 mmol/L tricine buffer (pH 8 with the addition of KOH). Chlorophyll concentration was measured spectrophotometrically as reported [56].
ATP synthesis was measured as the pH rise from 8.000 to 8.100 using a microelectrode connected to a Corning potentiometer with expanded scale. The pH change was recorded with a Gilson recorder. The reaction medium contained 100 mmol/L sorbitol, 5 mmol/L MgCl2, 10 mmol/L KCl and 1 μmol/L K+-tricine at pH 8.0 in the presence of 1 mmol/L ADP and 3 mmol/L KH2PO4. Methylviologen (0.05 mol/L) was added as electron acceptor for the Hill reaction. The effect of compounds 12, 13 and 23 on the ATP synthesis was evaluated at 100 μmol/L, 200 μmol/L, 300 μmol/L, 400 μmol/L, and 500 μmol/L. All mixtures were illuminated with actinic light of a projector lamp (GAP 2660) passed through a 5 cm aqueous solution of 2% CuSO4 as filter [57].
3.4. Cytotoxicity screening
3.4.1. Cell lines and cell cultures
The cytotoxicity of compounds 12-14 and 16-23 was tested against HL-60 (human leukemia), MDA-MB-435 (human breast cancer), HCT-8 (human colon) and SF-295 (human central nervous system) cell lines obtained from the National Cancer Institute (Bethesda, MD, USA). Cells cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin at 37 °C under a 5% CO2 atmosphere.
Heparinized blood (from healthy, non-smoker donors who had not taken any drug at least 15 days prior to sampling) was collected and Peripheral Blood Mononuclear Cells (PBMC) were isolated by a standard method of density-gradient centrifugation over Histopaque-1077. PBMC were washed and resuspended in RPMI 1640 medium supplemented with 20% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin at 37 °C under a 5% CO2 atmosphere. Phytohemaglutinin (2 %) was added at the beginning of culture. After 24 h of culture, cells were treated with the test compounds 12-14 and 16-23.
3.4.2. MTT assay
Tumor cell growth was quantified by the ability of living cells to reduce the yellow dye 3-(4,5-dimethyl-2-thiozolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a purple formazan product [38]. For the experiments, cells were plated in 96-well plates (0.7 × 105 cells/mL for MDA-MB-435, HCT-8 and SF-295 cell lines, and 0.3 × 106 cells/mL for leukemia cells). After 24 h, the test compounds (0.39 to 25 μg/mL) dissolved in DMSO (0.1%), were added to each well and incubated for 72 h. DMSO (0.1%) and doxorubicin were used as negative and positive controls, respectively. Thereafter, the plates were centrifuged and then the medium was replaced by fresh medium (150 μL) containing 0.5 mg/mL MTT. Three hours later, the MTT formazan product was dissolved in 150 µL DMSO, and absorbance was measured using a multiplate reader (Spectra Count, Packard, Ontario, Canada). Drug effect was quantified as the percentage of control absorbance of the reduced dye at 595 nm in relation to control wells.
3.4.3. Alamar Blue assay
In order to investigate selectivity of compounds toward a normal proliferating cell, the Alamar Blue assay was performed with PBMC after 72 h drug exposure [39]. Briefly, PBMC were plated in 96-well plates (3 × 105 cells/well in 100 µL of medium). After 24 h, the compounds (0.09–25 µg/mL) dissolved in DMSO were added to each well (using the HTS - high-throughput screening - Biomek 3000 - Beckman Coulter, Inc., Fullerton, California, USA) and incubated for 72 h. Doxorubicin was used as positive control. Twenty four hours before the end of the incubation, 10 µL of stock solution (0.312 mg/mL) of the Alamar Blue (resazurin - Sigma Aldrich Co. - St. Louis, MO, USA) was added to each well. The absorbance was measured using a multiplate reader (DTX 880 Multimode Detector, Beckman Coulter, Inc). The drug effect was quantified as the percentage of control absorbance at 570 nm and 595 nm.
4. Conclusions
A series of 2,5-bis(alkylamino)-1,4-benzoquinone analogues of sorgoleone (1) were synthesized and biologically evaluated in terms of their phytotoxicity and cytotoxicity. The assessment of phytotoxicity revealed that the compounds can act as plant growth regulators with various patterns of activity. Moreover, differences were noticed concerning the effects of the compounds on monocotyledonous and dicotyledonous species. Further investigation on the phytotoxicity of the 2,5-bis(alkylamino)-1,4-benzoquinones showed that they are capable of inhibiting the ATP synthesis in isolated chloroplasts. The cytotoxicity assays revealed that the compounds 2,5-bis(ethylamino)-1,4-benzoquinone 12 and 2,5-bis(1-(2-aminoethyl)piperidino)-1,4-benzoquinone 20 exhibited activity against all of the human cell lines used in the biological evaluation. The 2,5-bis(ethylamino) derivative 12 exhibited the most selective cytotoxicity against the tumor cell lines tested and is not toxic to normal cells.
Acknowledgements
We are grateful to the following Brazilian Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for research fellowships (to L.C.A.B. and U.A.P.) and financial support; and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) and FINEP for financial support. We are also grateful to Dr. Blas Lotina-Hennsen for the ATP synthesis inhibition biological assays.
Footnotes
Sample Availability: Contact the authors.
References and notes
- 1.Oliveros-Bastidas A.J., Macías F.A., Fernández C.C., Marín D., Molinillo J.M.G. Exudados dela raiz y su relevancia actual en las interacciones alelopaticas. Quim. Nova. 2009;32:198–213. doi: 10.1590/S0100-40422009000100035. [DOI] [Google Scholar]
- 2.Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 3.Walker T.S., Bais H.P., Grotewold E., Vivanco J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003;132:44–51. doi: 10.1104/pp.102.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin C., Yang X., Weston L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil. 2003;256:67–83. doi: 10.1023/A:1026290508166. [DOI] [Google Scholar]
- 5.Macías F.A., Molinillo J.M.G., Varela R.M., Galindo J.C.G. Allelopathy - A natural alternative for weed Control. Pest Manag. Sci. 2007;63:327–348. doi: 10.1002/ps.1342. [DOI] [PubMed] [Google Scholar]
- 6.Macías F.A., Galindo J.C.G., Molinillo J.M.G., Cutler H.G. Allelopathy – Chemistry and Mode of Action of Allelochemicals. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
- 7.Copping L.G., Duke S.O. Natural products that have been used commercially as crop protection agents - A review. Pest Manag.Sci. 2007;63:524–554. doi: 10.1002/ps.1378. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa L.C.A., Teixeira R.R., Montanari R.M. Phytotoxic Natural Products as Models for the development of crop protection agents. In: Epifano F., editor. Current Trends in Phytochemistry. Research Signpost; Kerala, India: 2008. pp. 21–59. [Google Scholar]
- 9.Chang M., Netzly D.H., Butler L.G., Lynn D.G. Chemical regulation of distance. Characterization of the first natural host germination stimulant for Striga asiatica. J. Am. Chem. Soc. 1986;108:7858–7860. doi: 10.1021/ja00284a074. [DOI] [PubMed] [Google Scholar]
- 10.Einhellig F.A., Souza I.F. Phytotoxicity of sorgoleone found in grain Sorghum root exudates. J. Chem. Ecol. 1992;18:1–11. doi: 10.1007/BF00997160. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales V.M., Kazimir J., Nimbal C., Weston L.A., Cheniae G.M. Inhibition of a photosystem II electron transfer reaction by the natural product sorgoleone. J. Agric. Food Chem. 1997;45:1415–1421. doi: 10.1021/jf960733w. [DOI] [Google Scholar]
- 12.Rasmussen J.A., Hejl A.M., Einhellig F.A., Thomas J.A. Sorgoleone from root exudate inhibits mitochondrial functions. J. Chem. Ecol. 1992;18:197–207. doi: 10.1007/BF00993753. [DOI] [PubMed] [Google Scholar]
- 13.Czarnota M.A., Paul R.N., Dayan, Nimbal C.I., Weston L.A. Mode of action, localization, of production, chemical nature, and activity of sorgolene: A potent PSII inhibitor in Sorghum spp. root exudates. Weed Technol. 2001;15:813–825. doi: 10.1614/0890-037X(2001)015[0813:MOALOP]2.0.CO;2. [DOI] [Google Scholar]
- 14.Streibig J.C., Dayan F.E., Rimando A.M., Duke S.O. Joint action of natural and synthetic photosystem II inhibitors. Pest. Sci. 1999;55:137–146. doi: 10.1002/(SICI)1096-9063(199902)55:2<137::AID-PS885>3.0.CO;2-D. [DOI] [Google Scholar]
- 15.Hejl A.M., Koster K.L. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J. Chem. Ecol. 2004;30:2181–2191. doi: 10.1023/B:JOEC.0000048782.87862.7f. [DOI] [PubMed] [Google Scholar]
- 16.Kubo I., Chaudhuri S.K. Structure of maesaquinone. Bioorg. Med. Chem. Lett. 1994;4:1131–1134. doi: 10.1016/S0960-894X(01)80242-0. [DOI] [Google Scholar]
- 17.Silva M.N. da, Ferreira V.F., de Souza M.C.B.V. Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na β-lapachona e derivados. Quim. Nova. 2003;26:407–416. doi: 10.1590/S0100-40422003000300019. [DOI] [Google Scholar]
- 18.Pan S.S., Andrews P.A., Glover C.J. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J. Biol. Chem. 1984;259:959–966. [PubMed] [Google Scholar]
- 19.Tomasz M., Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 1997;76:73–87. doi: 10.1016/S0163-7258(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 20.Dettrakul S., Surerum S., Rajviroongit S., Kittakoop P. Biomimetic transformation and biological activities of globiferin, a terpenoid benzoquinone from Cordia globifera. J. Nat. Prod. 2009;72:861–865. doi: 10.1021/np9000703. [DOI] [PubMed] [Google Scholar]
- 21.Marinho-Filho J.D., Bezerra D.P., Araújo A.J., Montenegro R.C., Pessoa C., Diniz J.C., Viana F.A., Pessoa O.D., Silveira E.R., de Moraes M.O., Costa-Lotufo L.V. Oxidative stress induction by (+)-cordiaquinone J triggers both mitochondria-dependent apoptosis and necrosis in leukemia cells. Chem. Biol. Interact. 2010;183:369–379. doi: 10.1016/j.cbi.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa L.C.A., Costa A.V., Veloso D.P., Lopes J.L.C., Hernandez-Terrones M.G., King-Diaz B., Lotina-Hennsen B. Phytogrowth-inhibitory lactones derivatives of glaucolide B. Z Naturforsch. 2004;59c:803–810. doi: 10.1515/znc-2004-11-1207. [DOI] [PubMed] [Google Scholar]
- 23.Chaves F.C., Barbosa L.C.A., Demuner A.J., Silva A.A. New helminthosporal analogues with plant-growth regulatory properties synthesized via oxyallyl cation. Z. Naturforsch. 2006;61b:1287–1294. [Google Scholar]
- 24.Barbosa L.C.A., Demuner A.J., Maltha C.R.A., da Silva P.S., Silva A.A. Sintese e avaliação da ativadade fitotóxica de novos análogos oxigenados do ácido helmintospórico. Quim. Nova. 2003;26:655–660. doi: 10.1590/S0100-40422003000500006. [DOI] [Google Scholar]
- 25.Demuner A.J., Barbosa L.C.A., Veloso D.P., Howarth O.W. Synthesis and plant growth regulatory activity of 6α,7β-dihydroxyvouacapan-17β-oic acid derivatives. Aust. J. Chem. 1998;51:61–66. doi: 10.1071/C97062. [DOI] [Google Scholar]
- 26.Barbosa L.C.A., Demuner A.J., Alvarenga E.S., Oliveira A., King-Diaz B., Lotina-Hennsen B. Phytogrowth- and photosynthesis-inhibiting properties of nostoclide analogues. Pest Manag.Sci. 2006;62:214–222. doi: 10.1002/ps.1147. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa L.C.A., Demuner A.J., Maltha C.R.A., Teixeira R.R., Souza K.A.P., Bicalho K.U. Phytogrowth activity of 3-(3-chlorobenzyl)-5-arylidenefuran-2(5H)-ones. Z. Naturforsch. 2009;64b:245–251. [Google Scholar]
- 28.Barbosa L.C.A., Rocha M.E., Teixeira R.R., Maltha C.R.A., Forlani G. Synthesis of 3-(4-bromobenzyl)-5-(aryl methylene)-5H-furan-2-ones and their activity as inhibitors of the photosynthetic electron transport chain. J. Agric. Food Chem. 2007;55:8562–8569. doi: 10.1021/jf072120x. [DOI] [PubMed] [Google Scholar]
- 29.Teixeira R.R., Barbosa L.C.A., Forlani G., Piló-Veloso D., Carneiro J.W.M. Synthesis of photosynthesis-inhibiting nostoclide analogues. J. Agric. Food Chem. 2008;56:2321–2329. doi: 10.1021/jf072964g. [DOI] [PubMed] [Google Scholar]
- 30.Lima L.S., Barbosa L.C.A., Alvarenga E.S., Demuner A.J., Silva A.A. Synthesis and phytotoxicity evaluation of substituted para-benzoquinones. Aust. J. Chem. 2003;56:625–630. [Google Scholar]
- 31.Barbosa L.C.A., Alvarenga E.S., Demuner A.J., Virtuoso L.S., Silva A.A. Synthesis of new phytogrowth-inhibitory substituted aryl-p-benzoquinones. Chem. Biod. 2006;3:553–567. doi: 10.1002/cbdv.200690059. [DOI] [PubMed] [Google Scholar]
- 32.Bayen S., Barooah N., Sarma R.J., Sen T.K., Karmakar A., Baruah J.B. Synthesis, structure and electrochemical properties of 2,5-bis(alkyl/arylamino)1,4-benzoquinones and 2-arylamino-1,4-naphthoquinones. Dye Pigment. 2007;75:770–775. doi: 10.1016/j.dyepig.2006.07.033. [DOI] [Google Scholar]
- 33.Hassan S.S.M., Iskander M.L., Nashed N.E. Spectrophotometric determination of aliphatic primary and secondary amines by reaction with p-benzoquinone. Talanta. 1985;32:301–305. doi: 10.1016/0039-9140(85)80084-9. [DOI] [PubMed] [Google Scholar]
- 34.Tindale C.R. Reactions of biogenic amines with quinones. Aust. J. Chem. 1984;37:611–617. doi: 10.1071/CH9840611. [DOI] [Google Scholar]
- 35.Nithianandam V.S., Erhan S. Quinone-amine polymers: 18. A novel method for the synthesis of poly(alkyl aminoquinone)s. Polymer. 1998;39:4095–4098. doi: 10.1016/S0032-3861(97)10301-9. [DOI] [Google Scholar]
- 36.Einhellig F.A., Schon M.K., Rasmussen J.A. Synergistic effects of four cinnamic acid compounds on grain sorghum. J. Plant Growth Regul. 1982;1:251–258. [Google Scholar]
- 37.Hennsen B.L., Achine L., Ruvalcaba N.M., Ortiz A., Hernández J., Farbán N., Martínez M. A. 2,5-Diamino-p-benzoquinone derivatives as photosystem I electron acceptors: Synthesis and electrochemical and physicochemical properties. J. Agric. Food Chem. 1998;46:724–730. doi: 10.1021/jf970979g. [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method. 1983;16:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S.A., Gogal R.M.J., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to 3H-thymidine incorporation assay. J. Immunol. Method. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhi-Jun Y., Sriranganathan N., Vaught T., Arastu S.K., Ahmed S.A. A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J. Immunol. Method. 1997;210:25–39. doi: 10.1016/S0022-1759(97)00171-3. [DOI] [PubMed] [Google Scholar]
- 41.Chari R.V.J. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008;41:98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- 42.O′Brien P.J. Molecular mechanisms of quinone cytotoxicity. Chem.-Biol. Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 43.Perrin D.D., Armarego W.L.F. Purification of Laboratory Chemicals. 3rd. Pergamon; Oxford, UK: 1988. [Google Scholar]
- 44.Brondani D.J., Bieber L.W. Regioselective lithiation and alkylation of 2-methoxy-hydroquinone. Quim. Nova. 1995;18:144–146. [Google Scholar]
- 45.Zhou Q., Swager T.M. Probing delocalization across alkyne-containing linkages: Synthesis and cyclic voltammetry of bridged phenylenediamines. J. Org. Chem. 1995;60:7096–7100. doi: 10.1021/jo00127a011. [DOI] [Google Scholar]
- 46.Wellington K.W., Steenkamp P., Brady D. Diamination by N-coupling using a commercial laccase. Bioorg. Med. Chem. 2010;18:1406–1414. doi: 10.1016/j.bmc.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Garreau Y. Oxidation products of gentisic acid in the presene of primary amines. Bull. Soc. Chim. Fr. 1950:501–502. [Google Scholar]
- 48.Garreau Y. [Interaction of] phenols and sulfites. Ann. Chim. Applicata. 1938;10:485–558. [Google Scholar]
- 49.Torigoe M. Cancerocidal substances. XIII. Effect of some quinones and related compounds upon the Yoshida sarcoma. Pharm. Bull. 1955;3:337–342. doi: 10.1248/cpb1953.3.337. [DOI] [PubMed] [Google Scholar]
- 50.Makarova A.N., Berlin A.Y. Reaction of ethylenimino-1,4-benzoquinones with amines. I. Reaction between ethylenimino-1,4-benzoquinones and secondary amines. Zh. Org. Khim. 1959;29:666–672. [Google Scholar]
- 51.Cavalitto C.J., Soria A.E., Hoppe J.O. Amino- and ammonium-alkylaminobenzoquinones as curarimimetic agents. J. Am. Chem. Soc. 1950;72:2661–2665. doi: 10.1021/ja01162a088. [DOI] [Google Scholar]
- 52.Barbosa L.C.A., Pereira U.A., Teixeira R.R., Maltha C.R.A., Fernandes S.A., Forlani G. Synthesis and phytotoxic activity of ozonides. J. Agric. Food Chem. 2008;56:9434–9440. doi: 10.1021/jf802077e. [DOI] [PubMed] [Google Scholar]
- 53.Barbosa L.C.A., Maltha C.R.A., Cusati R.C., Teixeira R.R., Rodrigues F.F., Silva A.A., Drew M.G.B., Ismail F.M.D. Synthesis and biological evaluation of new ozonides with improved plant growth regulatory activity. J. Agric. Food Chem. 2009;57:10107–10115. doi: 10.1021/jf902540z. [DOI] [PubMed] [Google Scholar]
- 54.Gomes F.P. Curso de Estatística Experimental. 13th. Nobel; Piracicaba, Brasil: 1990. [Google Scholar]
- 55.Macías M.L., Rojas I.S., Matar R., Lotina-Hennsen B. Effect of selected coumarins on spinach chloroplast photosynthesis. J. Agric. Food Chem. 1999;47:2137–2140. doi: 10.1021/jf981121+. [DOI] [PubMed] [Google Scholar]
- 56.Strain H.H., Coppe B.T., Svec W.A. Analytical procedures for the isolation, identification, estimation and investigation of the chlorophylls. In: San Pietro A., editor. Methods of Enzymology. Academic Press; New York, NY, USA: 1971. p. 452. [Google Scholar]
- 57.Demuner A.J., Barbosa L.C.A., Veiga T.A.M., Barreto R.W., King-Diaz B., Lotina-Hennsen B. Phytotoxic constituents from Nimbya alternantherae. Bioch. Syst. Ecol. 2006;34:790–795. doi: 10.1016/j.bse.2006.06.008. [DOI] [Google Scholar]