Abstract
(5S,10R)-5-methyl-10,11-dihydro-5H-dibenzo(A,D)cyclohepten-5,10-imine hydrogen maleate (MK-801) is an N-methyl-D-aspartate non-competitive antagonist that possesses useful biological properties, including anticonvulsant and anesthetic activities. Studies have indicated the rapid antidepressant effects of MK-801 in animal models. However, there are no reports concerning a sustained antidepressant effect in the chronic unpredictable mild stress (CUMS) model. Furthermore, the antidepressant mechanism remains unclear. The aim of the present study was to examine the effects of MK-801 (0.1 mg/kg) and rapastinel (10 mg/kg) on depression-like behavior in CUMS mice and measure the protein expression of brain-derived neurotrophic factor (BDNF), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (GluA1) and phosphorylated mammalian target of rapamycin (p-mTOR). In the tail suspension and forced swim tests, MK-801 significantly attenuated the increased immobility time in CUMS mice compared with the vehicle group. In the sucrose preference test, a single-dose injection of MK-801 significantly ameliorated the decreased sucrose preference in CUMS mice compared with the vehicle group. Western blot analyses indicated that MK-801 significantly attenuated the decreased BDNF, GluA1 and p-mTOR protein levels in the medial prefrontal cortex (mPFC), dentate gyrus (DG) and CA3 of the hippocampi of CUMS mice. Conversely, this compound had no effect on increased BDNF, GluA1 and p-mTOR protein levels in the nucleus accumbens of CUMS mice. Therefore, the present study revealed the sustained antidepressant effects of MK-801 in the CUMS model. Furthermore, synaptogenesis and neuronal regeneration in the prelimbic regions of mPFC, DG and CA3 of the hippocampus may be implicated as mechanisms that promote a sustained antidepressant response.
Keywords: antidepressant; (5S,10R)-5-methyl-10,11-dihydro-5H-dibenzo(A,D)cyclohepten-5,10-imine hydrogen maleate; rapastinel; chronic unpredictable mild stress; brain-derived neurotrophic factor; synaptogenesis
Introduction
In addition to being the most prevalent mental disorder, depression is one of the leading causes of disability across the world (1). Major depressive disorder is among the most severe and debilitating psychiatric illnesses (2). Although serotonin-norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors are generally effective in the treatment of depression, it can take several weeks for the patients to experience the complete therapeutic benefits (3). In addition, ~1/3 of these patients fail to respond to the current pharmacotherapy and there is a high rate of relapse among those who do (4). Therefore, the development of novel therapeutic agents capable of inducing rapid and sustained antidepressant responses is urgently required to treat patients with depression who are resistant to the currently available pharmacological agents.
The pharmacological agent (5S,10R)-5-methyl-10,11-dihydro-5H-dibenzo(A,D)cyclohepten-5,10-imine hydrogen maleate (MK-801) is a high affinity, noncompetitive antagonist of N-methyl-D-aspartate (NMDA) (5). Previous results have indicated the antidepressant action of rapastinel (formerly GLYX-13) in preclinical models of depression (6). However, the reports of the antidepressant activity of MK-801 in rodents are inconsistent. MK-801 at 0.1 mg/kg has been demonstrated to significantly decrease the immobility time during the forced swimming test (FST) in mice (7). Notably, MK-801 has been indicated to have a short half-life, persisting for 3 h following the administration of a single dose (7). Furthermore, MK-801 at 0.03 and 0.1 mg/kg exhibited antidepressant activity that lasted for 1 h in mice, whereas the antidepressant effects were not evident at 24 h in FST (8). MK-801 at 0.1 mg/kg significantly enhances the decreased sucrose consumption 2 days following single-dose administration in the sucrose preference test (SPT) (8). However, no anti-anhedonia effect was detected 4 or 7 days following the administration of the single dose in a social defeat stress model (9). These findings suggest that MK-801 induces a rapid antidepressant effect in the social defeat stress model, although the effect was not long lasting (9).
The US Food and Drug Administration granted rapastinel the Fast Track designation in 2014 (10). Studies have demonstrated that rapastinel, an NMDA receptor glycine-site functional partial agonist, induces a rapid and long-lasting antidepressant-like effect without psychotomimetic side effects in animal models (11). In another study, it was revealed that the antidepressant effect of rapastinel persisted for 5 days following treatment with a single dose in the social defeat stress model of depression (12). A recent placebo-controlled study suggested that rapastinel at 5 and 10 mg/kg produces a rapid and sustained antidepressant effect following a single intravenous infusion in patients with depression who had not responded to other antidepressants, without eliciting any psychotomimetic or other notable side effects (13).
The aim of the present study was to demonstrate that MK-801 elicits a sustained antidepressant effect in the established chronic unpredictable mild stress (CUMS) mouse model. The antidepressant effects of MK-801 and rapastinel were evaluated in mice subjected to behavioral tests in the CUMS model of depression. As brain-derived neurotrophic factor (BDNF), a subtype of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (GluA1) and phosphorylated mammalian target of rapamycin (p-mTOR) signaling are implicated in the mechanisms underlying the antidepressant action of the NMDA receptor antagonists (11,14,15), western blot analysis was performed to examine the effects of MK-801 and rapastinel on the protein expression of BDNF, GluA1 and p-mTOR in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), dentate gyrus (DG) and CA3 of the hippocampi regions (12,16).
Materials and methods
Animals
A total of 40 young, healthy male CD1 (ICR) mice (body weight, 22–25 g; age, 6–8 weeks) born and reared in the animal facility of Renmin Hospital of Wuhan University (Wuhan, China) were used in the present study. All animals were maintained in a room at 22±2°C with 60±5% relative humidity and a 12-h light/dark cycle (lights on between 07:00-19:00 h). Mice had ad libitum access to water and food when the stressors were not applied. The stressors were applied to mice outside their living area in a separate procedure room.
The present study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of Wuhan University.
Drugs and drug administration
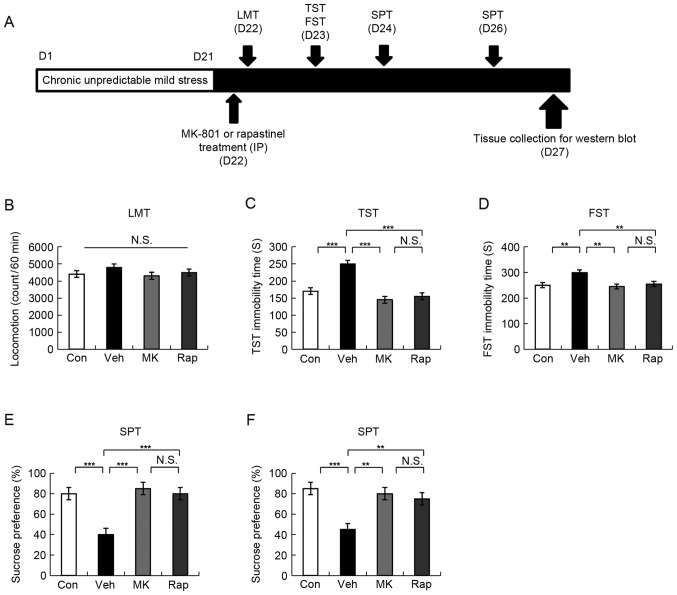
On the day of intraperitoneal injection a single dose of vehicle (10 ml/kg; 0.9% saline), MK-801 (0.1 mg/kg, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and rapastinel (10 mg/kg, Tocris Bioscience, Bristol, UK) were administered to mice (Fig. 1A). Previously reported doses of MK-801 (0.1 mg/kg) and rapastinel (10 mg/kg) were used (7–9,11). If a drug exhibited antidepressant activity that lasted for >24 h in FST or 2 days in SPT mice, it was considered long-lasting (8,9).
Figure 1.
Schedule of CUMS model, drug administration, behavioral tests and brain sampling. (A) CUMS was performed for 21 days. Stressed mice were used in the subsequent experiments. Vehicle, MK-801 (0.1 mg/kg), or rapastinel (10 mg/kg) was administered intraperitoneally (D22). LMT, TST and FST were performed 2, 4 and 8 h after injection of a single dose, respectively (D22-23). A 1% SPT test was performed 2 (D24) and 4 days (D26) after a single-dose injection. The collection of the brain regions was performed at D27. (B) LMT, (C) TST, (D) FST, and (E and F) 1% SPT results were determined. Values were presented as the mean ± standard error of the mean (10 mice/group). **P<0.01 and ***P<0.001 as indicated. Con, control; Veh, vehicle; MK, MK-801; Rap, rapastinel; CUMS, chronic unpredictable mild stress; LMT, locomotion test; TST, tail suspension test; FST, forced swim test; SPT, sucrose preference test; D, day; N.S., not significant.
CUMS mice model
The CUMS procedure was performed as previously described (11). CUMS mice model consisted of a range of unpredictable stressors, which alone are insufficient to induce sustained effects. The animal model consisted of random chronic exposure to a variety of unpredictable stressors, which are listed in Table I. To ensure the application of unpredictable stress and prevent habituation, all stressors were randomly scheduled during a 7-day experimental period and repeated three times throughout the 21-day experimental period, the SPT was used to evaluate the successful establishment of the CUMS model as previously described (11). Control mice (the housing conditions, age, sex and weight of the control mice were the same as the study group) were bred in a separate room and did not come in contact with the stressed groups. There were four treatment groups as follows (n=10 mice/group): Control (10 ml/kg; distilled water), vehicle (10 ml/kg; distilled water), MK-801 (0.1 mg/kg) and rapastinel (10 mg/kg).
Table I.
Schedule of stressor used in chronic unpredictable mild stress model.
| Day | Duration | Stressor |
|---|---|---|
| 1, 8, 15 | 24 h | Water deprivation |
| 2, 9, 16 | 24 h | Food deprivation |
| 2 h | Physical restraint | |
| 3, 10, 17 | 24 h | Water deprivation |
| 4, 11, 18 | 24 h | Soiled cage |
| 5 min | Forced swimming at 4°C | |
| 5, 12, 19 | 24 h | Food deprivation |
| 1 h | Exposure to an empty bottle | |
| 6, 13, 20 | 24 h | Soiled cage |
| Overnight | Overnight illumination | |
| 7, 14, 21 | 24 h | Exposure to a foreign object |
| 5 min | Cage tilt (45°C) |
Behavioral tests
All behavioral tests were performed as reported previously (17–19). The tests were performed in a quiet room. All experimental mice were placed in the behavioral test room for ~30 min prior to the test and were returned to the housing room soon after the tests.
The locomotion test (LMT) was performed using an animal movement analysis system SCANETMV-40 (Melquest Co., Ltd., Toyama, Japan). Mice were placed in transparent Plexiglas cages (560×560×330 mm). Under house lighting, cumulative exercise was recorded for 60 min as previously described (18,19) and the mice cages were cleaned between testing sessions. The LMT was performed 2 h following the injection of a single dose (D22).
During the tail suspension test (TST), the experimental mice were taken away from the living area and a piece of adhesive tape was placed ~2 cm from the tip of the tail. The tape was used to hang mice individually on a hook. The immobility time of tail suspension for each mouse, which was determined by a skilled observer, was video recorded for 10 min as previously described (17,18). When mice hung passively and completely motionless, they were considered immobile. TST was performed 4 h following the injection of a single dose (D23).
The FST was performed to assess immobility time in mice. Mice were placed individually in a cylinder (31×23 cm), which contained ~15 cm of water (23±1°C). The experimental mice were assessed using an automated forced-swim apparatus SCANETMV-40 (Melquest Co., Ltd.). When a mouse remained within the same rectangle for 0.3 sec after the rectangle was set up, the mouse was considered immobile. Immobility time was calculated from the activity time using the apparatus analysis software (Melquest Co., Ltd., Toyama, Japan) as follows: Total time-active time. The cumulative immobility time of the mice was recorded for 6 min (17,19). FST was performed 8 h following the injection of a single dose (D23).
To perform the SPT, mice were exposed to 1% sucrose solution and water for ~48 h, deprived for 4 h and then exposed to two identical bottles containing water and 1% sucrose solution for 1 h. The bottles containing sucrose and water were weighed prior to and following 1 h of exposure. Subsequently, the sucrose preference was evaluated as follows: The bottle containing sucrose was weighed prior to 1 h of exposure (S1), the bottle containing water was weighed prior to 1 h of exposure (W1), the bottle containing sucrose was weighed following 1 h of exposure (S2), the bottle containing water was weighed following 1 h of exposure (W2), the sucrose preference %=(S1-S2)/[(W1-W2) + (S1-S2)] ×100%. A 1% SPT test was performed 2 (D24) and 4 days (D26) following a single-dose injection.
Western blot analysis of BDNF, GluA1, and p-mTOR protein expression
Western blot analysis was performed as reported previously (17). Mice were sacrificed and their brains were immediately harvested. Coronal sections ~1 mm thick were cut and bilateral tissue punches of mPFC, NAc, DG and CA3 of the hippocampi were selected on ice using a SZ-LED Kenis light microscope, and stored at −80°C. Tissue samples were homogenized in Laemmli lysis buffer (Gold Biotechnology Inc., St. Louis, MO, USA). The samples were centrifuged at 3,000 × g at 4°C for 8 min to obtain the supernatants. Protein concentration was determined using the DC protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and samples were incubated for 5 min at 95°C with an equal volume of 4% SDS, 125 mM Tris/HCl, 0.1% bromophenol blue (pH 6.8), 20% glycerol and 10% β-mercaptoethanol. Aliquots (10 µg) were separated by 10% SDS-PAGE. Subsequently, proteins were transferred onto polyvinylidene difluoride membranes using a Mini Trans-Blot Cell. Membranes were blocked with 2% bovine serum albumin (Bio-Rad Laboratories, Inc.) in Tris buffered saline with 0.1% Tween-20 (TBST) for ~1 h at 22±2°C. Blots were incubated with primary antibodies against BDNF (1:500 dilution; cat. no. H-117, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GluA1 (cat. no. ab31232; dilution 1.67 µl/ml, Abcam, Cambridge, MA, USA), β-actin (1:10,000 dilution, Sigma-Aldrich; Merck KGaA) and p-mTOR (1:1,000 dilution, Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The following day, blots were washed three times in TBST and incubated with horseradish peroxidase conjugated secondary anti-rabbit antibody (cat. no. sc-2357; 1:5,000 dilution; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at room temperature. Following a final three washes with TBST, bands were detected using enhanced chemiluminescence (Bio-Rad Laboratories, Inc.) and a western blotting detection system. The images were captured and the optical density of the bands was analyzed using a LAS3000 imaging and detection system (Fujifilm Corporation, Tokyo, Japan).
Statistical analysis
Data were presented as the mean ± standard error of the mean. PASW Statistics 19 (formerly SPSS Statistics; SPSS, Inc., Chicago, IL, USA) was used for analysis and the groups were compared using one-way analysis of variance followed by Fishers least significant difference post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of intraperitoneal administration of MK-801 and rapastinel in the CUMS model
MK-801 provides a rapid antidepressant effect in the social defeat stress model (9). Notably, rapastinel has been demonstrated to produce a rapid and long-lasting antidepressant effect in CUMS and social defeat stress models (9,11,12). In the present study, the effects of MK-801 and rapastinel were assessed in the established CUMS model (Fig. 1A).
LMT indicated no statistically significant difference among the four groups (Fig. 1B). In the TST and FST, 0.1 mg/kg MK-801 and 10 mg/kg rapastinel significantly attenuated the increased immobility times in stressed mice (P<0.001 and P<0.005; Fig. 1C and D, respectively). The SPT results demonstrated that the sucrose preference of mice treated with a single injection MK-801 or rapastinel was significantly increased compared with that of the vehicle-treated group (P<0.001 and P<0.01; Fig. 1E (D24) and F (D26), respectively). These behavioral data suggest that MK-801 elicited a rapid and long-lasting (5 days) antidepressant effect in the CUMS model, which was consistent with the antidepressant effect of rapastinel.
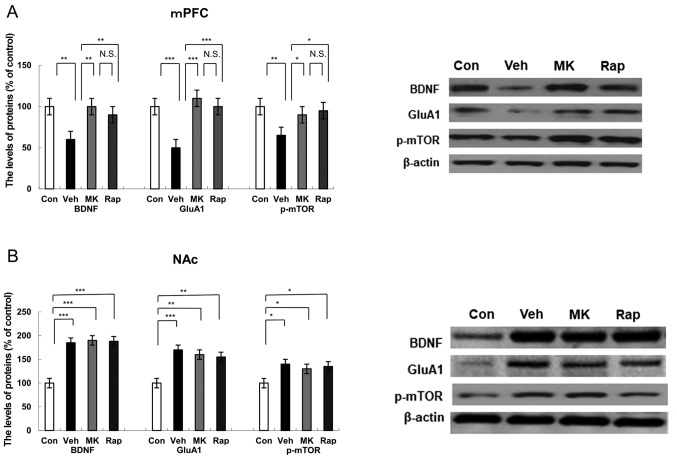
Protein expression of BDNF, GluA1 and p-mTOR in the mPFC brain regions following intraperitoneal administration of MK-801 and rapastinel
Western blot analysis was performed to detect the protein expression of BDNF, synaptogenesis marker GluA1 (14), and p-mTOR in the mPFC 5 days after the administration of a single dose of MK-801 and rapastinel (Fig. 2A). Results indicated that CUMS significantly reduced the protein expression of BDNF (P<0.01), GluA1 (P<0.001) and p-mTOR (P<0.01) compared with the control group in the mPFC (Fig. 2A). Notably, MK-801 and rapastinel significantly attenuated the decreased protein expression of BDNF (P<0.01), GluA1 (P<0.001) and p-mTOR (P<0.05) in the mPFC (Fig. 2A).
Figure 2.
Effects of MK-801 and rapastinel on BDNF, GluA1 and p-mTOR protein expression in the mPFC and NAc. Protein expression of BDNF, GluA1 and p-mTOR in the (A) mPFC and (B) NAc was determined using western blot analysis. Values are expressed as a percentage of those of the control mice and presented as the mean ± standard error of the mean (n=6 or 7). *P<0.05, **P<0.01, ***P<0.001 as indicated. Con, control; Veh, vehicle; MK, MK-801; Rap, rapastinel; BDNF, brain-derived neurotrophic factor; GluA1, Glutamate A1; p-mTOR, phosphorylated mammalian target of rapamycin; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; N.S., not significant.
Protein expression of BDNF, GluA1, p-mTOR in the NAc following intraperitoneal administration of MK-801 and rapastinel
Western blot analysis was used to assess protein expression of BDNF, GluA1 and p-mTOR in the NAc (Fig. 2B). CUMS significantly increased the protein expression of BDNF (P<0.001), GluA1 (P<0.001) and p-mTOR (P<0.05) in the NAc group compared with the control (Fig. 2B). Notably, MK-801 and rapastinel had no significant effect on the increased protein expression of BDNF, GluA1 and p-mTOR compared with the vehicle group (Fig. 2B).
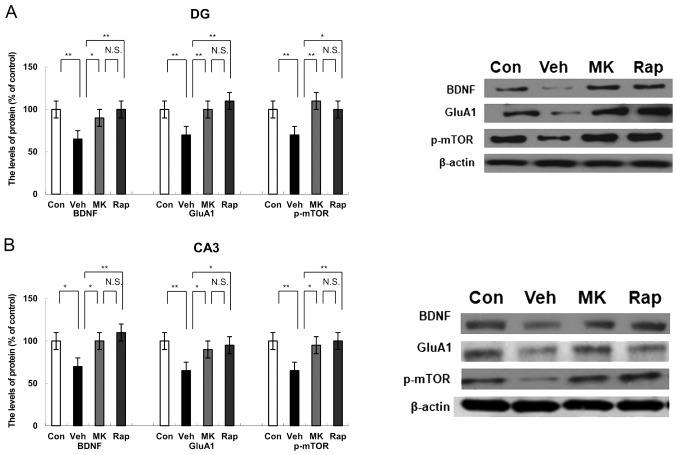
Protein expression of BDNF, GluA1 and p-mTOR in the DG of the hippocampus following intraperitoneal administration of MK-801 and rapastinel
Western blot analysis indicated the protein expression of BDNF, GluA1 and p-mTOR in the DG of the hippocampus. CUMS significantly decreased the protein expression of BDNF, GluA1 and p-mTOR compared with the control group in the DG of the hippocampus (all P<0.01; Fig. 3A). Notably, MK-801 and rapastinel significantly attenuated the decreased expression of BDNF, GluA1 and p-mTOR protein in the DG of the hippocampus (P<0.05 and P<0.01; Fig. 3A).
Figure 3.
Effects of MK-801 and rapastinel on the BDNF, GluA1 and p-mTOR protein expression in the DG and CA3 of the hippocampus regions. (A) Protein expression of BDNF, GluA1 and p-mTOR in the (A) DG and (B) CA3 of the hippocampus regions was determined using western blot analysis. Values were expressed as a percentage of that of control mice and presented as the mean ± standard error of the mean (n=6 or 7). *P<0.05 and **P<0.01, as indicated. Con, control; Veh, vehicle; MK, MK-801; Rap, rapastinel; BDNF, brain-derived neurotrophic factor; GluA1, Glutamate A1; p-mTOR, phosphorylated mammalian target of rapamycin; DG, dentate gyrus.
Protein expression of BDNF, GluA1 and p-mTOR in the CA3 region of hippocampus following intraperitoneal administration of MK-801 and rapastinel
Western blot analysis was performed to measure the protein expression of BDNF, GluA1 and p-mTOR in the CA3 region of the hippocampus. CUMS significantly reduced the protein expression of BDNF (P<0.05), GluA1 (P<0.01) and p-mTOR (P<0.01) compared with the control group within the CA3 of the hippocampus (Fig. 3B). However, MK-801 and rapastinel significantly attenuated the decreased protein expression of BDNF, GluA1 and p-mTOR compared with the vehicle group (P<0.05 and P<0.01; Fig. 3B).
Discussion
The present study was performed to further elucidate the mechanisms responsible for the long-lasting antidepressant effects observed in the CUMS model following single-dose administration of MK-801. In the established CUMS model, MK-801 significantly increased the protein expression of BDNF, GluA1 and p-mTOR in the mPFC, DG and CA3 of the hippocampus, resulting in a sustained antidepressant effect. Notably, the protein expression of BDNF in the mPFC, DG and CA3 of the hippocampus were significantly lower compared with the control mice. Furthermore, the administration of a single dose of MK-801 increased the protein expression level of BDNF in the mPFC, DG and CA3 of the hippocampus compared with the vehicle control in CUMS model mice. The expression of GluA1 and p-mTOR proteins in the CUMS model mice was significantly lower compared with in the control mice; however, MK-801 significantly increased the expression of GluA1 and p-mTOR proteins in the mPFC, DG and CA3 of the hippocampus in the CUMS model mice when measured 5 days following drug administration. Notably, MK-801 evoked the same long-lasting antidepressant response as rapastinel, which is currently being tested in a phase II clinical development program as an adjunctive therapy for major depressive disorder (clinicaltrials.gov identifier NCT01684163). On the basis of these findings, it was suggested that BDNF-TrkB, GluA1 neurotransmitter and p-mTOR signaling in the PFC and hippocampus may serve vital roles in the sustained antidepressant effect of MK-801.
NMDA receptors are predominantly identified at excitatory synapses and are ubiquitously expressed in the central nervous system (20). These receptors have become targets for drug development for the treatment of depression (20,21). Ketamine, an NMDA receptor antagonist, is one of the most appealing antidepressants for treatment-resistant depression (22,23). However, it is typically associated with psychotomimetic side effects. Ketamine is a racemic mixture containing equal parts of S-ketamine and R-ketamine (18). It has been reported that R-ketamine is a potent, long-lasting and safe antidepressant (18). In vivo imaging studies have revealed reduced glutamate levels in the PFC/anterior cortex of patients with depression (24–26). Postmortem data suggested that NMDA receptor protein expression is altered in the PFC of patients with depression (27). Similarly, CP-101,606 (an NR2B subunit-selective NMDAR antagonist) was reported to reduce depression scores (28). As MK-801 is an NMDA receptor noncompetitive antagonist, it is also reasonable to assume that its long-lasting antidepressant-like effects involve an NMDA receptor-mediated process akin. Although MK-801 has been considered to increase locomotor activity in the past (7), no significant effects at doses of 0.1 mg/kg were indicated in the present study, which is consistent with a recent report (9).
Consistent with the result of a previous report (8), the present study indicated that MK-801 had a rapid antidepressant effect (9). To the best of our knowledge, this is the first study to report the long-lasting antidepressant effects of MK-801 in the CUMS model. In the present study, a single-dose intraperitoneal injection of MK-801 produced a long-lasting (5-day) antidepressant effect in the CUMS model to a similar extent as rapastinel. However, the precise mechanisms underlying this effect remain unclear. A previous study revealed that the etiology of depression is associated with the PFC and hippocampus (12). Notably, mice and humans exhibit functional homology in these two regions of the brain (29). A recent study suggested changes in the NAc may be associated with depression and its functional abnormalities may be primarily concentrated in the shell rather than the nucleus (30). Therefore, to understand the antidepressant effect of MK-801, the mPFC, NAc, DG and CA3 of the hippocampus were examined in the present study.
BDNF is associated with the pathophysiology of depression and the BDNF gene may be responsible for susceptibility to depression (15). It has been reported that the expression of BDNF is conspicuously reduced in the PFC and hippocampi of patients with depression, whereas the expression of BDNF is significantly increased following antidepressant treatment (31–33). A previous study suggested that depression is associated with changes in brain neurotransmitters, including dopamine, 5-hydroxytryptamine, glutamate and γ-aminobutyric acid (34). Furthermore, changes in the steady state concentration or imbalance of neurotransmitters may be associated with depression (34). The glutamate system represents a novel target for the treatment of depression, inhibiting the release of neurotransmitters and regulates postsynaptic responses (16). Previous studies have identified that blocking the mTOR signaling pathway may reduce the antidepressant effect of NMDA receptor antagonists and prevent neuronal regeneration in animal depression models (11,20). p-mTOR is the activated form of mTOR. In the present study, a noticeable decrease in the protein expression of BDNF, GluA1 and p-mTOR in the mPFC, DG and CA3 of the hippocampus were indicated, while a significant increase in the expression of BDNF, GluA1 and p-mTOR proteins in the NAc of CUMS mice was determined. These findings suggest that the occurrence and development of depression may be mediated by the BDNF-TrkB, glutamate neurotransmitter and mTOR signaling pathways in these regions. These results are consistent with those reported by Shirayama et al (35). According to a previous report, synaptogenesis and neuronal regeneration serve a role in the prolonged antidepressant effect associated with NMDA receptor antagonists (14,16). The present results suggested that a single dose of MK-801 or rapastinel significantly attenuated the decrease in the expression of BDNF, GluA1 and p-mTOR proteins in the mPFC, DG and CA3 of the hippocampus of the CUMS model. It is likely that the sustained increase in the expression of BDNF, GluA1 and p-mTOR protein in the mPFC, DG and CA3 of the hippocampus is involved in the long-lasting (5-day) effect of MK-801 and rapastinel. However, further detailed studies are required to fully understand the role of synaptogenesis and neuronal regeneration in the long-lasting antidepressant response. The present study also identified that the expression of BDNF, GluA1 and p-mTOR proteins did not significantly change in the NAc following antidepressant treatment with MK-801 or rapastinel, which suggests that MK-801 or rapastinel cannot inhibit p-mTOR signaling pathway or block the release of GluA1 in NAc. However, further research is required.
In conclusion, the present study demonstrated that a single dose of MK-801 produced a sustained antidepressant effect in the established CUMS model of depression, and that MK-801 elicits a rapid and sustained antidepressant effect similar to rapastinel. Furthermore, it is likely that increased synaptogenesis and neuronal regeneration in the mPFC, DG and CA3 of the hippocampus may be involved in the sustained antidepressant response. Therefore, the present findings suggest that the NMDA antagonist MK-801 may be considered as a rapid and long-lasting therapeutic agent in patients with depression, once the safety and efficacy of MK-801 are proven in a clinical setting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 8157051331).
Availability of data and materials
The datasets during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
J-CC conceived and designed the experiments. B-KY performed the experiments. JQ and YN analyzed the data.
Ethics approval and consent to participate
The present study was approved by the Institutional Animal Care and Use Committee of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing interests.
References
- 1.Monteggia LM, Zarate CA., Jr Antidepressant actions of ketamine: From molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci. 2015;16:7796–7801. doi: 10.3390/ijms16047796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaki S, Fukumoto K. Potential of glutamate-based drug discovery for next generation antidepressants. Pharmaceuticals (Basel) 2015;8:590–606. doi: 10.3390/ph8030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, Kasper S. European group for the study of resistant depression (GSRD)-where have we gone so far: Review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012;22:453–468. doi: 10.1016/j.euroneuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B, Ren Q, Ma M, Chen QX, Hashimoto K. Antidepressant effects of (+)-MK-801 and (−)-MK-801 in the social defeat stress model. Int J Neuropsychopharmacol. 2016;19(pii):pyw080. doi: 10.1093/ijnp/pyw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moskal JR, Burgdorf JS, Stanton PK, Kroes RA, Disterhoft JF, Burch RM, Khan MA. The development of rapastinel (Formerly GLYX-13); A rapid acting and long lasting antidepressant. Curr Neuropharmacol. 2017;15:47–56. doi: 10.2174/1570159X14666160321122703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, Liu A, Wang Q, Zhou W. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol. 2014;18(pii):pyu110. doi: 10.1093/ijnp/pyu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, Ma M, Chen QX, Hashimoto K. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016;233:3647–3657. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM GLYX-13 Clinical Study Group, corp-author. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 14.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18:pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohgi Y, Futamura T, Hashimoto K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med. 2015;15:206–221. doi: 10.2174/1566524015666150330143008. [DOI] [PubMed] [Google Scholar]
- 17.Ren Q, Ma M, Ishima T, Morisseau C, Yang J, Wagner KM, Zhang JC, Yang C, Yao W, Dong C, et al. Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proc Natl Acad Sci USA. 2016;113:E1944–E1952. doi: 10.1073/pnas.1601532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: A rapid-acting and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K. Comparison of ketamine, 7,8-dihydroxyflavone and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232:4325–4335. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodge D, Mercier MS. Ketamine and phencyclidine: The good, the bad and the unexpected. Br J Pharmacol. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haj-Mirzaian A, Kordjazy N, Haj-Mirzaian A, Ostadhadi S, Ghasemi M, Amiri S, Faizi M, Dehpour A. Evidence for the involvement of NMDA receptors in the antidepressant-like effect of nicotine in mouse forced swimming and tail suspension tests. Psychopharmacology (Berl) 2015;232:3551–3561. doi: 10.1007/s00213-015-4004-0. [DOI] [PubMed] [Google Scholar]
- 25.Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, Drevets WC, Theodore WH. 5-HT1A receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry. 2007;62:1258–1264. doi: 10.1016/j.biopsych.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 29.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K. BDNF-TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl Psychiatry. 2015;5:e666. doi: 10.1038/tp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol. 2015;18:pyu121. doi: 10.1093/ijnp/pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Yang C, Ren Q, Zhang JC, Chen QX, Shirayama Y, Hashimoto K. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci. 2016;266:765–769. doi: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Neurosci. 2013;263:367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 35.Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol. 2015;25:2449–2458. doi: 10.1016/j.euroneuro.2015.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the present study are available from the corresponding author on reasonable request.