Abstract
Activation of Toll-like receptor 4 (TLR4) and its accessory proteins myeloid differentiation protein 2 (MD-2) can trigger immune and inflammatory activities, and contribute to developing chronic inflammatory diseases. The formation of the TLR4/MD-2 complex after binding to lipopolysaccharide (LPS) leads to the activation of downstream signaling pathway. The present study was designed to reveal the effect of the soluble form of the extracellular TLR4 domain and MD-2 (sTLR4/sMD-2) complex lacking the intracellular and transmembrane domains on various aspects of LPS-induced inflammation in vivo and in vitro. It was demonstrated that the sTLR4/sMD-2 complex inhibited the LPS-induced production of tumor necrosis factor-α, interleukin-8 and C-X-C motif chemokine ligand 1 (CXCL1) in THP-1 cells. In addition, it was revealed that the sTLR4/sMD-2 complex significantly reduced LPS-induced acute lung injury (ALI) with a reduction of total cells and neutrophil count, pro-inflammatory cytokines and chemokine CXCL1 in bronchoalveolar lavage fluid. Moreover, the sTLR4/sMD-2 complex inhibited the number of inflammatory cells in the lung of treated animals. These novel mechanisms emphasized the important role of sTLR4/sMD-2 complex in ALI and suggested sTLR4/sMD-2 complex could provide an anti-inflammatory strategy for treating inflammatory diseases.
Keywords: LPS, sTLR4/sMD-2 complex, THP-1, ALI, cytokine, CXCL1
Introduction
Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria, is involved in various disorders including acute lung injury (ALI) that leads to the accumulation of inflammation by inducing various inflammatory cells activation (1). Toll-like receptor 4 (TLR4) is a member of the Toll-like receptor family, which mediates regulation of endotoxin induced pro-inflammatory cellular responses (2–4).
TLR4 contains an intracellular domain that is involved in the activation of the cellular signaling pathways, a transmembrane region and a leucine-rich extracellular domain (5,6). Upon LPS binding, with the help of myeloid differentiation protein 2 (MD-2), a multimer complex composed of TLR4/MD-2-LPS is formed (7,8), which initiates signaling cascades and results in the activation of pro-inflammatory factors such as NF-κB and the IFN regulatory factors.
A critical step in the response to infection is to activate the innate immune response. LPS plays an importance role in sepsis and septic shock pathogenesis, because more than half of the patients have intermediate or higher levels of endotoxin in a novel endotoxin activity assay (EAA) on the day of ICU admission (9–11). Excessive activation of innate immune responses leads to the pathogenesis of many inflammatory diseases (12), so it is critical for innate immunity to be strictly controlled and activated when necessary. Inhibition of TLR4 signaling has become a hot topic in numerous researches. Eritoran is a competitive inhibitor of LPS and has been expected as a protective agent for endotoxin shock. Another method of down-regulating TLR4 signaling is to produce an inhibitory isoform by alternatively splicing specific genes encoding essential signaling components such as IL-1R-associated kinase 2, TLR3, MD-2 and MyD88 (13–17). For instance, Gray P found the the interaction of MD-2 with TLR4 could be competitively inhibited in vitro by a novel isoform of human MD-2 (18). Kondo Y identified that Gb4 is an endogenous ligand for TLR4/MD-2, which competes with LPS for binding to TLR4/MD-2, suggesting that Gb4 can be a promising drug for the treatment of LPS-induced endotoxin shock induced by Gram-negative bacteria (19). Mitsuzawa H testified soluble forms of extracellular TLR4 domain (sTLR4) and MD-2 (sMD-2) inhibited LPS binding on cell surface and attenuated LPS-induced lung injury in mice (20).
In the present study, we sought to make further efforts to define the anti-inflammatory effects of sTLR4/sMD-2 complex in the LPS-induced inflammation in vitro and in vivo. Our results demonstrated that sTLR4/sMD-2 complex suppressed the expression of inflammatory cytokines and chemokine CXCL1 in LPS-stimulated macrophages and reduced pulmonary inflammation caused by LPS in mice. Taken together, these results demonstrated that sTLR4/sMD-2 complex alleviates ALI through inhibition of pro-inflammatory cytokines and chemokine CXCL1 expression and this mechanism indicated sTLR4/sMD-2 complex serves as an anti-inflammatory agent for treating inflammatory disease.
Materials and methods
Protein expression and purification
The entire preparation process of extracellular TLR4 domain and MD-2, including construction of expression vectors of sTLR4 and MD-2, removal of endotoxin, preparation and purification of sTLR4/sMD2 complex according to the molar ratio of 1:1, was carried out as described previously [21]. The molar ratio of the sTLR4/sMD-2 complex (1:1) in treated mice was the same as that in treated cells.
THP-1 cell culture and treatment
Macrophage-like cell line THP-1 was obtained from China Cell Line Bank (Shanghai, China). The cells were cultured in six-well plates (BD Biosciences, Franklin Lakes, NJ, USA) at a density of 5×106 cells/ml. To induce THP-1 cells to the mature macrophages-like state, cells were cultured in RPMI 1640 containing 10% FBS with 100 ng/ml PMA for 24 h and further incubated in the culture medium without PMA for another 24 h. Then, cells were incubated with medium alone (Con), LPS (1.0 µg/ml), LPS + sTLR4 (LPS 1.0 µg/ml +sTLR4 5.0 µg/ml), LPS + sMD-2 (LPS 1.0 µg/ml + sMD-2 1.25 µg/ml), LPS + sTLR4/sMD-2 (LPS 1.0 µg/ml +sTLR4/sMD-2 6.25 µg/ml). After 24 h, cell supernatant was collected and total RNA was extracted.
Reverse transcription semi-quantitative polymerase chain reaction (PCR)
Total RNAs were extracted from cells using RNAiso Plus reagent according to the manufacturer's instructions. Primers used in our study were listed in Table I. The 25 µl PCR reactions included Premix TaqTM (Ex Taq v.2.0 plus dye; Takara Biotechnology Co., Ltd., Dalian, China), 20 µM primers, cDNA and dd H2O. The PCR reaction was carried out by the manufacturer's instructions. The electrophoresis image of PCR products were analyzed by the AlphaImager gel analysis system (ProteinSimple, San Jose, CA, USA). Each analysis was repeated three times. The semiquantitative value was expressed as the ration of the integrated optical density, as previously reported (21).
Table I.
Primer sequences used for RT-sqPCR.
| Gene | Primers sequence |
|---|---|
| GAPDH | |
| Forward | 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ |
| Reverse | 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ |
| IL-8 | |
| Forward | 5′-GACATACTCCAAACCTTTCCACC-3′ |
| Reverse | 5′-CAACCCTACAACAGACCCACAC-3′ |
| TNF-α | |
| Forward | 5′-GCCCCAATCCCTTATTTACCC-3′ |
| Reverse | 5′-GGCGATTACAGACACAACTCCC-3′ |
| CXCL1 | |
| Forward | 5′-ACGCATTTACTGTCACGGTTC-3′ |
| Reverse | 5′-GTTGTATGGGGCATTGACTTTC-3′ |
RT-sqPCR, reverse transcription semi-quantitative polymerase chain reaction; IL-8, interleukin-8; TNF-α, tumor necrosis factor- α; CXCL1, C-X-C motif chemokine ligand 1.
Murine model of LPS-induced ALI
Health male BALB/c mice with the body weight of 22–28 g were obtained from the Experimental Animal Center of Guangxi Medical University. All of the animals were kept in sterile conditions. Mice were randomly divided into five groups (n=5/group), i.e. phosphate buffered saline-treated group (PBS), LPS-treated group (LPS), sTLR4+LPS-treated group (LPS+sTLR4), sMD-2+LPS-treated group (LPS+sMD-2) and sTLR4/sMD-2+LPS-treated group (LPS+sTLR4/sMD-2). Before surgery, all animals were anaesthetized by i.p. with 60 mg/kg sodium pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After successful endotracheal intubation, LPS (400 µg/kg in 50 µl of PBS) was instilled intratracheally with sTLR4 (650 µg/kg), sMD-2 (175 µg/kg) or sTLR4/sMD-2 (825 µg/kg). Control mice received intratracheal instillation of sterile PBS alone. The severity of lung injury was evaluated by the wet lung-to-body weight ratio, histopathology changes of the lung tissues and cellular profiles in the bronchoalveolar lavage fluid (BALF) after LPS administration for 16 h. Experimental mice were used strictly in accordance with the national animal experimental requirements, and the study was approved by the Ethical Committee for Animal Experiments from the Fourth Affiliated Hospital of Guangxi Medical University (Liuzhou, China).
BALF collection
After LPS infection for 16 h, mice were euthanized by anesthetic overdose with sodium pentobarbital (120 mg/kg, i.p.). BALF was collected by washing the lungs four times with 0.5 ml of ice-cold saline. Then the cells were centrifuged at 400 g for 10 min at 4°C. The supernatant was frozen at −80°C for further analysis. Subsequently, the sedimentary cells were resuspended in PBS and the total counts of cells were counted using a hemocytometer, and the May-Giemsa staining (Thermo Fisher Scientific, Inc., Waltham, MA, USA) method was used for manual classification count. We only counted the number of inflammatory cells, including neutrophils, monocytes, macrophages and lymphocytes for the total cells in BALF. A professional technician performed cell staining and counting manually. At least 300 cells/slides were counted no less than twice and the mean was calculated in each experiment.
Pulmonary index
The lung tissue samples were excised and extraneous tissues were cleared away. Then the lung tissue were weighed and the wet lung-to-body weight ratio was calculated to assess the severity of lung inflammation.
Enzyme-linked immunosorbent assay (ELISA)
To detect the TNF-α, IL-8 and IL-6 protein levels, the cell supernatant and/or BALF of mice were determined by ELISA kits from RayBiotech, Inc., (Norcross, GA, USA). Quantitation of secreted CXCL1 protein levels was analyzed by ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA).
Histopathology
The right inferior lung lobe were fully fixed in 10% neutral buffered formalin for one week, followed by paraffin-embedding in line with the standard procedures. All paraffin-embedded tissues were sectioned to 4 µm thick, and stained with hematoxylin and eosin for examination under Nikon Eclipse E800 microscope (×200). The lung injury score was evaluated by one pathologist in a blinded fashion as previously described (22). The extent of the pathological lesions was scaled from 0 to 3 according to the criteria shown in Table II.
Table II.
Extent of the pathological lesions grade.
| Score | Alveolar septae | Alveolar hemorrhage | Intra-alveolar fibrin | Intra-alveolar infiltrations per field |
|---|---|---|---|---|
| 0 | All are thin and delicate | No hemorrhage | No intra-alveolar fibrin | <5 intra-alveolar cells |
| 1 | Congested alveolar septae in less than 1/3 of the field | Erythrocyte per alveolus in 1 to 5 alveoli | Fibrin strands in less than 1/3 of the field | 5–10 intra-alveolar cells |
| 2 | Congested alveolar septae in 1/3 to 2/3 of the field | At least 5 erythrocyte per alveolus in 5 to 10 alveoli | Fibrin strands in 1/3 to 2/3 of the field | 10–20 intra-alveolar cells |
| 3 | Congested alveolar septae in greater than 2/3 of the field | At least 5 erythrocytes per alveolus in more than 10 alveoli | Fibrin strands in greater than 2/3 of the field | >20 intra-alveolar cells |
Immunohistochemical staining
All paraffin-embedded tissues were cut in 4 µm thick for immunohistochemical analysis. 0.3% hydrogen peroxide in methanol was used to block endogenous peroxidase activity. Then sections were treated with 0.1 M citrate buffer (pH 6.0) as an antigen retrieval system. Anti-MPO antibody was used for immunostaining (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The avidin biotin peroxidase complex method was used to perform immunostaining and the immunohistochemical analyses were performed with the chromogen diaminobenzidine.
Statistical analysis
Data were presented as mean ± SD. The results were statistically evaluated by one-way analysis of variance followed by the LSD post hoc test. All data were analyzed using with SPSS (v.17.0) software. P<0.05 was considered to indicate a statistically significant difference.
Results
sTLR4/sMD-2 complex competes with cell surface TLR4/MD-2 complex in binding LPS
Our laboratory has successfully constructed extracellular TLR4 domain and sMD-2 [21] and we have reported the binding assay of LPS to THP-1 cells in previous paper by Zou et al (21). The fluorescence intensity was significantly decreased in sTLR4/sMD-2 complex treated group instead of sTLR4 or MD-2 alone. These results suggested that recombinant sTLR4/sMD-2 complex competes with TLR4/MD-2 complex on cell surface in binding LPS.
sTLR4/sMD-2 complex inhibies the expression of pro-inflammatory cytokines and chemokines induced by LPS in THP-1 cells
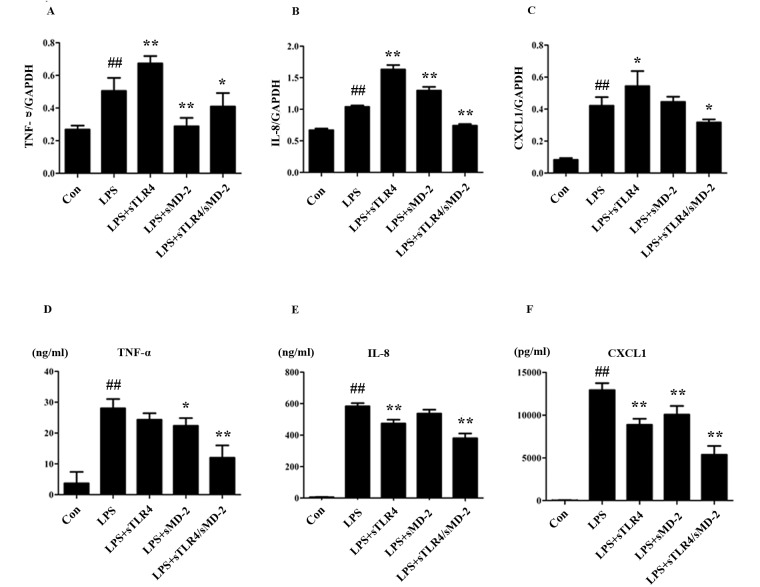
Cytokine microarray analysis of the changes in transcription levels caused by LPS-treated THP-1 cells showed an up-regulation of TNF-α, IL-8, MIP-1α and MIP-1β (23). We examined the expression of TNF-α, IL-8 and CXCL1 in LPS stimulated THP-1 cells after being treated with sTLR4, sMD-2 and sTLR4/sMD-2 complex. After 24 h, there was a significant increase of TNF-α, IL-8 and CXCL1 on mRNA and protein levels in LPS treated group, whereas the mRNA and protein levels of TNF-α, IL-8 and CXCL1 were significantly inhibited in sTLR4/sMD-2 complex treated group (Fig. 1). However, administration of sTLR4 or sMD-2 alone caused inconsistent mRNA expression of TNF-α, IL-8, and CXCL1 (Fig. 1A-C). Furthermore, administration of sTLR4 or sMD-2 alone gave a trend for decreasing the expression of TNF-α, IL-8 and CXCL1 protein induced by LPS (Fig. 1D-F). Of note, the anti-inflammatory effect of sTLR4/sMD-2 complex treatment group was better than that of sTLR4 or sMD-2 alone group. Together, these findings suggested that sTLR4/sMD-2 complex could suppress LPS-induced pro-inflammatory mediators and chemokines production in THP-1 cells.
Figure 1.
sTLR4/sMD-2 complex inhibits the expression of pro-inflammatory cytokines and chemokines induced by LPS in THP-1 cells. THP-1 cells (5×106 cells/ml) were incubated with medium alone (Con), LPS (1.0 µg/ml), LPS + sTLR4 (LPS 1.0 µg/ml + sTLR4 5.0 µg/ml), LPS + sMD-2 (LPS 1.0 µg/ml + sMD-2 1.25 µg/ml), LPS + sTLR4/sMD-2 (LPS 1.0 µg/ml + sTLR4/sMD-2 6.25 µg/ml) for 24 h. Total RNA was extracted, and TNF-α, IL-8 and CXCL1 expression were determined by reverse transcription semi-quantitative polymerase chain reaction. Expression was normalized to GAPDH. (A) The mRNA expression of TNF-α. (B) The mRNA expression of IL-8. (C) The mRNA expression of CXCL1. Culture media were collected, (D) TNF-α, (E) IL-8 and (F) CXCL1 protein expression were measured by ELISA. Values are mean ± SD (n=3). ##P<0.01 vs. the Con group; *P<0.05 and **P<0.01 vs. the LPS group.
sTLR4/sMD-2 complex attenuates the LPS-induced ALI
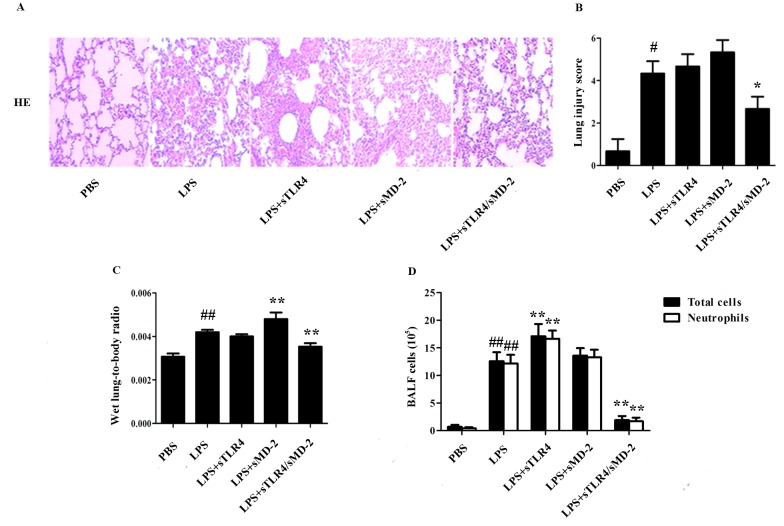
To evaluate the potential role of sTLR4/sMD-2 complex in the pathological changes of lung in LPS-treated ALI mice, lung histological changes were evaluated after administration of LPS with sTLR4, sMD-2 or sTLR4/sMD-2 complex. The results showed marked inflammatory infiltrate, thick alveolar septum and severe interstitial edema in LPS-induced ALI mice. When sTLR4 or sMD-2 was separately treated, no significant decrease in LPS-induced pulmonary inflammation was observed. In contrast, administration of sTLR4/sMD-2 complex effectively reduced the airway inflammation (Fig. 2A). Furthermore, the semiquantitative histopathology score system was used to evaluate the severity of lung injury as shown in Table II (22). We found that administration of sTLR4/sMD-2 complex could significantly reduce lung injury score (Fig. 2B). The pulmonary index was also significantly decreased in sTLR4/sMD-2 complex treated mice. In contrast, lung injury score and pulmonary index ratio in the sTLR4 or sMD-2 groups did not decrease significantly compared to that in the LPS group (Fig. 2B and C).
Figure 2.
sTLR4/sMD-2 complex attenuats the LPS-induced ALI. Mice were anesthetized and treated with PBS, LPS (400 µg/kg), sTLR4 (LPS 400 µg/kg + sTLR4 650 µg/kg), sMD-2 (LPS 400 µg/kg + sMD-2175 µg/kg), sTLR4/MD-2 (LPS 400 µg/kg + sTLR4/sMD-2825 µg/kg) after tracheostomy. After 16 h, the lung tissue and BALF were collected. (A) Representative images of HE stained lung sections from five experimental groups. Original magnification, ×200. Each photograph represents three independent experiments. (B) Lung injury score was determined. (C) The pulmonary index of wet lung-to-body weight ratio. (D) Total cells and neutrophil counts in BALF were detected to evaluate pulmonary inflammation induced by LPS. #P<0.05 and ##P<0.01 vs. the PBS group; *P<0.05 and **P<0.01 vs. the LPS group.
Then we evaluated the potential role of sTLR4/sMD-2 complex in the changes of total cells and neutrophil count in BALF of LPS-treated mice. LPS administration resulted in dramatically increased total inflammatory cell count (P<0.05), most of which were neutrophils (Fig. 2D). Notably, we found that sTLR4/sMD-2 complex could significantly decrease the total inflammatory cell and neutrophil count in BALF, whereas sTLR4 or sMD-2 alone did not have the same effect (Fig. 2D).
sTLR4/sMD-2 complex decreases cytokines concentrations in BALF and MPO-positive cells in lung tissues
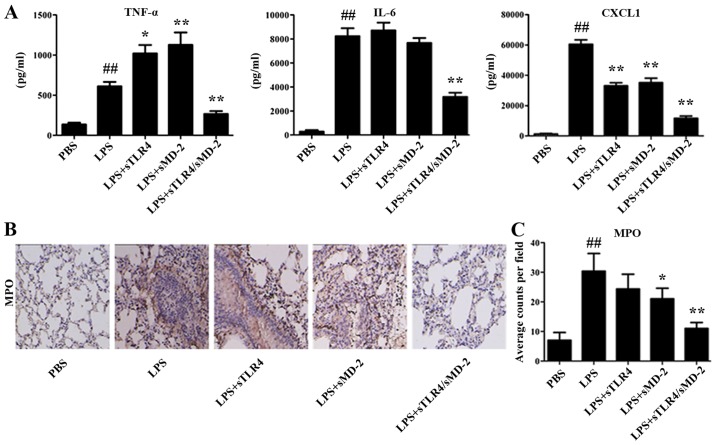
The proinflammatory cytokines TNF-α, IL-6 and chemokine CXCL1 in BALF were quantified using method of ELISA. We found that the increased expression of TNF-α, IL-6 and CXCL1 in BALF cells were significantly suppressed by sTLR4/sMD-2 complex treatment (Fig. 3A). Administration of sTLR4 or sMD-2 alone decreased the expression of CXCL1 in BALF cells, but the effects were worse than that of sTLR4/sMD-2 complex. However, administration of sMD-2 alone significantly increased the expression of TNF-α in BALF cells, which was not consistent with results in THP-1 cells. We believed that sMD-2 can combine with LPS directly and induce homodimerization of the TLR4/MD-2 complex to trigger a proinflammatory signaling cascade when treated with a high concentration (400 µg/kg in 50 µl of PBS) of LPS in vivo. And TNF-α is an early inflammatory factor, so the expression of TNF-α increases first. However, when we used a low concentration (1 µg/ml) of LPS in vitro, no extra LPS was combined with sMD-2, so the addition of sMD-2 have no effects. Immunohistochemical staining also revealed a large amount of inflammatory cells infiltrated in lung tissue. The production of MPO, which is an indicator of neutrophil infiltration, was also detected in lung tissue. As shown in Fig. 3B and C, the number of MPO-positive expression in the LPS group was greater than that in PBS group (P<0.01). We found a significant reduction of MPO-positive cells in the sMD-2 group compared with the LPS group (P<0.05), whereas sTLR4/sMD-2 complex markedly decreased MPO-positive cells (P<0.01). In summary, sTLR4/sMD-2 complex could effectively protect against ALI induced by LPS.
Figure 3.
sTLR4/sMD-2 complex decreased cytokines concentrations in BALF and MPO-positive cells in lung tissues. (A) The pro-inflammatory cytokine TNF-α, IL-6 and chemokine CXCL1 induced by LPS in BALF were determined. (B) Lung sections were stained for MPO. Five sections were analyzed per animal (n=5) and 8~10 high power fields were counted (five mice/group, ×200). (C) Positive staining was quantified by Image-Pro Plus software. Each graph represents one of three independent experiments performed. ##P<0.01 vs. the PBS group; *P<0.05 and **P<0.01 vs. the LPS group.
Discussion
ALI is associated with an acute inflammatory process and is characterized by increased vascular permeability, fibrin deposition, and a large quantity of edema fluid accumulations in alveoli (24). LPS inhalation resulted in significant lung injury. To bind to LPS, the extracellular domain of TLR4 interacts with secreted protein MD-2 (25,26). MD-2 combines with LPS directly at its hydrophobic cavity and this recognition of LPS induces homodimerization of the TLR4/MD-2 complex, which forms 1:1 of the LPS-TLR4/MD-2 complex (27). The formation of the LPS-TLR4/MD-2 complexes can recruit intracellular adaptor protein MyD88 to activate downstream signaling pathways, such as MAPKs and NF-κB (28). Macrophages can release a large number of inflammatory mediators after LPS stimulation (29). Therefore, the inflammatory model of the macrophage induced by LPS can be used to evaluate the anti-inflammatory effects of various reagents. The effects of sTLR4/sMD-2 complex have not been elucidated on THP-1 cells. So we tried to explore the underlying mechanism of the therapeutic effects of sTLR4/sMD-2 complex in ALI.
The main characteristics of acute pulmonary inflammation are protein leakage, production of inflammatory mediators and neutrophil influx (30). Inflammatory mediators TNF-α and IL-6 were reported to play a vital role in LPS-induced ALI (31). In this study, we investigated the effects of sTLR4/sMD-2 complex on the inflammatory factors of LPS-stimulated THP-1 cells. We found that administration of sTLR4/sMD-2 complex significantly inhibited the mRNA and protein level of TNF-α, IL-8 and CXCL1 induced by LPS. These results indicated that sTLR4/sMD-2 complex may be useful for treating inflammation diseases with an overproduction of pro-inflammatory cytokines.
Studies have demonstrated that tissue macrophages synthesize neutrophil chemoattractants CXCL1/CXCL2 in response to LPS, which contributes to recruiting neutrophils into tissues (32). When ALI occurs, the tissue macrophages in the lungs produce CXCL1 to recruit neutrophil cells in the infection site for killing pathogens. In this study, we found that treatment with sTLR4/sMD-2 complex markedly attenuated LPS-stimulated CXCL1 expression in THP-1 cells and BALF. So it is important to inhibit CXCL1 production by sTLR4/sMD-2 complex for treatment of ALI. The levels of MPO, a granule-derived mediator, is increased in BALF samples obtained from mice with ALI (33). Furthermore, our results showed that sTLR4/sMD-2 complex significantly decreased LPS-induced MPO production. To analyze the severity of lung edema, the lung W/D ratio was measured. We found that sTLR4/sMD-2 complex treatment decreased the wet lung-to-body weight ratio, which suggested it suppressed the excess accumulation of fluid in lung tissues. These results showed that sTLR4/sMD-2 complex can act as an anti-inflammatory reagent against LPS-induced ALI.
Early studies showed that sTLR4/sMD-2 complex attenuates LPS induced-ALI (20). In the present study, we found sTLR4/sMD-2 complex inhibited the expression of TNF-α and CXCL1 on the cell surface and in the cell supernatant of THP-1 cells treated with LPS at higher concentration in vitro. Secondly, our results also showed that the administration of sTLR4/sMD-2 complex decreased wet lung-to-body weight ratio and lung injury score, pro-inflammatory cytokines production of IL-6 and CXCL1 in BALF and MPO activity. Moreover, sTLR4/sMD-2 complex significantly alleviated LPS-induced lung histopathological changes, whereas sTLR4 and sMD-2 alone had no effect. Furthermore, sTLR4/sMD-2 complex had the ability to protect against ALI even when treated with high concentrations (400 µg/kg) of LPS. These results are all the new findings of this study, which provides powerful evidence to support sTLR4/sMD-2 complex's role as a potential treatment for preventing ALI induced by LPS. Therapeutic effects of the sTLR4/sMD-2 complex in other biological fields still need to be investigated in the future studies.
Acknowledgements
The authors would like to thank Mr. Hao Wu, Mr. Qi Sun, Mr. Dong Wei, Ms. Min Yi, Mr. Xi Qin, Mr. Siqiong Pan, Mr. Ni Zheng, Mr. Ting Li and Mr. Meiying Lu (The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, Guangxi, China) for their excellent technical assistance. We also thank Mr. Yujie Huang (The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, Guangxi, China) for her work in revising this manuscript.
Funding
This study was supported by grants from Key Laboratory Construction of Tumor Diseases Prevention in Liuzhou, Guangxi (grant no. 2014G020403) and National Natural Science Foundation of China (grant no. 81160269).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
JM and SD contributed to the conception and design of the study. JM and YZ drafted the manuscript. JM, YZ, JC and FQ performed the research. XC and XC performed the data analyses. SD reviewed the manuscript and gave his approval to the submitted and final versions. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were reviewed and approved by the Ethical Committee for Animal Experiments from the Fourth Affiliated Hospital of Guangxi Medical University (Liuzhou, China). All efforts were made to minimize the suffering of the experimental mice.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Ulich TR, Fann MJ, Patterson PH, Williams JH, Samal B, Del Castillo J, Yin S, Guo K, Remick DG. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. Am J Physiol. 1994;267:L442–L446. doi: 10.1152/ajplung.1994.267.4.L442. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Rietschel ET. Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 3.Means TK, Golenbock DT, Fenton MJ. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–232. doi: 10.1016/S1359-6101(00)00006-X. [DOI] [PubMed] [Google Scholar]
- 4.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/S0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med. 2001;29(7 Suppl):S2–S7. doi: 10.1097/00003246-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway CA., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/S0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 7.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 9.Marshall JC. Endotoxin in the pathogenesis of sepsis. Contrib Nephrol. 2010;167:1–13. doi: 10.1159/000315914. [DOI] [PubMed] [Google Scholar]
- 10.Opal SM. Endotoxins and other sepsis triggers. Contrib Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, et al. Diagnostic and prognostic implications of endotoxemia in critical illness: Results of the MEDIC study. J Infect Dis. 2004;190:527–534. doi: 10.1086/422254. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 13.Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: Naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682–6686. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- 14.Ohta S, Bahrun U, Tanaka M, Kimoto M. Identification of a novel isoform of MD-2 that downregulates lipopolysaccharide signaling. Biochem Biophys Res Commun. 2004;323:1103–1108. doi: 10.1016/j.bbrc.2004.08.203. [DOI] [PubMed] [Google Scholar]
- 15.Hardy MP, O'Neill LA. The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J Biol Chem. 2004;279:27699–27708. doi: 10.1074/jbc.M403068200. [DOI] [PubMed] [Google Scholar]
- 16.Leeman JR, Gilmore TD. Alternative splicing in the NF-kappaB signaling pathway. Gene. 2008;423:97–107. doi: 10.1016/j.gene.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 18.Gray P, Michelsen KS, Sirois CM, Lowe E, Shimada K, Crother TR, Chen S, Brikos C, Bulut Y, Latz E, et al. Identification of a novel human MD-2 splice variant that negatively regulates Lipopolysaccharide-induced TLR4 signaling. J Immunol. 2010;184:6359–6366. doi: 10.4049/jimmunol.0903543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo Y, Ikeda K, Tokuda N, Nishitani C, Ohto U, Akashi-Takamura S, Ito Y, Uchikawa M, Kuroki Y, Taguchi R, et al. TLR4-MD-2 complex is negatively regulated by an endogenous ligand, globotetraosylceramide. Proc Natl Acad Sci USA. 2013;110:4714–4719. doi: 10.1073/pnas.1218508110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuzawa H, Nishitani C, Hyakushima N, Shimizu T, Sano H, Matsushima N, Fukase K, Kuroki Y. Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J Immunol. 2006;177:8133–8139. doi: 10.4049/jimmunol.177.11.8133. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y, Qin F, Chen J, Meng J, Wei L, Wu C, Zhang Q, Wei D, Chen X, Wu H, et al. sTLR4/MD-2 complex inhibits colorectal cancer in vitro and in vivo by targeting LPS. Oncotarget. 2016;7:52032–52044. doi: 10.18632/oncotarget.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Z, Li Q, Han Y, Liang Y, Xu Z, Ren T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators Inflamm. 2012;2012:540794. doi: 10.1155/2012/540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison LM, van den Hoogen C, van Haaften WC, Tesh VL. Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides. Infect Immun. 2005;73:403–412. doi: 10.1128/IAI.73.1.403-412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 25.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 27.Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: Higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 29.Lee JK, Sayers BC, Chun KS, Lao HC, Shipley-Phillips JK, Bonner JC, Langenbach R. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Part Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt Y, Kurdowska A, Allen TC. Acute lung injury: A clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 31.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 33.Dooley JL, Abdel-Latif D, St Laurent CD, Puttagunta L, Befus D, Lacy P. Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1091–L1102. doi: 10.1152/ajplung.90471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.