Abstract
Stroke survivors often experience social isolation, which can lead to post-stroke depression (PSD) and post-stroke anxiety (PSA) that can compromise neurogenesis and impede functional recovery following the stroke. The present study aimed to investigate the effects and mechanisms of post-stroke social isolation-mediated PSD and PSA on hippocampal neurogenesis and cognitive function. The effects of the natural antidepressant hyperforin on post-stroke social isolation-mediated PSD and PSA were also investigated. In the present study, a model of PSD and PSA using C57BL/6J male mice was successfully established using middle cerebral artery occlusion combined with post-stroke isolated housing conditions. It was observed that PSD and PSA were more prominent in the isolated mice compared with the pair-housed mice at 14 days post-ischemia (dpi). Mice isolated 3 dpi exhibited decreased transforming growth factor-β (TGF-β) levels and impairment of hippocampal neurogenesis and memory function at 14 dpi. Intracerebroventricular administration of recombinant TGF-β for 7 consecutive days, starting at 7 dpi, restored the reduced hippocampal neurogenesis and memory function induced by social isolation. Furthermore, intranasal administration of hyperforin for 7 consecutive days starting at 7 dpi improved PSD and PSA and promoted hippocampal neurogenesis and memory function in the isolated mice at 14 dpi. The inhibition of TGF-β with a neutralizing antibody prevented the effects of hyperforin. In conclusion, the results revealed a previously uncharacterized role of hyperforin in improving post-stroke social isolation-induced exaggeration of PSD and PSA and, in turn, promoting hippocampal neurogenesis and cognitive function via TGF-β.
Keywords: hippocampus, neurogenesis, post-stroke depression and anxiety, social isolation, transforming growth factor-β
Introduction
Post-stroke depression (PSD) and post-stroke anxiety (PSA) are serious and often interrelated complications of stroke. PSD is the most common psychiatric disorder following ischemic stroke and occurs in ~1/3 of all stroke survivors (1); PSA prevalence is ~20-25% (2). Comorbidity of PSD and PSA was reported to be 12.3% of stroke survivors at 3 months post-stroke (3). PSD is positively correlated with PSA (2), and comorbid depression and anxiety worsen the prognosis and severity of depressive symptoms (4). PSD and PSA can compromise rehabilitation outcomes, lead to poorer cognitive and physical outcomes and can negatively impact patient quality of life following a stroke (2,5), making this a serious and costly public health issue, warranting investigations into antidepressant preventive and curative therapies.
Pre-stroke social isolation gives rise to cognitive impairment of functional recovery in humans and has been associated with an increased risk of PSD and PSA (6-9). An experimental study provided direct evidence that isolating mice immediately following stroke leads to PSD and PSA, which may contribute to decreased general locomotor activity and histological outcomes in the stroke hemisphere (10). Several socially isolated patients are not clinically identified until they seek medical attention following a stroke, and stroke survivors experience and perceive higher levels of social isolation compared with age-matched healthy individuals (11). Therefore, it is essential to investigate treatment strategies for post-stroke isolation-mediated PSD and PSA, subsequent neurogenesis, and neurological and cognitive dysfunction. Translational efforts targeting socio-emotional factors in post-stroke functional recovery may widen the intervention options for social support and may be an interesting addition to current therapeutic strategies for acute stroke. However, the role and underlying mechanisms of post-stroke social isolation in hippocampal neurogenesis and post-stroke memory deficits remain to be fully elucidated.
Pharmacological treatment with antidepressants initiated timely following a stroke can prevent the development of PSD (12-14). It has also been reported that antidepressant pharmacotherapy promotes long-term functional recovery following a stroke, including everyday activities, and cognitive and executive functioning (15-17). Studies have identified hyperforin as the major active compound in St. John’s Wort, a plant used in self-medicating mild to moderate forms of depression (18,19). A previous study reported that 14-day delayed treatment with hyperforin in the recovery phase of a stroke promotes angiogenesis and improves functional recovery following an ischemic stroke (20). Hippocampal neurogenesis is closely associated with cognitive function and mood following a stroke (21). It remains to be elucidated whether delayed 7-day administration of the natural antidepressant hyperforin to post-stroke socially isolated mice during stroke recovery can promote hippocampal neurogenesis and restore memory function via the inhibition of PSD and PSA.
Although there is controversy regarding the level of transforming growth factor-β (TGF-β) in depression, studies have demonstrated that patients with major depression disorder (MDD) exhibit reduced serum levels of TGF-β (22,23) and a decrease in TGF-β network-associated gene transcripts in the choroid plexus (24). A 6-week period of treatment with the antidepressants fluoxetine, venlafaxine or paroxetine increases the levels of TGF-β1 (25). TGF-β members promote the proliferation of neuroepithelial stem cells, neurite outgrowth and synapse formation (26). Whether the inhibition of TGF-β is involved in post-stroke social isolation-mediated PSD and PSA and the attenuation of hippocampal neurogenesis and memory function remains to be elucidated.
The purpose of the present study was to investigate whether delayed 7-day treatment with hyperforin to post-stroke socially isolated mice can inhibit PSD and PSA via TGF-β, enhance neurogenesis in the hippocampal dentate gyrus (DG) and consequently improve impaired memory following ischemic stroke.
Materials and methods
Experimental animals
Adult male C57BL/6J mice (age, 8-10 weeks; weight, 23-25 g) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The animals were maintained at temperature of 23±11°C with 50±10% relative humidity in specific pathogen-free conditions and had access to food and water ad libitum. All procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (publication no. 80-23) revised 1996, and were approved by the Animal Care and Use Committee for experimental animals of Tongji Medical College (Wuhan, China).
Middle cerebral artery occlusion (MCAO) procedure and housing conditions
Focal cerebral ischemia was induced by MCAO as previously reported (27). The mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (8 mg/kg). Under an operating microscope, the right external carotid artery was incised and a 6-0 silicone-coated nylon monofilament with a rounded tip was inserted into the internal carotid artery until it reached the bifurcation of the MCA for occlusion for 60 min, followed by reperfusion. The sham-operated mice underwent an identical surgical procedure without ischemia. A laser-Doppler probe (Periflux system 5000; Perimed AB, Stockholm, Sweden) was placed on the skull (5 mm lateral and 2 mm posterior to the bregma) to monitor the regional cerebral blood flow (rCBF) during MCAO. Mice with <25% rCBF reduction following occlusion and ~80% rCBF increase upon reperfusion were included in further analyses.
During the MCAO, the rectal temperature was maintained at 37±0.5°C. Immediately following surgery and on a daily basis thereafter, the MCAO mice in all housing groups received a subcutaneous injection of normal saline (NS; 1% v/w).
Each male mouse was randomly allocated to housing groups in standard clear plastic cages (27×17×12.5 cm) either individually (socially isolated, SI) or with an ovariectomized female mouse (pair-housed, PH) immediately following ischemia for 14 continuous days.
Experimental groups and drug administration
Experiment 1: The mice were randomly divided into six groups (Fig. 1A): Group 1) Sham operated SI mice (sham + SI; n=12 for behavioral assessment); group 2) sham operated PH mice (sham + PH; n=12 for behavioral assessment); group 3) post-stroke SI mice [MCAO + SI; n=12 for behavioral assessment; n=6 for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis; n=6 for western blotting (WB); and n=6 for immunofluorescence (IF)]; group 4) post-stroke PH mice (MCAO + PH; n=12 for behavioral assessment; n=6 for RT-qPCR; n=6 for WB; and n=6 for IF); group 5) post-stroke SI mice treated with recombinant mouse (r) TGF-β (MCAO + SI + rTGF-β; n=12 for behavioral assessment and n=6 for IF); and group 6) post-stroke SI mice treated with PBS (MCAO + SI + PBS; n=12 for behavioral assessment and n=6 for IF). Immediately following surgery, a cannula was inserted into the left lateral ventricle (0.1 mm posteriorly, 0.9 mm lateral to the midline and 3.1 mm deep from the bregma) in groups 5 and 6 using a stereotaxic instrument (RWD Life Science Co., Ltd., Shenzhen, China) for intracerebroventricular (i.c.v.) injection (28). rTGF-β (2.5 µg/kg; Sino Biological, Inc., Beijing, China) or sterile PBS was administrated i.c.v. into the left lateral ventricle of the SI mice using a sterile 26-G Hamilton microsyringe (80330; Hamilton Company, Reno, NV, USA) every 24 h for 7 continuous days commencing at 7 days post-ischemia (dpi).
Figure 1.
Schematic representation of the animal grouping. Transient focal cerebral ischemia was induced by a filament occlusion of the right middle cerebral artery. (A) Experiment 1: Mice were PH or SI housed for 14 days immediately following stroke. mRNA expression (RT-qPCR), protein expression (IF, WB) and behavioral analyses were performed to examine the effects of post-stroke social isolation on post-stroke depression and anxiety, hippocampal neurogenesis and memory function. Mediated effects of TGF-β on post-stroke social isolation were also investigated. (B) Experiment 2: RT-qPCR, IF, WB and behavioral analyses were performed to evaluate the effects of hyperforin on the treatment of post-stroke social isolation-induced post-stroke depression and anxiety, reduced hippocampal neurogenesis and memory deficits. Effects of TGF-β on hyperforin-treated post-stroke social isolation were also investigated. dpi, days post-ischemia; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; WB, western blotting; IF, immunofluorescence; TGF-β, transforming growth factor-β; anti-TGF-β, TGF-β-neutralizing antibody; rTGF-β, recombinant mouse TGF-β; OVX, ovariectomized female mice; PH, pair-housed male and OVX; SI, socially isolated.
Experiment 2: The mice were randomly divided into five groups (Fig. 1B): Group 1) Post-stroke SI mice treated with hyperforin (MCAO + SI + hyperforin; n=6 for RT-qPCR and n=6 for WB); group 2) post-stroke SI mice treated with NS (MCAO + SI + NS; n=6 for RT-qPCR and n=6 for WB); group 3) post-stroke SI mice treated with NS plus PBS (MCAO + SI + NS + PBS; n=12 for functional assays and n=6 for IF); group 4) post-stroke SI mice treated with hyperforin plus TGF-β-neutralizing antibody (MCAO + SI + hyperforin + anti-TGF-β; n=12 for functional assays and n=6 for IF); and group 5) post-stroke SI mice treated with hyperforin plus PBS (MCAO + SI + hyperforin + PBS; n=12 for functional assays and n=6 for IF). Hyperforin (Cayman Chemical Company, Ann Arbor, MI, USA) was dissolved in ethanol at 20 µg/µl. The stock solution was diluted with NS to 0.5 µg/µl and intranasally administered to alternating nostrils with a 2 min interval between each application, every 24 h for 7 days (starting at 7 dpi). Drops (2 µl) were administered to the surface of each nare, allowing the mice to inhale each drop into the nasal cavity (29). A total of 10 µl treatment was delivered over 5 min. The animals in groups 2 and 3 were treated accordingly using NS. TGF-β-neutralizing antibody (1 µg; Abcam, Cambridge, UK) was dissolved in PBS and i.c.v. injected into the left lateral ventricle 30 min prior to the application of hyperforin.
5-Bromo-2′-deoxyuridine (BrdU) labeling
The mice were injected intraperitoneally twice with S-phase marker BrdU (50 µg/g body weight in NS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with 8 h between injections at 13 dpi. The mice were anesthetized and transcardially perfused 1 day following the final injection to analyze BrdU-labeling of the recently proliferated cells by immunofluorescence staining and observed under a fluorescence microscope (BX51; Olympus Corporation, Tokyo, Japan).
Behavioral assessments Forced swim task (FST)
Depression-type behavior in the mice (n = 12/group) was assessed using the FST at 14 dpi. The mice were placed into an opaque cylinder tank (diameter, 24 cm; depth, 53 cm), containing 30 cm3 of 25°C water and the duration of immobility was scored over 6 min. Immobility was assessed during the final 4 min as previously described (10). Quantification of floating, vs. swim time was analyzed using Observer software (version 5; Exeter Software, Setauket, NY, USA).
Sucrose consumption test (SCT)
To examine depressive-type behavior, the SCT was performed at 14 dpi. Two identical 10-ml vials were placed on a custom-made wire cage top. At 3 days prior to MCAO, the individually-housed mice were provided with two 10-ml vials of water for 12 h and then were returned to their original housing condition and provided with their normal drinking water and cage tops. After 12 h, the mice were provided with two different 10-ml vials of 3% sucrose for 12 h. Following the completion of habituation, all mice were returned to their original housing condition and provided with their normal drinking water and cage tops. The mice were deprived of water for 12 h prior to the test and were then given access to 3% sucrose solution over 12 h. The volume of 3% sucrose consumed was recorded.
Open field test (OFT)
Anxiety-type behavior in the mice (n = 12/group) was assessed using the OFT at 14 dpi. The mice were placed in an open field apparatus (40×40×37.5 cm) and allowed to explore for 20 min. Locomotor activity was quantified using a photobeam activity system (FlexField; San Diego Instruments, San Diego, CA, USA). The relative level of activity occurring in the periphery, vs. center squares was recorded.
Y-maze task
Spatial reference memory was assessed using the continuous variant of the Y-maze spontaneous alternation procedure. The Y-maze task was used with three identical open arms (50×16×32 cm). The mice were placed in the center of the maze and allowed to explore the three maze arms freely for 8 min. The mice made correct alternations when they sequentially visited the three arms without repeated entry into a previously visited arm. The total arm entries during the session were counted. Spontaneous alternation was calculated as follows: Spontaneous alternation (%) = [alternations/(total arm entries - 2)] × 100.
Novel object recognition task (NORT)
To assess recognition memory, the mice were placed in a chamber (35×25×35 cm) made of gray plastic for 30 min 2 days prior to the NORT. During the training sessions, two identical objects were placed symmetrically in the center of the chamber and the mouse was allowed to explore for 10 min. The time spent exploring each object was recorded. During the retention tests, the mice were placed in the chamber, in which one of the familiar objects was replaced with a novel object, and the mice were allowed to explore freely for 5 min. The mice were considered to be exploring an object when they faced the object at a distance of ≤1 cm. A discrimination index was determined as the ratio of the time spent exploring any one object (training session) or the novel object (retention session) over the total time spent exploring both objects.
Step-through passive avoidance task
To assess the contextual memory impairment, a step-through passive avoidance apparatus containing light (14×10×25 cm) and dark (25×25×25 cm) compartments equipped with an electric grid floor was used. The mice were allowed to adjust to the apparatus the day preceding the trials. For the training trials, a mouse was placed in the light compartment. When the mouse crossed to the dark compartment with all four paws, the door closed and the mouse was punished by intermittent electric shocks (2 sec; intensity, 0.8 mA). Following 30 sec, the mouse was removed from the apparatus. The retention tests were conducted 24 h following the training session (without electric shock) and observed for ≤300 sec.
Western blotting
Brain tissues of the ischemic hemisphere was lysed in RIPA lysis buffer containing protease and phosphatase inhibitors (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). An equal quantity of protein (30-50 µg/lane) was resolved on a 10% SDS-PAGE gel (Beyotime Institute of Biotechnology, Shanghai, China) and blotted onto a PVDF membrane (Millipore; Merck KGaA) using an electrophoresis apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% non-fat milk/TBST to reduce nonspecific binding and subsequently incubated with specific rabbit anti-TGF-β monoclonal antibody (Abcam; cat. no. ab64715) at 1:1,000 dilution and anti-β-actin (1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-10731) overnight at 4°C. Following extensive washing with TBST, the membranes were incubated with an appropriate peroxidase-conjugated secondary antibody (1:3,000; Wuhan Sanying Biotechnology, Wuhan, China; cat. no. LP1001b) for 2 h at room temperature. Following three washes with TBST, chemiluminescent signals were visualized using electrochemiluminescence western blotting detection reagents (Millipore; Merck KGaA) and bands were captured using an UVP gel documentation system (UVP, LLC, Phoenix, AZ, USA). The band intensity was quantified using ImageJ software version 1.41 (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining
The mice were anesthetized and transcardially perfused with NS and 4% paraformaldehyde in PBS (pH 7.4). Following blocking in normal goat serum (Sigma-Aldrich; Merck KGaA) to reduce nonspecific binding, paraffin-embedded brain sections (thickness, 4-µm) were incubated overnight at 4°C with diluted monoclonal primary antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA) as follows: Rabbit Ki67 (1:100; cat. no. 9129), rabbit phosphorylated-histone H3 (PH3; 1:50; cat. no. 9706), mouse BrdU (1:100; cat. no. 5292), rabbit doublecortin (DCX; 1:50; cat. no. 14802) and rabbit NeuN (1:200; cat. no. 12943). Following washing with PBS, the slides were incubated with DyLight 549-conjugated goat anti-rabbit (cat. no. A23320) or DyLight 488-conjugated goat anti-mouse secondary antibodies (cat. no. A23210) at a 1:200 dilution (Abbkine Scientific Co., Ltd., Wuhan, China) at 37°C for 2 h. The antibodies were diluted in 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in PBS. Nuclei were stained with DAPI and slides were observed using a fluorescence microscope (BX51; Olympus Corporation). In the hippocampal DG, positive cells (Ki67+, PH3+ and BrdU+/DCX+) were counted in the granule cell layer of the dorsal DG of hippocampi (bregma from -2.3 to -4.5 mm).
RT-qPCR measurements
Total RNA from the post-ischemic hemisphere was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s protocol. cDNA was obtained using Taqman reverse transcriptase (Applied Biosystems; Thermo Fisher Scientific, Inc.). TGF-β and β-actin cDNA were amplified using Power SYBR-Green (Applied Biosystems; Thermo Fisher Scientific, Inc.). Two-step qPCR was performed (95°C for 15 sec, 60°C for 60 sec for 40 cycles) with specific primers for TGF-β (forward, 5′-GTGTGGAGCAACATGTGGAACTCTA-3′ and reverse, 5′-TTGGTTCAGCCACTGCCGTA-3′) and β-actin (forward, 5′-AAGGCCAACCGTGAAAAGAT-3′ and reverse, 5′-GTGGTACGACCAGAGGCATAC-3′). The relative quantitation value is expressed as 2−ΔΔCq, where ΔCq is the difference between the mean ΔCq value of duplicate measurements of the sample and β-actin control (30).
Statistical analysis
Data from the behavioral assessments (FST, SCT and OFT), RT-qPCR analysis and western blotting were analyzed by two-way analysis of variance (ANOVA) with surgery and housing condition as between subject factors. Other data were analyzed using one-way ANOVA to compare several groups, followed by Fisher’s Protected Least Significant Difference test for pairwise comparison of groups. The results are presented as the mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
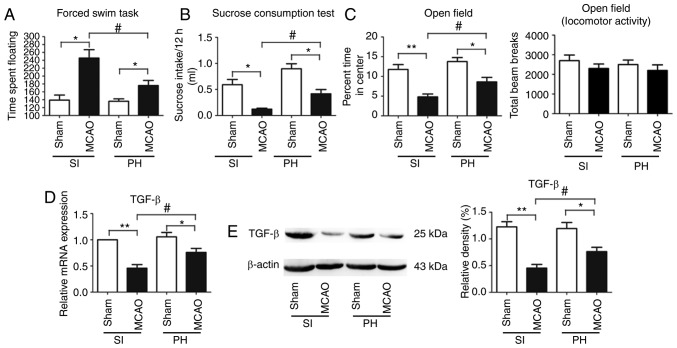
Post-stroke isolation leads to PSD and PSA
Depression-type behavior was evaluated using FST and SCT. The floating time in the FST was significantly increased (P<0.05; Fig. 2A) and consumption of sucrose was significantly decreased (P<0.05; Fig. 2B) in the post-stroke SI mice compared with the sham-operated SI mice at 14 dpi. Furthermore, compared with the sham-operated PH mice at 14 dpi, floating times in the post-stroke PH mice were significantly increased (P<0.05; Fig. 2A) and sucrose consumption was significantly decreased (P<0.05; Fig. 2B). The post-stroke PH mice exhibited a significant reduction in floating times (P<0.05; Fig. 2A) and a significant increase in sucrose consumption (P<0.05; Fig. 2B) compared with the post-stroke SI mice at 14 dpi.
Figure 2.
Post-stroke social isolation leads to PSD and PSA accompanied by reduced expression of TGF-β in ischemic hippocampi. Effects of post-stroke social isolation on depression-type behavior, determined via (A) forced swimming task and (B) sucrose consumption, and (C) anxiety-type behavior were evaluated at 14 dpi (n=12). TGF-β levels in the ischemic hippocampi in each group were measured by (D) reverse transcription-quantitative polymerase chain reaction and (E) western blot analyses at 14 dpi (n=6). Data are presented as the mean ± standard error of the mean and were analyzed by two-way analysis of variance followed by Tukey’s test. *P<0.05; **P<0.01; #P<0.05. MCAO, middle cerebral artery occlusion; SI, socially isolated; PH, pair-housed; PSD, post-stroke depression; PSA, post-stroke anxiety; TGF-β, transforming growth factor-β; dpi, days post-ischemia.
Anxiety-type behavior was measured by the OFT. The post-stroke SI mice spent significantly less time in the center of the open field chamber than the sham-operated SI mice at 14 dpi (P<0.01; Fig. 2C). The percentage of time spent in the center of the open field chamber was further significantly reduced in the post-stroke PH mice compared with the sham-operated PH mice at 14 dpi (P<0.05; Fig. 2C). Post-stroke PH had a significant effect on OFT; the post-stroke PH mice spent a significantly increased duration in the center of the open field chamber compared with the post-stroke SI mice (P<0.05; Fig. 2C). No significant difference was observed in locomotor activity or exploration among the four groups in the OFT (P>0.05; Fig. 2C).
Post-stroke isolation decreases the expression of TGF-β in the ischemic hippocampus during stroke recovery
The mRNA and protein expression levels of TGF-β in the ischemic hippocampi were significantly decreased in the post-stroke SI mice compared with those in the post-stroke PH mice at 14 dpi (P<0.05; Fig. 2D and E).
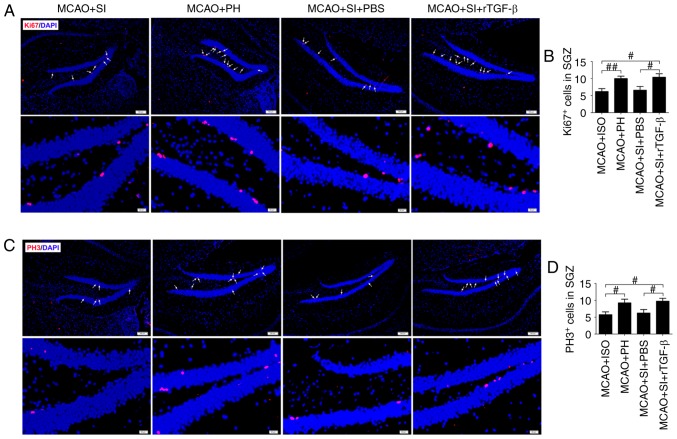
rTGF-β treatment restores the reduced hippocampal neurogenesis induced by social isolation during stroke recovery
The numbers of general proliferation marker Ki67+ and M-phase-specific marker PH3+ cells in the ischemic hippocampi were significantly decreased in the post-stroke SI mice compared with those in the post-stroke PH mice at 14 dpi (P<0.01 and P<0.05, respectively; Fig. 3A-D). The administration of rTGF-β to the post-stroke SI mice significantly increased the numbers of Ki67+ and PH3+ cells in the ischemic hippocampus compared with those in the post-stroke SI mice treated with PBS at 14 dpi (P<0.05; Fig. 3A-D). The post-stroke SI mice treated with rTGF-β exhibited similar numbers of Ki67+ and PH3+ cells in the ischemic hippocampi as the post-stroke PH mice (P>0.05; Fig. 3A-D).
Figure 3.
Post-stroke social isolation inhibits hippocampal neurogenesis. (A) Representative fluorescence images and (B) quantitative determination of Ki67-labeled cells; and (C) fluorescence images and (D) quantitative determination of PH3-labeled cells in the ischemic hippocampus at 14 days post-ischemia. Nuclei were stained with DAPI; scale bar=100 µm. White arrows indicate Ki67- or PH3-positive cells. Data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance followed by Fisher’s Protected Least Significant Difference test; n=6. #P<0.05 and ##P<0.01. MCAO, middle cerebral artery occlusion; rTGF-β, recombinant mouse transforming growth factor-β; SGZ, subdentate gyrus zone; SI, socially isolated; PH, pair-housed; PH3, phosphor-histone H3.
Cell proliferation was assessed at 13 dpi following treatment of the mice with BrdU to label S-phase cells; on 14 dpi, the dividing cells were analyzed. It was observed that the post-stroke SI mice exhibited a significantly decreased number of BrdU+ cells in the ischemic hippocampus compared with the post-stroke PH mice (P<0.01; Fig. 4A and B). The post-stroke SI mice administered with PBS had significantly fewer BrdU+ cells in the ischemic hippocampus compared with the post-stroke SI mice administered with rTGF-β (P<0.05), which themselves had a similar number of BrdU+ cells in the ischemic hippocampus as the post-stroke PH mice (P>0.05; Fig. 4A and B).
Figure 4.
Post-stroke social isolation inhibits neuronal differentiation in the ischemic hippocampus. (A) Representative images of double-immunostained DCX+/BrdU+ cells in the ischemic hippocampus at 14 days post-ischemia of each group; scale bar=50 µm. (B) Quantitative determination of BrdU+ and DCX+/BrdU+ cells in the ischemic hippocampus of each group (n=6). White arrows indicate DCX/BrdU double positive cells. Data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance followed by Fisher’s Protected Least Significant Difference test. #P<0.05 and ##P<0.01. MCAO, middle cerebral artery occlusion; rTGF-β, recombinant mouse transforming growth factor-β; SGZ, subdentate gyrus zone; SI, socially isolated; PH, pair-housed; BrdU, 5-bromo-2′-deoxyuridine; DCX, doublecortin.
DCX, a neuron-specific microtubule-associated protein, expresses specifically in neuroblasts and functions as a marker for neuronal precursors and neurogenesis. Quantitative determination of BrdU/DCX double-labeled cells was performed to assess adult hippocampal neurogenesis. Significantly lower numbers of BrdU+/DCX+ cells in the ischemic hippocampus were observed in the post-stroke SI mice compared with the post-stroke PH mice at 14 dpi (P<0.05; Fig. 4A and B). Furthermore, significantly lower numbers of BrdU+/DCX+ cells were observed in the ischemic hippocampus of the PBS-treated post-stroke SI mice compared with the rTGF-β-treated post-stroke SI mice (P<0.05), which themselves exhibited a similar number of BrdU+/DCX+ cells in the ischemic hippo-campus as the post-stroke PH mice (P>0.05; Fig. 4A and B).
rTGF-β improves the social isolation-induced exaggeration of impaired memory-associated behaviors following a stroke
The percentage of correct alternations in the Y-maze was significantly lower in the post-stroke SI mice compared with the post-stroke PH mice at 14 dpi (P<0.05; Fig. 5A); no significant change in the number of total arm entries was observed (P>0.05; Fig. 5B). The post-stroke SI mice treated with rTGF-β exhibited a significant increase in the percentage of correct alternations compared with the PBS-treated post-stroke SI mice (P<0.05; Fig. 5A); no significant change in the number of total arm entries was observed (P>0.05; Fig. 5B).
Figure 5.
Post-stroke social isolation impairs memory-associated behaviors during stroke recovery following ischemic stroke. (A) Spontaneous alternation rate in the Y-maze task was measured at 14 dpi (n=12). (B) Number of total arm entries in the Y-maze task among for groups at 14 dpi (n=12). (C) Time spent exploring the familiar objects in all groups during the training session at 14 dpi (n=12). (D) Time spent exploring the familiar and novel objects in all groups during the test session at 14 dpi (n=12). (E) Step-through latency times without shocks in all groups at 14 dpi (n=12). (F) Step-through latency times at 24 h following shocks during the retention test at 14 dpi (n=12). Data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance followed by Fisher’s Protected Least Significant Difference test. #P<0.05 and ##P<0.01. MCAO, middle cerebral artery occlusion; rTGF-β, recombinant mouse transforming growth factor-β; SI, socially isolated; PH, pair-housed; dpi, days post-ischemia.
In the NORT, the mice spent a comparable duration exploring each object, yielding a discrimination index of ~50% in all groups during the training session (Fig. 5C). During the retention trial performed 1 h following training, the post-stroke PH mice spent a significantly longer duration exploring the novel object compared with the familiar object (P<0.01; Fig. 5D), whereas the post-stroke SI mice explored the objects equally (P>0.05; Fig. 5D). The post-stroke SI mice treated with rTGF-β exhibited a significant preference for the novel object compared with the PBS-treated post-stroke SI mice (P<0.05; Fig. 5D).
In the passive-avoidance task, no significant differences were observed in step-through latency times without shocks among groups (P>0.05; Fig. 5E). Post hoc analysis of the animals assessed following training revealed that the post-stroke PH mice exhibited significant higher step-through latency times 24 h following shocks compared with the post-stroke SI mice at 14 dpi (P<0.05; Fig. 5F). Treatment with rTGF-β significantly restored the reduced step-through latency times in the post-stroke SI mice during the retention test (P<0.05; Fig. 5F).
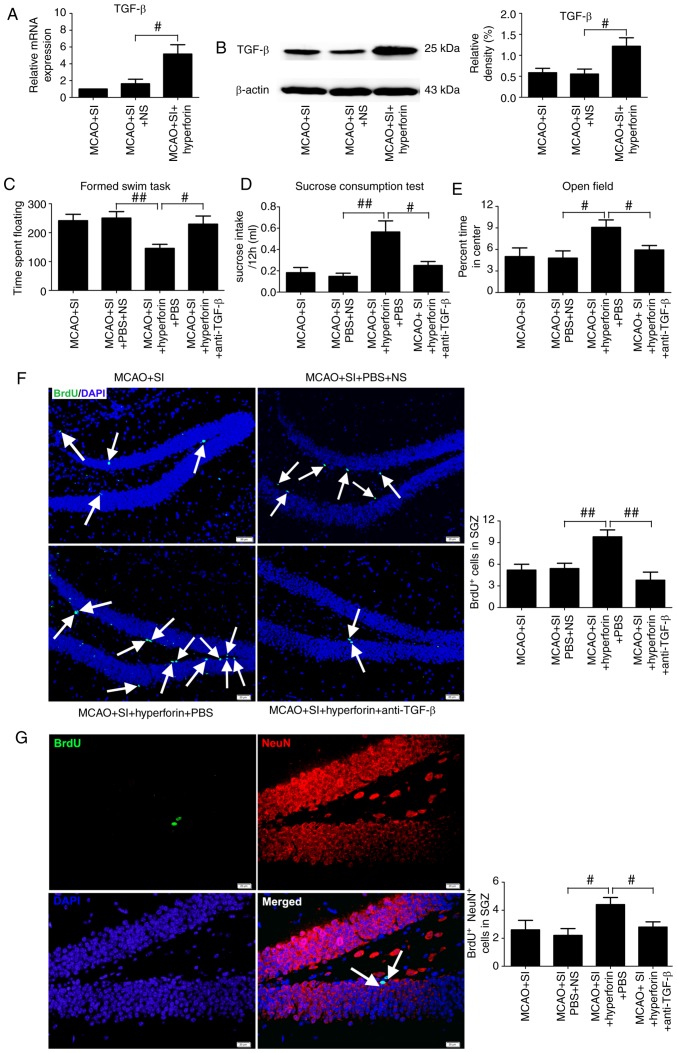
Hyperforin improves the social isolation-induced exaggeration of PSD and PSA via TGF-β
Intranasal administration of hyperforin to the post-stroke SI mice significantly increased the mRNA and protein levels of TGF-β in the ischemic hippocampi compared with those in the NS-treated post-stroke SI mice at 14 dpi (P<0.05; Fig. 6A and B).
Figure 6.
Hyperforin treatment inhibits PSD and PSA, and restores reduced hippocampal neurogenesis induced by social isolation. TGF-β levels in ischemic hippocampi from each group were measured by (A) reverse transcription-quantitative polymerase chain reaction and (B) western blot analyses at 14 dpi (n=6). Effects of hyperforin in depression-type behavior, determined via (C) forced swimming task and (D) sucrose consumption, and (E) anxiety-type behavior were assessed at 14 dpi (n=12). Quantitative determination of (F) BrdU-labeled cells (scale bar=50 µm) and (G) BrdU/NeuN-double-labeled cells (scale bar=20 µm) in the ischemic hippocampus from each group (n=6). White arrows in (F) indicate BrdU-positive cells. White arrows in (G) indicate BrdU/NeuN double positive cells. Data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance followed by Fisher’s Protected Least Significant Difference test. #P<0.05 and ##P<0.01. anti-TGF-β, transforming growth factor-β-neutralizing antibody; MCAO, middle cerebral artery occlusion; SGZ, subdentate gyrus zone; SI, socially isolated; dpi, days post-ischemia; BrdU, 5-bromo-2′-deoxyuridine.
The floating times in the FST were significantly decreased (P<0.01; Fig. 6C) and sucrose consumption was significantly increased (P<0.01; Fig. 6D) in the hyperforin-treated post-stroke SI mice compared with the NS-treated post-stroke SI mice at 14 dpi. Application of a TGF-β-neutralizing antibody significantly reversed the hyperforin-mediated decrease in floating times (P<0.05; Fig. 6C) and increase in sucrose consumption (P<0.05; Fig. 6D) in the post-stroke SI mice.
The percentage of time spent in the center of the open field chamber was significantly higher in the hyperforin-treated post-stroke SI mice compared with that in the NS-treated post-stroke SI mice at 14 dpi (P<0.05; Fig. 6E). TGF-β-neutralizing antibodies reversed the hyperforin-mediated increase in the time spent in the center of the open field chamber in the post-stroke SI mice (P<0.05; Fig. 6E).
Hyperforin promotes decreased hippocampal neurogenesis in social isolation following stroke via TGF-β
Intranasal administration of hyperforin to the post-stroke SI mice led to a significant increase in the number of BrdU+ and BrdU+/NeuN+ cells in ischemic hippocampi compared with that in the NS-treated post-stroke SI mice at 14 dpi (P<0.01 and P<0.05, respectively; Fig. 6F and G). TGF-β-neutralizing antibody treatment reversed the hyperforin-mediated increase in BrdU+ and BrdU+/NeuN+ cells in the ischemic hippocampi of post-stroke SI mice at 14 dpi (P<0.01 and P<0.05, respectively; Fig. 6F and G).
Hyperforin improves impaired memory-associated behaviors in social isolation following a stroke via TGF-β
The percentage of correct alternations in the Y-maze was significantly higher in the hyperforin-treated post-stroke SI mice compared with the NS-treated post-stroke SI mice at 14 dpi (P<0.05; Fig. 7A) and no significant change in the number of total arm entries was observed (P>0.05; Fig. 7B). TGF-β-neutralizing antibody treatment significantly reversed the hyperforin-mediated increase in the percentage of correct alternations in the post-stroke SI mice at 14 dpi (P<0.05; Fig. 7A), without significant changes to the number of total arm entries (P>0.05; Fig. 7B).
Figure 7.
Hyperforin treatment restores the impaired memory function induced by social isolation. (A) Spontaneous alternation rate in the Y-maze task measured at 14 dpi (n=12). (B) Number of total arm entries in the Y-maze task for all groups at 14 dpi (n=12). (C) Time spent exploring familiar objects in all groups during the training session at 14 dpi (n=12). (D) Time spent exploring familiar and novel objects in all groups in the test session at 14 dpi (n=12). (E) Step-through latency times without shock for all groups at 14 dpi (n=12). (F) Step-through latency times at 24 h following shock during the retention test at 14 dpi (n=12). Data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance followed by Fisher’s Protected Least Significant Difference test. #P<0.05 and ##P<0.01. anti-TGF-β, transforming growth factor-β-neutralizing antibody; MCAO, middle cerebral artery occlusion; SI, socially isolated; dpi, days post-ischemia.
During the training session in the NORT, mice spent a comparable time exploring the same objects in all groups (P>0.05; Fig. 7C). During the retention trial performed 1 h following training, the hyperforin-treated post-stroke SI mice spent significantly more time exploring the novel object compared with the familiar object (P<0.05; Fig. 7D) and the NS-treated post-stroke SI mice explored the objects equally (P>0.05; Fig. 7D). The hyperforin-treated post-stroke SI mice treated with TGF-β-neutralizing antibody exhibited significantly impaired discrimination between familiar and novel objects at 14 dpi (P<0.05; Fig. 7D).
In the passive-avoidance task, no significant differences in step-through latency times without shock were observed among groups (P>0.05; Fig. 7E). Post hoc analysis following training revealed that hyperforin treatment significantly increased step-through latency times compared with those in the NS-treated post-stroke SI group at 14 dpi (P<0.01; Fig. 7F). TGF-β-neutralizing antibody treatment significantly reversed this hyperforin-mediated increase in step-through latency times (P<0.05; Fig. 7F).
Discussion
The results of the present study indicated that daily nasal administration of hyperforin during stroke recovery ameliorates the post-stroke isolation-mediated exaggeration of reduced hippocampal neurogenesis and impaired memory-associated behaviors following ischemic stroke. Several critical observations were made: i) Post-stroke social isolation-mediated PSD and PSA may reduce hippocampal neurogenesis and impaired memory function via TGF-β; ii) nasal administration of hyperforin improved PSD and PSA via TGF-β in post-stroke SI mice; iii) reduced hippocampal neurogenesis and impaired memory function in post-stroke SI mice was restored following hyperforin treatment via TGF-β.
Increasing evidence has demonstrated that social interaction is the most recognized mental and psychological factor influencing post-stroke mortality rate and functional recovery (31-33). Social interaction exerts prominent beneficial effects on infarct volume and functional recovery in both genders (34). Stroke survivors are at increased risk of experiencing social isolation, often due to mobility limitations. Considering the fact that many socially isolated patients are not clinically identified until they seek medical attention following a stroke, assessing the effects of post-stroke social isolation compared with pre-stroke social isolation on clinical outcomes is critical to developing therapies targeting socio-emotional factors in neural regeneration and functional recovery. Pre-stroke social isolation increases the volume of infarcts following ischemic stroke (34), and previous studies have demonstrated that infarct volume during the acute phase of a stroke significantly influences neurogenesis during stroke recovery. Infarct size is positively associated with neurogenesis following ischemic stroke (35). To avoid the effects of infarct volume on post-stroke neurogenesis, social isolation was implemented immediately following a stroke rather than pre-stroke. In the present study, a PSD and PSA model was successfully established by MCAO using male C57BL/6 mice combined with post-stroke isolated-housing initiated immediately following ischemia. The data obtained are consistent with findings from a previous study demonstrating that mice isolated immediately following a stroke exhibited PSD and PSA (10). Of note, no significant differences in PSD or PSA were observed between the sham groups in the SI or PH mice.
In the adult central nervous system (CNS), cell proliferation occurs throughout adulthood in two main neurogenic niches that contain neural stem cells: The subventricular zone of the lateral cerebral ventricles and the DG subgranular zone. These neurogenic regions harbor adult neural progenitor cells (36). Hippocampal neurogenesis is closely associated with cognitive function and mood following a stroke, and impaired hippocampal neurogenesis is accompanied by poor learning and memory (21). Evidence supports that PSD and PSA are associated with reduced neurogenesis following ischemic stroke (37,38). In addition, clinical studies have implicated the detrimental effect of PSD on the recovery of function and cognitive abilities (39,40). The present study demonstrated for the first time, to the best of our knowledge that post-stroke isolation inhibited hippocampal neurogenesis leading to memory deficits. These findings suggest that induced PSD and PSA may have important effects on hippocampal neurogenesis and memory function in post-stroke isolation.
Increasing evidence indicates that members of the TGF-β family, in addition to exerting well-established neurotrophic and neuroprotective effects in the CNS, are important in neuropsychiatric diseases, including anxiety, depression and other neuropsychiatric disorders (26). TGF-β network-associated gene transcripts in the choroid plexus and levels of TGF-β in the serum are markedly decreased in patients with MDD (22-24). In the present study, it was demonstrated that SI mice exhibited decreased TGF-β levels in their hippocampus during the stroke recovery phase. rTGF-β administration restored the reduced hippocampal neurogenesis and impaired memory function in the post-stroke SI mice. These observations suggested that the social isolation-induced attenuation of TGF-β following a stroke may be critical in reducing neurogenesis in the hippocampi and memory function, and inducing anxiety and depression.
Hyperforin, the main active ingredient of St John’s wort extract, exhibits antidepressant properties (18,19). A previous study demonstrated that, in addition to its neuroprotective role in ischemic injury in the acute phase following an ischemic stroke (41), hyperforin increases angiogenesis and improves functional recovery (20). In the present study, it was observed for the first time, to the best of our knowledge, that the nasal administration of hyperforin inhibited PSD and PSA in SI mice via TGF-β. Furthermore, during stroke recovery, these mice exhibited increased hippocampal neurogenesis and memory function, which was mediated by TGF-β. Taken together, these results demonstrated the important modulatory effects of TGF-β on the hyperforin-mediated inhibition of PSD and PSA, and its promoting effects on hippocampal neurogenesis and memory function in SI mice. Intranasal administration of hyperforin was selected in the present study due to its noninvasive, rapid onset, safe and effective characteristics (42,43). The treatment of CNS disorders is often restricted due to drug transport across the blood-brain barrier (BBB) and subsequently to the brain. The BBB provides a major obstacle in the delivery of drugs to the CNS, with ~100% of macromolecular drugs and >98% of small molecular drugs failing to cross and enter the brain at pharmacologically significant levels (44). Other than essential nutrients, only small lipid-soluble molecules with molecular weights <500 Da can effectively be transported across the BBB and reach efficacious concentrations in the brain (45). Although invasive approaches for delivering therapeutics to the brain, including i.c.v., have been applied to bypass the BBB, these approaches are not clinically practical due to their inconvenience, questionable safety and high cost. Non-invasive intranasal delivery has been demonstrated to circumvent the BBB and allows the direct and rapid delivery of large and/or charged therapeutics to the CNS (43,46). Non-invasive intranasal administration as an alternative to invasive delivery methods represents the most promising and novel method for delivering therapeutics directly to the brain (43). Therefore, the beneficial effects of hyperforin administration via a nasal route may be considered in developing therapeutic approaches for post-stroke social isolation-mediated PSD and PSA with reduced hippocampal neurogenesis and memory deficits.
In conclusion, the presented data demonstrate that post-stroke social isolation-mediated PSD and PSA may reduce hippocampal neurogenesis and impair memory function via TGF-β. The intranasal delivery of hyperforin during stroke recovery activated TGF-β, which may inhibit PSD and PSA and improve reduced hippocampal neurogenesis and memory deficits in SI mice. Therefore, hyperforin treatment via intranasal routes may be useful in treating post-stroke social isolation-mediated decreased memory function.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81601156 and 81571286).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
SY and JZ contributed to the experimental design. YZ, PY and HL established and performed the stroke model, functional assays and western blotting. HY performed immunocytochemistry and RT-qPCR analysis. JZ handled the imaging tools. SY and JZ analyzed and interpreted the data. JZ prepared the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23) revised 1996, and were approved by the Animal Care and Use Committee of experimental animals of Tongji Medical College (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hackett ML, Pickles K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 2.Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: A systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8:545–559. doi: 10.1111/j.1747-4949.2012.00906.x. [DOI] [PubMed] [Google Scholar]
- 3.Barker-Collo SL. Depression and anxiety 3 months post stroke: Prevalence and correlates. Arch Clin Neuropsychol. 2007;22:519–531. doi: 10.1016/j.acn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Morrison V, Pollard B, Johnston M, MacWalter R. Anxiety and depression 3 years following stroke: Demographic, clinical, and psychological predictors. J Psychosom Res. 2005;59:209–213. doi: 10.1016/j.jpsychores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br J Psychiatry. 2013;202:14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 6.Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- 7.Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: A prospective epidemiological study. J Am Geriatr Soc. 2004;52:774–778. doi: 10.1111/j.1532-5415.2004.52217.x. [DOI] [PubMed] [Google Scholar]
- 8.Ouimet MA, Primeau F, Cole MG. Psychosocial risk factors in poststroke depression: A systematic review. Can J Psychiatry. 2001;46:819–828. doi: 10.1177/070674370104600905. [DOI] [PubMed] [Google Scholar]
- 9.Meng C, Zhang JC, Shi RL, Zhang SH, Yuan SY. Inhibition of interleukin-6 abolishes the promoting effects of pair housing on post-stroke neurogenesis. Neuroscience. 2015;307:160–170. doi: 10.1016/j.neuroscience.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe LM, Doran SJ, Mwilambwe-Tshilobo L, Conti LH, Venna VR, McCullough LD. Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice. Behav Brain Res. 2014;260:162–170. doi: 10.1016/j.bbr.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebrahim S, Barer D, Nouri F. Use of the nottingham health profile with patients after a stroke. J Epidemiol Community Health. 1986;40:166–169. doi: 10.1136/jech.40.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen A, Lunde M, Poulsen DL, Sorensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44:216–221. doi: 10.1176/appi.psy.44.3.216. [DOI] [PubMed] [Google Scholar]
- 13.Niedermaier N, Bohrer E, Schulte K, Schlattmann P, Heuser I. Prevention and treatment of poststroke depression with mirtazapine in patients with acute stroke. J Clin Psychiatry. 2004;65:1619–1623. doi: 10.4088/JCP.v65n1206. [DOI] [PubMed] [Google Scholar]
- 14.Robinson RG, Jorge RE, Moser DJ, Acion L, Solodkin A, Small SL, Fonzetti P, Hegel M, Arndt S. Escitalopram and problem-solving therapy for prevention of poststroke depression: A randomized controlled trial. JAMA. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narushima K, Paradiso S, Moser DJ, Jorge R, Robinson RG. Effect of antidepressant therapy on executive function after stroke. Br J Psychiatry. 2007;190:260–265. doi: 10.1192/bjp.bp.106.025064. [DOI] [PubMed] [Google Scholar]
- 16.Acler M, Robol E, Fiaschi A, Manganotti P. A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol. 2009;256:1152–1158. doi: 10.1007/s00415-009-5093-7. [DOI] [PubMed] [Google Scholar]
- 17.Jorge RE, Acion L, Moser D, Adams HJ, Jr, Robinson RG. Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry. 2010;67:187–196. doi: 10.1001/archgenpsychiatry.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cervo L, Rozio M, Ekalle-Soppo CB, Guiso G, Morazzoni P, Caccia S. Role of hyperforin in the antidepressant-like activity of Hypericum perforatum extracts. Psychopharmacology (Berl) 2002;164:423–428. doi: 10.1007/s00213-002-1229-5. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Filippov V, Lishko P, Maximyuk O, Noldner M, Krishtal O. Hyperforin attenuates various ionic conductance mechanisms in the isolated hippocampal neurons of rat. Life Sci. 1999;65:2395–2405. doi: 10.1016/S0024-3205(99)00506-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Yao C, Chen J, Zhang Y, Yuan S, Lin Y. Hyperforin promotes post-stroke functional recovery through interleukin (IL)-17A-mediated angiogenesis. Brain Res. 2016;1646:504–513. doi: 10.1016/j.brainres.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Shetty AK. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: Can early neural stem cell grafting intervention provide protection. Epilepsy Behav. 2014;38:117–124. doi: 10.1016/j.yebeh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musil R, Schwarz MJ, Riedel M, Dehning S, Cerovecki A, Spellmann I, Arolt V, Muller N. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression-no influence of celecoxib treatment. J Affect Disord. 2011;134:217–225. doi: 10.1016/j.jad.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 23.Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: Effect of sertraline therapy. Clin Dev Immunol. 2007;2007;76396 doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner CA, Thompson RC, Bunney WE, Schatzberg AF, Barchas JD, Myers RM, Akil H, Watson SJ. Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci. 2014;8:238. doi: 10.3389/fnhum.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KM, Kim YK. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6:1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Krieglstein K, Zheng F, Unsicker K, Alzheimer C. More than being protective: Functional roles for TGF-β/activin signaling pathways at central synapses. Trends Neurosci. 2011;34:421–429. doi: 10.1016/j.tins.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Mao X, Zhou T, Cheng X, Lin Y. IL-17A contributes to brain ischemia reperfusion injury through calpain-TRPC6 pathway in mice. Neuroscience. 2014;274:419–428. doi: 10.1016/j.neuroscience.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Shan Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain Behav Immun. 2011;25:1658–1667. doi: 10.1016/j.bbi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Ma M, Ma Y, Yi X, Guo R, Zhu W, Fan X, Xu G, Frey WH, II, Liu X. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Hinojosa R, Haun J, Hinojosa MS, Rittman M. Social isolation poststroke: Relationship between race/ethnicity, depression, and functional independence. Top Stroke Rehabil. 2011;18:79–86. doi: 10.1310/tsr1801-79. [DOI] [PubMed] [Google Scholar]
- 32.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris PL, Robinson RG, Raphael B. Prevalence and course of depressive disorders in hospitalized stroke patients. Int J Psychiatry Med. 1990;20:349–364. doi: 10.2190/N8VU-6LWU-FLJN-XQKV. [DOI] [PubMed] [Google Scholar]
- 34.Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, Conti LH, McCullough LD. NF-κB contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol. 2012;124:425–438. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moraga A, Pradillo JM, Cuartero MI, Hernandez-Jimenez M, Oses M, Moro MA, Lizasoain I. Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia. FASEB J. 2014;28:4710–4718. doi: 10.1096/fj.14-252452. [DOI] [PubMed] [Google Scholar]
- 36.Gross CG. Neurogenesis in the adult brain: Death of adogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 37.Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Hippocampal neurogenesis and behavioural studies on adult ischemic rat response to chronic mild stress. Behav Brain Res. 2008;189:9–16. doi: 10.1016/j.bbr.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: Mechanisms, translation and therapy. J Cell Mol Med. 2012;16:1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong YJ, Kim WC, Kim YS, Choi KW, Son SY, Jeong YG. The relationship between rehabilitation and changes in depression in stroke patients. J Phys Ther Sci. 2014;26:1263–1266. doi: 10.1589/jpts.26.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mpembi MN, Miezi SM, Nzuzi TM, Massamba VK, Henrard S, De Partz MP, Peeters A, Macq J, Dubois V, Constant E. Clinical profile of post-cerebrovascular depression: Descriptive cross-sectional study in the rehabilitation center for people with disabilities of kinshasa (DR Congo) Pan Afr Med J. 2014;17:109. doi: 10.11604/pamj.2014.17.109.3296. In French. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, Zhang JC, Fu J, Chen F, Wang J, Wu ZL, Yuan SY. Hyperforin attenuates brain damage induced by transient middle cerebral artery occlusion (MCAO) in rats via inhibition of TRPC6 channels degradation. J Cereb Blood Flow Metab. 2013;33:253–262. doi: 10.1038/jcbfm.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman CD, Frey WH, II, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30:2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18. doi: 10.1016/S0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 44.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 45.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 46.Dhuria SV, Hanson LR, Frey WH., II Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.