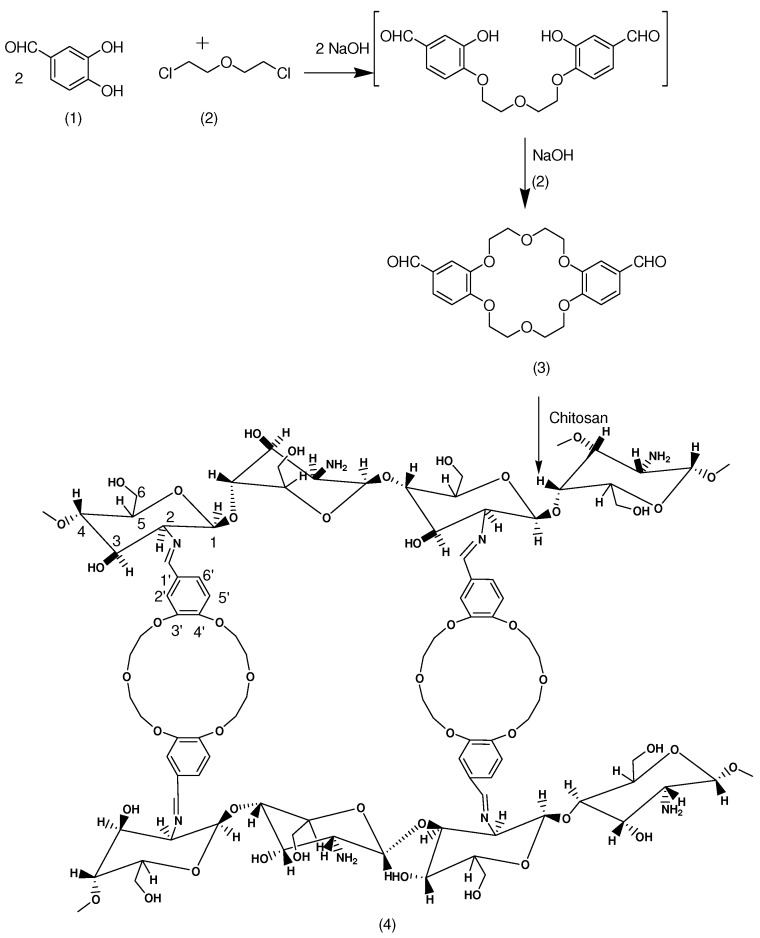
Abstract
Microwave irradiation was used to obtain a di-Schiff base type crosslinked chitosan dibenzocrown ether (CCdBE) via the reaction between the –NH2 and –CHO groups in chitosan and 4,4′-diformyldibenzo-18-c-6, respectively. The structure of the synthesized compound was characterized by elemental analysis, solid state 13C-NMR and FT-IR spectra analysis. The results showed that the mass fraction of nitrogen in the CCdBE derivative was much lower than those of chitosan. The FT-IR spectra of CCdBE revealed the expected chitosan-crown ether structure, as evidenced by the presence of the characteristic C=N and Ar peaks. The adsorption properties of CCdBE for Pd2+ and Hg2+ were investigated and the results demonstrated that the adsorbent has both desirable adsorption properties with a high particular adsorption selectivity for Hg2+ when in the presence of Pb2+ as well as selectivity coefficients for metal ions of KHg2+/Pb2+ = 8.00 and KHg2+/Pb2+ = 10.62 at pH values of 4 and 6, respectively. The reusability tests for CCdBE for Pb2+ adsorption showed that complete recovery of the ion was possible with CCdBE after 10-multiple reuses while CTS had no reusability at acidic solution because of its higher dissolution. The studied features of CCdBE suggested that the material could be considered as a new adsorbent. It is envisaged that the crosslinking of CTS into CCdBE would enhance practicality and effectiveness of adsorption in ion separation and removal procedures.
Keywords: chitosan, crown ether, adsorption
1. Introduction
Heavy metals are highly toxic at low concentrations and can accumulate in living organisms, causing several disorders and diseases [1,2]. As a result of industrialization and urbanization, the presence of heavy metal ions in water streams has increased greatly in the last fifty years. Removal of heavy metal ions from wastewater is essential because of their extreme environmental, public health and economic impacts [3].
The main techniques that have been used for metal content reduction from industrial waste are chemical precipitation, ion exchange, membrane filtration, electrolytic methods, reverse osmosis, solvent extraction, and adsorption [4,5,6]. However, these methods are limited by high operational costs and/or may also be inefficient in the removal of some toxic metal ions, particularly at trace level concentrations [7,8].
The use of chelation ion exchange for wastewater remediation has gained considerable attention in recent years. Chelation ion exchange, in contrast to simple ion exchange, has the advantage of only removing toxic metal ions while the harmless ions move on into the environment [9]. Some of the best chelation ion-exchange materials consist of different biopolymers and their derivatives because of the variety of functional groups, like –OH and –NH2, with which other chemical moieties, e.g., metal ions, can easily react and bond. These biopolymers, including cellulosics, alginates, proteins, chitin and chitin derivatives have remarkable capabilities of lowering metal ion concentrations to parts per billion levels [9,10]. For example, chitosan (CTS) is a deacetylated derivative of chitin that can adsorb metals due to its amino and hydroxyl groups. However, CTS can be dissolved in acidic media which limits its recycling in adsorption processes. Crosslinked chitosan synthesized by the reaction of CTS with hydrophobic crosslinking agents can overcome the disadvantages of CTS and still maintain good adsorption properties for many metal ions. Also, modifications to increase the number of binding sites and/or binding surfaces of chitosan have been made both by substitution on the amino group at C-2 or by crosslinking the polyglycans with small chemicals. Crosslinking CTS with biomass/biopolymers (e.g., alginate) [1,2], chelators such as ethylenediamine tetraacetic acid (EDTA) [11], fixatives such as glutaraldehyde (GA) [12] or polymers like polyvinyl alcohol (PVA) [13] creates a three-dimensional network within the biopolymer and increases the internal surface area for metal adsorption. Increase in structural and chemical stability of these crosslinked derivatives contributes to the resistance and endurance of acid [2] from surface and subsurface groundwater [14,15,16], thereby improving water/sewage purification treatments.
Because crown ethers have particular molecular structures, they have good and different complex selectivity for many metal ions. However, they are not recycled easily after use, so their applications are limited. If crown ethers were crosslinked to chitosan chains to give crown ether-crosslinked chitosan containing double structures and displaying the properties of chitosan and crown ethers, these novel chitosan derivatives would have stronger complex formation with better selectivity for metal ions than the corresponding crown ethers and chitosan separately [17].
Recently, microwave-assisted reactions have received much attention due to the higher conversions and shorter reaction times possible under microwave irradiation compared to those of conventional heating [18]. As a result, microwave irradiation as a chemical reaction means has been widely applied in various synthetic fields of chemistry such as organic and polymer synthesis [19]. In this work, we applied microwave technology to prepare a crosslinked chitosan dibenzocrown ether (CCdBE). This work thus serves as not only an expansion of the applications of microwave irradiation in reactions, but also as an expansion of the use of chitosan as an adsorbent for heavy metal removal. The chitosan derivative (CCdBE) was synthesized by the reaction of 4,4´-diformyldibenzo-18-crown-6 crown ether with CTS under microwave irradiation to give a N-Schiff base type crosslinked chitosan dibenzo-18-crown-6 (CCdBE) [20]. 4,4´-Diformyldibenzo-18-crown-6 was chosen because it was expected that the introduction of 4,4´-diformyldibenzocrown ether residues into chitosan could significantly enhance the adsorption ability compared with the parent CTS, making it possible for this CTS derivative to show promising applications in water treatment.
The structure of the synthesized compound was confirmed by FT-IR spectra analysis, solid state 13C-NMR, elemental analysis and X-ray powder diffraction analysis. The adsorption of lead and mercury ions by CCdBE was studied and it showed that the cross linking of CTS into CCdBE could enhance the practicality and effectiveness of adsorption in ion separation and removal procedures.
2. Results and Discussion
2.1. Synthesis and Structural Characterization of Chitosan-Crown Ether
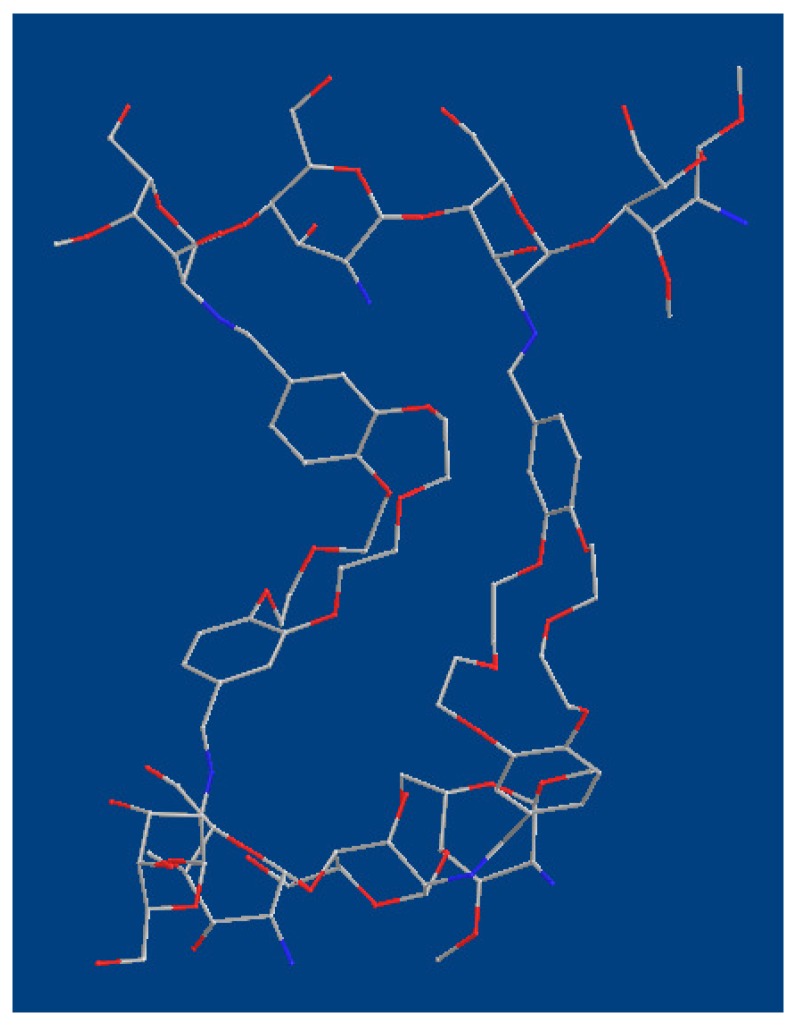
Chitosan crosslinked with dibenzo-18-crown-6 ether (CCdBE) was synthesized as shown in Scheme 1. The three-dimensional structure of the minimized conformation (Figure 1) of CCdBE showed that the crown ether rings were perpendicular to the crosslinked chitosan chains, which represent a series of parallel circular cavities passing over a cylindrical groove between the two crosslinked chitosan chains.
Scheme 1.
Reaction scheme for the synthesis of CcdBE.
Figure 1.
Three-dimensional (3D) view of CcdBE.
Structure elucidation of crown ether crosslinked-chitosan (CCdBE) was accomplished by elemental analysis, FT-IR spectral analysis, X-ray diffraction analysis and solid state 13C-NMR analysis. The CCdBE derivative did not dissolve in organic solvents such as dimethylsulfoxide (DMSO), chloroform, formamide, dimethylformamide (DMF) and trifluoroacetic acid.
2.1.1. Elemental Analysis
The elemental analysis results and degree of substitution (DS) of CTS and CCdBE are presented in Table 1. The nitrogen content of CCdBE was much lower than that of CTS. It was thought that the difference was attributable to the fact that the cross linker molecule (4´,4″-formyldibenzo-18-crown-6) does not have nitrogen atoms in its structure and although it had been grafted to chitosan by -CHO and -NH2 reaction, it only added carbon, oxygen and hydrogen atoms to CCdBE, decreasing the relative content of nitrogen in this molecule. Hydrogen content also decreased due to water elimination during the graft reaction and carbon content increased due to the high carbon content on cross linker molecule.
Table 1.
Elemental analysis of chitosan (CTS) and crosslinked chitosan (CCdBE).
| Compound | N% | C% | H% | DS% |
|---|---|---|---|---|
| CTS | 6.20 | 38.30 | 6.70 | - |
| CCdBE | 3.60 | 46.55 | 6.25 | 42 |
2.1.2. Infrared Analysis
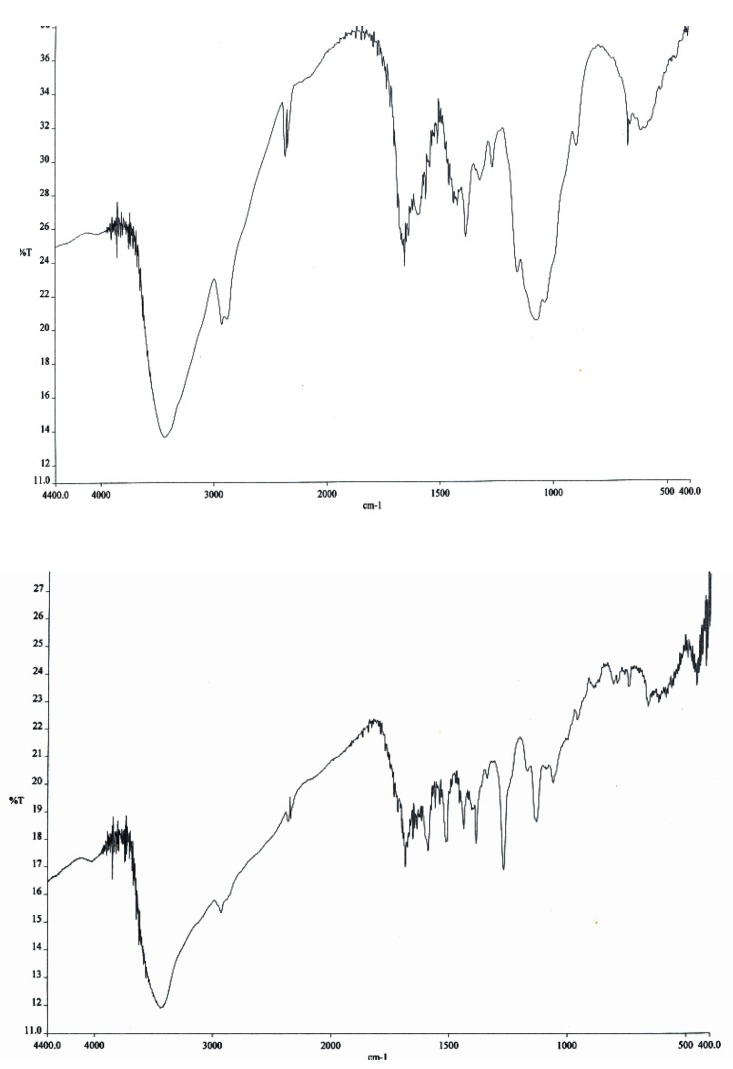
The IR spectra of chitosan and the derivative are shown in Figure 2. Although marked differences were not observed in the IR spectra, a characteristic C=N stretch vibration peak appeared at 1635 cm-1 due to the presence of the Schiff base groups produced in the course of the reaction (from chitosan to chitosan-crown ether).
Figure 2.
FTIR spectra of the chitosan (upper) and crosslinked chitosan (lower).
2.1.3. X-ray Diffraction Analysis
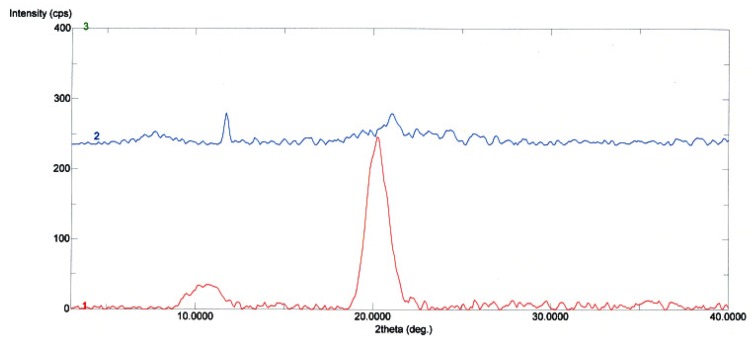
The X-ray diffraction patterns of chitosan (CTS) and crosslinked chitosan (CCdBE) are shown in Figure 3. It shows the characteristic peaks of chitosan (CTS) at 2θ = 10º and 20º, which indicate a high degree of crystallinity for this substance [21,22]. The results revealed that the peak at 2θ = 10º disappeared and the peak at 2θ = 20º decreased in crosslinked chitosan (CCdBE). It appeared that CCdBE didn’t exhibit any crystallinity peak. It was thought that the decrease in crystallinity of CCdBE could be attributed to deformation of the strong hydrogen bond in the chitosan backbone chain because the amino groups were substituted by 4´,4″-diformyldibenzo-18-crown-6. The obtained results were in reasonable agreement with the published data of Xin-Hu et al. [23] who suggested that the crystallinity of chitosan was decreased when it is converted into a N-Schiff base-type benzo-21-crown-7 chitosan [23].
Figure 3.
X-ray diffraction patterns of chitosan (in red) and crosslinked chitosan (blue).
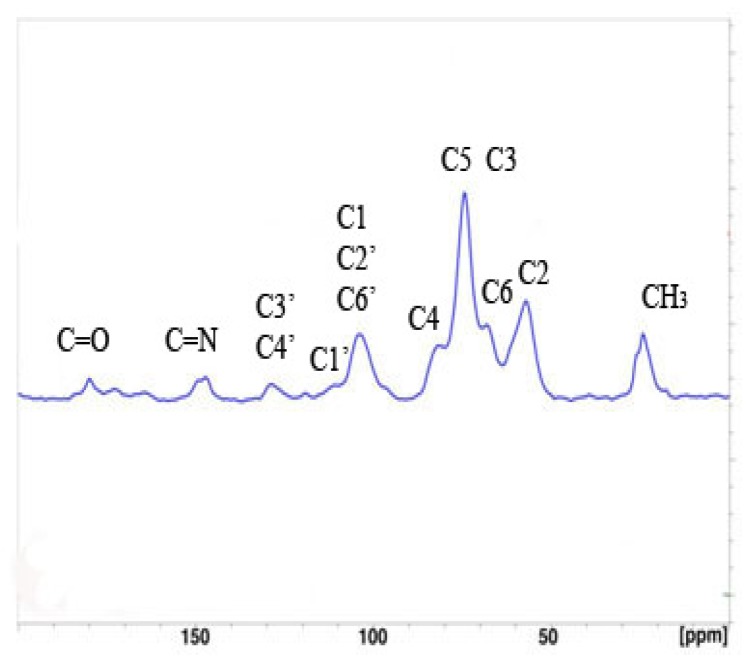
2.1.4. Solid-State 13C-NMR Analysis
The Solid-State 13C-NMR spectrum for crosslinked chitosan are shown in Figure 4. Characteristic aromatic carbon peak appeared at 127 ppm while the characteristic peak of carbon in C=N groups occurred at 148 ppm.
Figure 4.
Solid-state 13C NMR spectrum of crosslinked chitosan (CCdBE).
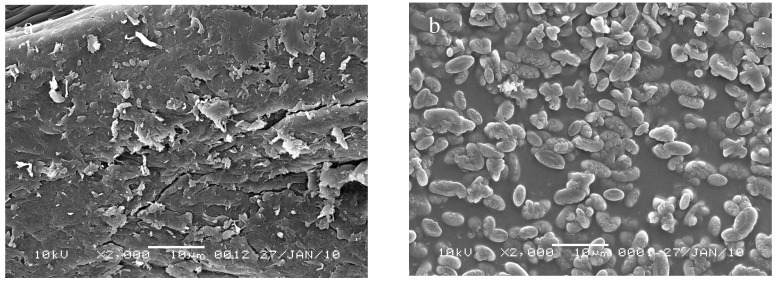
2.1.5. Scanning Electron Microscopy (SEM) Studies
Scanning electron microscope images of the surface of chitosan (CTS) and the crosslinked chitosan (CCdBE) are shown in Figure 5a,b. The surface morphology of chitosan (Figure 5a) was smooth, whereas the crosslinked chitosan (CCdBE, Figure 5b) exhibited a bean-like shaped granulated surface with high cavity-shaped porosities. The porous structure of (CCdBE) may be formed due to the bridging connection of diformyldibenzo-18-crown-6 molecules, hydrophobic in nature, between the different amine groups in CTS, hydrophilic surface, through the intra-molecular and/or inter-molecular crosslinking interactions that leads to deformation of the strong hydrogen bond in the chitosan backbone chain and resulted in an amorphous and a porous surface of CCdBE. The obtained results were confirmed using X-ray diffraction analysis.
Figure 5.
SEM of chitosan (a) and crosslinked chitosan (b).
2.2. Adsorption Behavior
2.2.1. Single Metal Ion Adsorption
The adsorption capacities for metal ions onto CTS and CCdBE are presented in Table 2. It was observed that the adsorption capacities of CCdBE for Pb2+ and Hg2+ were higher than that of CTS. This could be attributed to the three-dimensional network of CCdBE that provided a cavity tailored to the volumetric space of Pb2+ and Hg2+ to adsorption. In addition, it can be seen that CCdBE has a better adsorption capacity for Hg2+ which further suggest that CCdBE could selectively adsorb Hg2+.
Table 2.
Adsorption capacities of CTS and CCdBE for Pb2+ and Hg2+ at pHs 4 and 6.
| Adsorbent | Adsorption capacities (mmol/g) | |||
|---|---|---|---|---|
| pH 4 | pH 6 | |||
| Pb2+ | Hg2+ | Pb2+ | Hg2+ | |
| CTS | 0.82 | 0.33 | 0.94 | 1.48 |
| CCdBE | 0.99 | 1.10 | 1.18 | 1.58 |
2.2.2. Two Metal Ion Co-Adsorption
The selective adsorption of CTS and CCdBE for Hg2+ and Pb2+ from their solution is shown in Table 3. By comparing the selectivity of CCdBE for Pb2+ and Hg2+ with that of CTS, it can be found that the adsorption of CCdBE for Hg2+ is greater than that for Pb2+ at pHs 4 and 6, whereas the adsorption of Hg2+ byCTS was slightly higher than that of Pb2+ at pH 6 while the opposite occurred at pH 4.
Table 3.
Adsorption selectivity of CTS and CCdBE for Pb(II) and Hg(II) ions.
| Adsorbent | Adsorption capacities (mmol/g) | Selectivity coefficient (KHg2+/pb2+) | Adsorption capacities (mmol/g) | Selectivity coefficient (KHg2+/pb2+) | ||
|---|---|---|---|---|---|---|
| pH 4 | pH 6 | |||||
| Pb2+ | Hg2+ | Pb2+ | Hg2+ | |||
| CTS | 0.29 | 0.13 | 0.45 | 0.42 | 0.57 | 1.36 |
| CCdBE | 0.12 | 0.96 | 8.00 | 0.13 | 1.38 | 10.62 |
From the selectivity coefficient (k) results it can be observed that KHg/Pb has been improved 8-fold at pH 4 and 10.66-fold at pH 6 by crosslinking of CTS into CCdBE. The results further suggest that CCdBE can selectively recognize Hg2+. Furthermore, it is concluded that a selective separation of Pb2+ and Hg2+ could be affected by using the CCdBE.
2.2.3. Reusability
It was observed that the adsorption capacities of Pb2+ decreased slightly from 1.2 to 1.08 mmol/g with increasing reuse cycles, and the adsorption capacity for Pb2+ is quite high even after reusing it 10 times, which indicates that the crosslinking in CCdBE results results in good reusability properties.
3. Experimental Section
3.1. Materials
Chitosan of medium molecular weight with 75-85% deacetylation, 3,4-dihydroxybenzaldehyde bis(2-chloroethyl)ether and zinc chloride were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). All aqueous solutions were prepared with deionized water that had been passed through a Millipore Milli-Q Plus water purification system (Millipore, Bedford, MA, USA). The metal salts chosen for this study ([PbCl2 and Hg(NO3)·0.5 H2O] were reagent grade. The compound 2,3,11,12-[4,4´-diformyl dibenzo]-1,4,7,10,13,16-hexaoxacyclooctadeca-2,11-diene (3) was synthesized using a previously reported procedure [20].
3.2. Synthesis of Crosslinked Chitosan diBenzocrown Ether (CCdBE) (4)
Powdered chitosan (1.13 g, 0.005 mol glucosamine residues) was dissolved in 20 mL of 10% (wt) acetic acid solution prepared in water to which ethanol (20 mL) was added and stirred using a magnetic stirrer for 30 min at 40 ºC. Then, 4´,4″-diformyldibenzo-18-c-6 crown ether (3), which was dissolved in chloroform-ethanol mixture (1:1, 5 mL) was slowly added dropwise into the above solution under nitrogen. The reaction mixture was irradiated in a microwave oven (Daewoo MW 800 W domestic type oven) at 10% intensity for 10 min. The reaction mixture was cooled and neutralized with sodium carbonate solution. The liquid was decanted from the reaction mixture. The remaining residue was washed with distilled water, and subsequently the aqueous layer was decanted. Washing and decantation were repeated twice with water and ethyl alcohol. The residue was dried under a reduced pressure overnight giving a pale brown fibrous solid (85% yield).
3.3. Structural Characterization of Chitosan-Crown Ether
3.3.1. Elemental Analysis
The elemental analysis was performed on a Perkin Elmer CHNSO analyzer, model no. 2400 (Perkin Elmer, Inc., Waltham, MA, USA).
3.3.2. Infrared Spectra Analysis
IR analysis (Perkin Elmer FT-IR, Waltham, MA, USA) was employed to confirm the changes in functional groups for natural (CTS) and crosslinked chitosan (CCdBE). Infrared spectra (400-4000 cm-1) were recorded using 100-mg KBr discs containing 2% of chitosan (CTS) or crosslinked chitosan (CCdBE).
3.3.3. X-ray Diffraction Analysis
The powder X-ray diffraction patterns of CTS and CCdBE were recorded with a Rigaku Ultima IV X-ray diffractometer (Osaka, Japan) using X-ray tube Cu (1.540562). Samples were irradiated with monochromatized Cu-Kα radiation and analyzed between 2θ angles of 5 and 40. The voltage, the current, and the time per step were 40 mV, 55 mA and 1s, respectively. Powder X-ray diffraction patterns were measured in order to evaluate the crystalline/amorphous character differences between CTS and CCdBE.
3.3.4. Solid-state 13C-NMR Analysis
Solid state 13C-NMR spectra were recorded at the Center for Pharmaceutical Biotechnology (University of Illinois at Chicago, IL, USA) on a Bruker Avance operating at 500 MHz 1H frequency with a Bruker magic angle spinning probe with 4 mm diameter rotors. CPMAS spectra were collected at 9 kHz spinning speed and 55 kHz Two-Pulse Phase Modulation (TPPM) decoupling.
3.3.5. Scanning Electron Microscopy (SEM) Studies
The shape and surface characteristics of CTS and CCdBE were observed using a scanning electron microscope (SEM). Samples were mounted on an aluminum stub using a double-sided adhesive carbon tape and the flakes were then sputter-coated with gold palladium (Au/Pd) using a vacuum evaporator (Edwards). The coated samples were then scanned and photomicrographs were taken with a Jeol (Tokyo, Japan) JSM1-5510 SEM instrument.
3.4. Adsorption Experiments
3.4.1. Single Metal Ion Adsorption Experiment
A sample of CTS or CCdBE (25 mg) was added to a metal acetate solution (100.0 mL of 10 ppm initial M2+ concentration) with a given pH (pH 4 and 6) adjusted with 0.1 m/L acetic acid. The solution was shaken for 24 h at 25 ºC and then filtered. The adsorption capacities of metal ions were obtained from initial and final concentrations of metal ions in the acetate solution as determined by a Shimadzu atomic adsorption spectrophotometer, model AA-460-13 (Shimadzu Corporation, Tokyo, Japan).
3.4.2. Two Metal Ion Co-Adsorption Experiment
A sample of CTS or CCdBE (25 mg) was added to acetate solution containing Pb2+ and Mg2+ ions (100.0 mL, initial concentration of single species 5 ppm) at pH values of 4 and 6. The solution was shaken for 24 h at 25 ºC and then filtered. The contents of M2+ were determined from initial and final concentrations of metal ions in the acetate solution as determined by atomic adsorption spectrophotometry.
3.4.3. Reusability Experiment
The crosslinked chitosan (CCdBE) adsorbed Pb2+ was dipped into stirring 0.1 mol/L HCl for 1 h at 25 ºC to remove Pb2+, and then treated with 0.1 mol/L NaOH for 5-8 h. Finally it was filtered and washed in turn with water, ethanol and ether. The CCdBE obtained was used in adsorption experiment and the process was repeated 10 times.
4. Conclusions
Microwave irradiation was utilized in the synthesis of a N-Schiff base-type crosslinked chitosan crown ether (CCdBE) by the reaction of 4´,4″-diformyldibenzo-18-crown-6 with chitosan. Its adsorption selectivity and reusability was determined. The adsorption capacity of the obtained CCdBE was much higher for Hg2+ than that for Pb2+. The reported crosslinking method could retain higher adsorption capacity of CTS and, at the same time, improve the acidic resistance of CTS with a desirable selectivity towards mercury ions over lead ions.
Acknowledgements
The authors acknowledge the generous financial support from the Center of Excellence for Research in Engineering Materials (grant # 430-CEREM-03), King Saud University and Ministry of Higher Education, Saudi Arabia.
Footnotes
Sample Availability: Samples of compounds 3 and 4 are available from the authors.
References and Notes
- 1.Gotoh T., Matsushima K., Kikuchi K.I. Adsorption of Cu and Mn on covalently crosslinked alginate gel beads. Chemosphere. 2004;55:57–64. doi: 10.1016/j.chemosphere.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Gotoh T., Matsushima K., Kikuchi K.I. Preparation of alginate-chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere. 2004;55:135–140. doi: 10.1016/j.chemosphere.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Cao J., Tan Y., Che Y., Xin H. Novel complex gel beads composed of hydrolyzed polyacrylamide and chitosan: An effective adsorbent for the removal of heavy metal from aqueous solution. Bioresource Technol. 2010;101:2558–2561. doi: 10.1016/j.biortech.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 4.Monier M., Ayad D.M., Sarhan A.A. Adsorption of Cu(II), Hg(II), and Ni(II) ions by modified natural wool chelating fibers. J. Hazard. Mater. 2010;176:348–355. doi: 10.1016/j.jhazmat.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Wu F.C., Tseng R.L., Juang R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001;35:613–618. doi: 10.1016/S0043-1354(00)00307-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhoua L., Wang Y., Liu Z., Huang Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009;161:995–1002. doi: 10.1016/j.jhazmat.2008.04.078. [DOI] [PubMed] [Google Scholar]
- 7.Evans J.R., Davids W.G., MacRae J.D., Amirbahman A. Kinetics of cadmium uptake by chitosan-based crab shells. Water Res. 2002;36:3219–3226. doi: 10.1016/S0043-1354(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 8.Rangel-Mendez J.R., Monroy-Zepeda R., Leyva-Ramos E., Diaz-Flores P.E., Shirai K. Chitosan selectivity for removing cadmium (II), copper (II), and lead (II) from aqueous phase: pH and organic matter effect. J. Hazard. Mater. 2009;162:503–511. doi: 10.1016/j.jhazmat.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 9.Deans R.J., Dixon B.G. Uptake of Pb2+ and Cu2+ by novel biopolymers. Water Res. 1992;26:469–472. doi: 10.1016/0043-1354(92)90047-8. [DOI] [Google Scholar]
- 10.Kurita K., Sannan T., Iwakura Y. Studies on Chitin. VI. Binding of Metal Cations. J. Appl. Polym. Sci. 1979;23:511–515. doi: 10.1002/app.1979.070230221. [DOI] [Google Scholar]
- 11.Loretz B., Bernkop-Schnurch A. In vitro evaluation of chitosan-EDTA conjugate polyplexes as a nanoparticulate gene delivery system. AAPS J. 2006;8:E756–E764. doi: 10.1208/aapsj080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masri M.S., Randall V.G., Pittman A.G. Removal of metallic ions by partially polyamine polymers. Polym. Preprints-Am. 1978;19:483–488. [Google Scholar]
- 13.Jin L., Bai R. Mechanisms of lead adsorption of chitosan/PVA hydrogel beads. Langmuir. 2002;18:9765–9770. doi: 10.1021/la025917l. [DOI] [Google Scholar]
- 14.Schmuhl R., Krieg H.M., Keizer K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: kinetics and equilibrium studies. Water SA. 2001;27:1–8. [Google Scholar]
- 15.Guibal E., Jansson-Charrier M., Saucedo I., Le Cloirec P. Enhancement of metal ion sorption performances of chitosan: effect of the structure on the diffusion properties. Langmuir. 1995;11:591–598. doi: 10.1021/la00002a039. [DOI] [Google Scholar]
- 16.No H.K., Meyers S.P.J. Crawfish chitosan as a coagulant in recovery of organic compounds from seafood processing streams. J. Agric. Food Chem. 1989;37:580–583. doi: 10.1021/jf00087a002. [DOI] [Google Scholar]
- 17.Yi Y., Wang Y., Liu H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr. Polym. 2003;53:425–430. doi: 10.1016/S0144-8617(03)00104-8. [DOI] [Google Scholar]
- 18.Xing R.G., Liu S., Yu H.H., Guo Z.Y., Wang P.B., Li C.P., Li Z., Li P.C. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005;340:2150–2153. doi: 10.1016/j.carres.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Sha Y.W., Wang Y., Ge J., Wang X. Application of microwave irradiation in the synthesis of heterocyclic compounds. Chin. J. Org. Chem. 2001;21:102–115. [Google Scholar]
- 20.Wada F., Hirayama H., Namiki H., Kikukawa K., Matsuda T. New applications of crown ethers. II. Synthesis of 4′-Formylbenzocrown Ethers. Bull. Chem. Soc. Jpn. 1980;53:1473–1474. doi: 10.1246/bcsj.53.1473. [DOI] [Google Scholar]
- 21.Samuels R.J. Solid-state characterization of the structure of chitosan films. J. Polym. Sci. Part B: Polym. Phys. 1981;19:1081–1105. [Google Scholar]
- 22.Yen M.-T., Yang J.-H., Mau J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009;75:15–21. doi: 10.1016/j.carbpol.2008.06.006. [DOI] [Google Scholar]
- 23.Tang X.-H., Tan S.-Y., Wang Y.-T. Study of the synthesis of chitosan derivatives containing benzo-21-crown-7 and their adsorption properties for metal ions. J. Appl. Polym. Sci. 2001;83:1886–1891. [Google Scholar]