Abstract
MicroRNA (miR)-214 has been demonstrated to suppress gluconeogenesis by targeting activating transcription factor 4 (ATF4), which regulates gluconeogenesis by affecting the transcriptional activity of forkhead box protein O1 (FoxO1). Our previous study revealed that the upregulation of maternally expressed gene 3 (MEG3), a long noncoding RNA, enhanced hepatic insulin resistance via increased FoxO1 expression. The present study aimed to explore whether miR-214 and ATF4 were involved in the MEG3-mediated increase of FoxO1 expression. MEG3, miR-214 and ATF4 expression were examined by reverse transcription quantitative polymerase chain reaction and western blot analysis. The interaction among MEG3, miR-214 and ATF4 was analysed using the luciferase reporter assay. MEG3-targeting small interference RNAs were injected into high-fat diet (HFD)-fed mice to verify the role of MEG3 in hepatic insulin resistance in vivo. MEG-3 and ATF4 were demonstrated to be upregulated and miR-214 was indicated to be downregulated in the livers of HFD-fed and ob/ob mice. In mouse primary hepatocytes, palmitate time-dependently increased MEG3 and ATF4 but decreased miR-214 expression levels. Furthermore, MEG3 served as a competing endogenous RNA (ceRNA) for miR-214 to facilitate ATF4 expression, while miR-214 inhibition and ATF4 overexpression reversed the MEG3 knockdown-mediated decrease in the expression of FoxO1 and FoxO1-downstream targets phosphoenolpyruvate carboxykinase and glucose-6-phosphatase catalytic subunit. In HFD-fed mice, MEG3 knockdown substantially improved impaired glucose and insulin tolerance, while down-regulating HFD-induced ATF4 expression and upregulating HFD-suppressed miR-214 expression. In conclusion, MEG3 promoted hepatic insulin resistance by serving as a ceRNA of miR-214 to facilitate ATF4 expression. These data provide insight into the molecular mechanism of MEG3 involvement in the development of type 2 diabetes mellitus.
Keywords: maternally expressed gene 3, microRNA-214, activating transcription factor 4, hepatic insulin resistance, competing endogenous RNA
Introduction
Type 2 diabetes mellitus (T2DM) accounts for ~90% of all DM cases (1), a global epidemic posing a serious health burden to society (2). Insulin resistance is the primary cause of T2DM, and has therefore become the primary target of T2DM treatment (3,4). It is well known that the liver is the primary glycogen storage site and serves a central role in glucose homeostasis (5,6). Once glucose homeostasis is disrupted, additional insulin is secreted to control the elevated glucose levels, further promoting insulin resistance (7). However, the cellular and molecular mechanisms responsible for hepatic insulin resistance are not yet known.
Long noncoding RNAs (lncRNAs) are commonly defined as non-protein-coding RNA transcripts measuring >200 nucleotides (8). Previous studies have indicated that lncRNAs are involved in the regulation of hepatic glucose and lipid metabolism, with dysregulated lncRNA expression potentially associated with T2DM pathogenesis (9,10). One particular lncRNA, maternally expressed gene 3 (MEG3), is an imprinted gene belonging to the imprinted delta like non-canonical notch ligand 1 (DLK1)-MEG3 locus located on human chromosome 14q32.3 (11). In mice, MEG3 is referred to as gene trap locus 2 and is located on distal chromosome 12 (11). MEG3 has been implicated as a putative tumour suppressor due to the loss of MEG3 expression in a range of cancer types, and its ability to inhibit tumour cell proliferation (11). Our previous study demonstrated that MEG3 overexpression significantly enhanced hepatic insulin resistance via increased forkhead box protein O1 (FoxO1) expression (5), which is a key transcription factor in regulating hepatic gluconeogenesis (12). However, the mechanisms by which MEG3 increases FoxO1 expression remain unclear.
lncRNAs may serve as competing endogenous RNAs (ceRNAs) that either segregate or competitively bind microRNAs (miRNAs) to regulate target mRNA expression (13). miRNAs are a class of small RNA molecules measuring 18-24 nucleotides that regulate gene expression post-transcriptionally via mRNA destabilization or translational repression (14). Fan et al (15) revealed that miR-214 is a direct target of MEG3. An additional study demonstrated that miR-214 suppressed gluconeogenesis by targeting activating transcription factor 4 (ATF4), a potential coactivator of FoxO1 in the regulation of gluconeogenesis (16). Li et al (17) stated that ATF4 deficient (ATF4−/−) mice exhibited decreased levels of fasting blood glucose compared with the wild-type (ATF4+/+) mice and that ATF4 served a role in high- carbohydrate diet-induced insulin resistance.
Increased FoxO1 activity resulted in hyperglycaemia- associated insulin resistance by promoting the transcription of two key gluconeogenic enzymes, namely glucose-6-phosphatase catalytic subunit (G6pc) and phosphoenolpyruvate carboxykinase (Pepck) (18,19). G6pc and Pepck are rate-limiting enzymes that are highly upregulated during fasting, but suppressed in the fed state and by insulin (5). Based on these aforementioned studies, we hypothesized that MEG3 serves as a ceRNA of miR-214 to facilitate ATF4 expression, resulting in the promotion of FoxO1 expression and its downstream gluconeogenic enzymes G6pc and Pepck, thereby increasing gluconeogenesis and promoting insulin resistance. To address this, the present study evaluated the expression of MEG3, miR-214 and ATF4 in ob/ob and high-fat diet (HFD)-fed mice using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. Leptin-deficient ob/ob mice are overweight, develop insulin resistance, and serve as a model for T2DM. Furthermore, their interactions were examined using the luciferase reporter assay and their effects on insulin resistance in T2DM were also investigated. The results of the present study demonstrated that MEG3 promoted hepatic insulin resistance by serving as a ceRNA of miR-214 to facilitate ATF4 expression.
Materials and methods
Animals and treatment
Male C57BL/6 (3-5-week-old) leptin-deficient ob/ob (T2DM model), and control mice (8 weeks) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All animals were housed under controlled temperatures (25±1°C) and humidity (50%), with 12 h light-dark cycles and ad libitum access to food and water. All animal experiments were approved by the Ethics Committees of the First Affiliated Hospital of University of Science and Technology of China (Hefei, China). All experimental procedures were performed in strict accordance with the Institutional Animal Care and Use Committees of Anhui Provincial Hospital and the First Affiliated Hospital of University of Science and Technology of China.
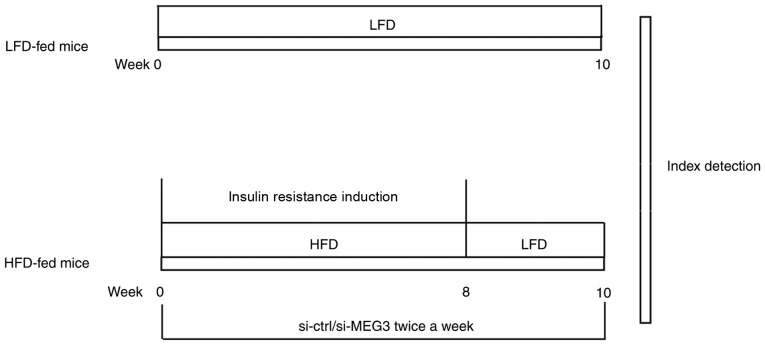
To induce insulin resistance, four-week-old male C57BL/6 mice were fed an HFD (containing 45 kcal% fat; cat. no., D12451; Research Diets; New Brunswick, NJ, USA) or low-fat diet (LFD; containing 10 kcal% fat) for 8 weeks. Next, liver tissues were isolated and examined for the expression of MEG3, miR-214, and ATF4 using RT-qPCR and western blot analysis. HFD-fed male C57BL/6 mice also received an 800 µl injection of the small interfering (si)-MEG3 fragment (si-MEG), described subsequently, in PBS (100 µg/ml) via the tail vein twice a week for 10 weeks. At week 10, glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed. A summary of the experimental model is presented in Fig. 1.
Figure 1.
Summary of the experimental animal model. Mice in the HFD group were fed HFD for 8 weeks from weeks 0-8 to induce insulin resistance. Mice in the HFD group also received an injection of the si-MEG3 fragment via the tail vein twice a week for 10 weeks from weeks 0-10. In parallel experiments, mice in the LFD group were fed LFD for 10 weeks from weeks 0-10. At week 10, GTTs and ITTs were performed. The relative expression of MEG3, miR-214, and ATF4 in mouse liver tissues was evaluated by reverse transcription-quantitative polymerase chain reaction. ATF4 expression in mouse liver tissues was measured by western blotting. LFD, low-fat diet; HFD, high-fat diet; si, small interfering RNA; ctrl, control; MEG3, maternally expressed gene 3; GTTs, glucose tolerance tests; ITTs, insulin tolerance tests.
GTTs and ITTs
For GTTs, mice received an intraperitoneal (IP) injection of D-glucose (2 g/kg) following overnight fasting, as described previously (20). For ITTs, mice received an IP injection of 0.75 units/kg insulin, and insulin levels were measured via a radioimmunoassay (RIA) using an ultrasensitive rat insulin RIA kit (100% cross-reactivity with mouse insulin; Linco; EMD Millipore, Billerica, MA, USA) following 4 h of fasting. Blood glucose levels were measured using a Bayer Glucometer Elite monitor (Bayer AG, Leverkusen, Germany).
Plasmid construction and cell transfection
To overexpress MEG3 or ATF4, the full-length MEG3 or ATF4 cDNA fragments (20 ng; Shanghai GenePharma Co., Ltd., Shanghai, China) were cloned into the pcDNA 3.1 plasmid (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), generating pcDNA3.1-MEG3 or pcDNA3.1-ATF4 plasmids. An empty pcDNA3.1 vector was used as the control. Primary hepatocytes were transfected with these constructs using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), following the manufacturer’s protocol. To knock down MEG3 expression, small interfering RNA si-MEG3 (5′-AUU GGA GGU GAG GAA GGA AAG CAG C-3′) was designed and synthesized by Shanghai GenePharma Co., Ltd. A scramble siRNA (5′-UUC UCC GAA CGU GUC ACG UTT-3′) was used as the negative control (si-Ctrl). Primary hepatocytes were transfected with 50 nM siRNAs using Lipofectamine® 2000, according to the manufacturer’s protocol.
The miRNA double-stranded mimics for miR-214 or miR-214 inhibitors were purchased from Shanghai GenePharma Co., Ltd. When cells grew to 80-90% confluence, miR-214 mimics (sequence: 5′-ACA GCA GGC ACA GAC AGG CAG U-3′) were transfected into primary mouse hepatocytes using Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in 12-well plates at a dose of 0.5 ng/well.
Following transfection for 48 h, primary hepatocytes were harvested for RT-qPCR analysis to examine the knockdown or overexpression efficiency.
Isolation and culture of mouse primary hepatocytes
Hepatocytes were isolated from male C57BL/6 mice using the two-step collagenase perfusion method, as previously described (21). Isolated hepatocytes were cultured for 5 days in medium 199 (Gibco; Thermo Fisher Scientific, Inc.), as previously described (22). Prior to all experiments, hepatocytes were serum starved for 5 h. Cells were stimulated with either 0.5 mM palmitate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or transfected with the DNA plasmids.
RNA extraction and RT-qPCR
RT-qPCR was used to detect the relative expression of MEG3, miR-214, ATF4, FoxO1, G6pc and Pepck in mouse liver tissues or primary mouse hepatocytes, as described in our previous study (5). In brief, total RNA was extracted from liver tissues or primary hepa-tocytes using TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. Total RNA was then reverse transcribed using the iScript kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and qPCR reactions were performed on the CFX96™ real-time PCR detection system (Bio-Rad Laboratories, Inc.) using Power SYBR-Green RT-qPCR reagents. PCR assays were performed using a TaqMan Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers used were as follows: MEG3 forward (F), 5′-CTG CCC ATC TAC ACC TCA CG-3′; reverse (R), 5′-CTC TCC GCC GTC TGC GCT AGG GGC T-3′; miR-214 F, 5′-GCA CAG CAG GCA CAG ACA-3′; miR-214 R, 5′-CAG AGC AGG GTC AGC GGT A-3′; ATF4 F, 5′-CCT GAA CAG CGA AGT GTT GG-3′; ATF4 R, 5′-TGG AGA ACC CAT GAG GTT TCA A-3′; FoxO1 F, 5′-AAG AGG CTC ACC CTG TCG C-3′; FoxO1 R, 5′-GCA TCC ACC AAG AAC TTT TCC-3′; G6pc F, 5′-GTG CAG CTG AAC GTC TGT CTG TC-3′; G6pc R, 5′-TCC GGA GGC TGG CAT TGT A-3′; Pepck F, 5′-CTT CTC TGC CAA GGT CAT CC-3′; Pepck R, 5′-TTT TGG GGA TGG GCA C-3′; GAPDH F, 5′-ACC ACA GTC CAT GCC ATC AC-3′; GAPDH R, 5′-TCC ACC ACC CTG TTG CTG TA-3′; 18S F, 5′-AGG GTT CGA TTC CGG AGA GG-3′; 18S R, 5′-CAA CTT TAA TAT ACG CTA T TG G-3′. The thermocycling conditions used were as follows: Initial denaturation at 95°C for 3 min; followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec, and a final extension of 72°C for 5 min. The relative expression levels of miR-214 and MEG-3 were normalized to 18S, while ATF4, FoxO1, G6pc and Pepck were normalized to GAPDH expression. The 2−ΔΔCq method was used to detect mRNA expression levels (23).
Western blot analysis
Western blot analysis was used to detect ATF4, FoxO1, G6pc and Pepck protein expression in mouse liver tissues or primary mouse hepatocytes. Separated liver tissues or primary hepatocytes were lysed in ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), supplemented with protease inhibitors, for 60 min to extract total liver or cell proteins. Protein concentrations were quantified using a Bio-Rad protein assay with a Bio-Rad Protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following centrifugation at 12,000 x g for 5 min at 4°C, an equal amount (20 µg/lane) of protein from each lysate was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc.). The PVDF membranes were then blocked with 5% fat-free milk for 1 h at room temperature and sequentially incubated at 4°C overnight with the corresponding primary antibodies against ATF4 (1:1,000; cat. no. ab23760; Abcam, Cambridge, MA, USA), FoxO1 (1:1,000; cat. no. ab39670; Abcam), Pepck (1:200; cat. no. sc-377027; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and G6pc (1:1,000; cat. no. TA334651; OriGene Technologies, Inc., Rockville, MD, USA). Subsequent to washing three times with TBS + 0.1% Tween, the membranes were then incubated at room temperature with appropriate horseradish peroxidase-coupled secondary antibody (1:1,000; cat. no. NA931-1ML; GE Healthcare, Chicago, IL, USA) for 1 h. Blots were developed using an enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.) and band intensity was quantified with Quantity One software (4.62 version; Bio-Rad Laboratories, Inc.). β-actin served as a loading control.
Luciferase activity assay
The luciferase activity assay was performed as previously described (13), with minor alterations. Fragments of MEG3 and the 3′-untranslated region (UTR) of ATF4, containing the predicted wild-type (Wt) or mutated (Mut) binding sites of miR-214, were amplified from mouse genomic DNA by PCR using DreamTaq DNA Polymerase (Thermo Fisher Scientific, Inc.). The primers were as follows: ATF4-Wt 3′UTR construct primer F, 5′-CCA GAA TTC GTA GTC AGG TGC TTT GTG-3′; ATF4-Wt 3′UTR construct primer R, 5′-CCA CTC GAG AAC ATC ACT TTC ATG GA-3′; ATF4-Mut primer F, 5′-AGT CTT GTG TTG CTG TGT TTG CTG TAA TAA ATT ATT TTG TAG T-3′; ATF4-Mut primer R, 5′-ACT ACA AAA TAA TTT ATT ACA GCA AAC ACA GCA ACA CAA GAC T-3′; MEG3 construct primer F, 5′-CCA GAA TTC AGG ATG GCA AAG GAT GAA-3′; MEG3 construct primer R, 5′-CCA CTC GAG TTT AGG GAG GTG TCG GTG-3′; MEG3 Mut primer F, 5′-CTT CTT GAA AGG CCT GTC TAC ACT TGC TGT CTT CCT TCC TCA CCT CCA ATT TCC TC-3′; and MEG3 Mut primer R, 5′-GAG GAA ATT GGA GGT GAG GAA GGA AGA CAG CAA GTG TAG ACA GGC CTT TCA AGA AG-3′. The thermocycling conditions used were as follows: Initial denaturation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 1 min, and a final extension at 72°C for 5 min. The PCR products were then cloned into a pMIR-REPORT lucif-erase reporter vector (Ambion; Thermo Fisher Scientific, Inc.). The constructs were termed MEG3-Wt, MEG3-Mut, ATF4-Wt and ATF4-Mut constructs. The corresponding mutants were generated using a QuikChange Site Directed Mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA). For the luciferase reporter assay, 293 cells were co-transfected with 200 ng luciferase reporter vector constructs, 25 ng pRL-TK (expressing Renilla luciferase as the internal control), and 20 µM miR-214 mimic or miR-negative control (NC), using Lipofectamine® 2000. A Dual-Luciferase Reporter assay kit (Promega Corporation, Madison, WI, USA) was applied to analyse luciferase activity at 24 h post-transfection.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package standard version 16.0 (SPSS, Inc., Chicago, IL, USA). The data are presented as the mean ± standard deviation from three independent experiments. Unpaired Student’s t-tests were used to analyse differences between two groups. A one-way analysis of variance followed by Tukey’s post hoc test was used to analyse differences among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
MEG3, miR-214 and ATF4 expression in HFD-fed and ob/ob mice
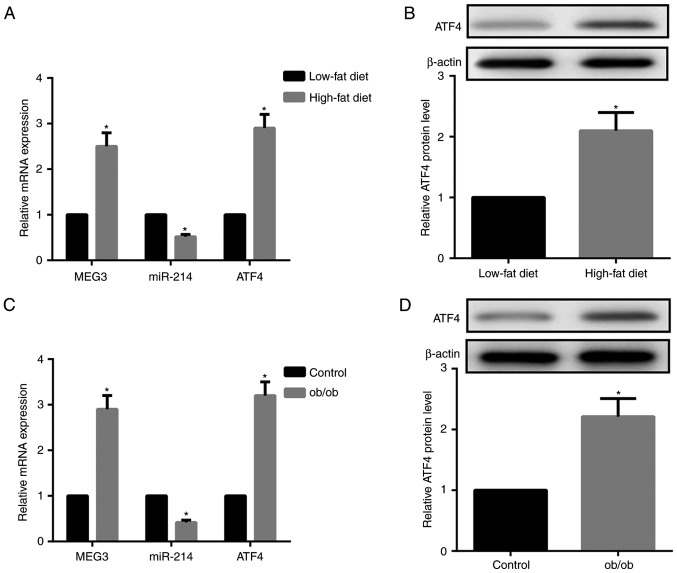
As demonstrated in Fig. 2A and B, HFD-fed mice exhibited increased MEG3 expression and decreased miR-214 expression in liver tissues compared with LFD-fed mice. Furthermore, ATF4 mRNA and protein expression levels in the liver tissues were significantly increased in HFD-fed compared to LFD-fed mice. The expression patterns of MEG-3, miR-214 and ATF4 in the liver tissues of ob/ob mice, a model of severe obesity, insulin resistance and T2DM caused by leptin deficiency, was consistent with those in HFD-fed mice (Fig. 2C and D). Together, HFD-fed and ob/ob mice exhibited upregulated MEG3 and ATF4 expression, but downregulated miR-214 expression compared with the LFD-fed mice and control mice, respectively.
Figure 2.
HFD-fed and ob/ob mice demonstrate upregulated MEG3 and ATF4, downregulated miR-214. (A) Compared with LFD-fed mice, RT-qPCR results indicated that MEG3 and ATF4 mRNA expression were upregulated, while miR-214 expression was downregulated, in liver tissues from HFD-fed mice. (B) Western blot analysis revealed that ATF4 protein expression in the liver tissues from HFD-fed mice was upregulated compared with LFD-fed mice. (C) Compared with the control mice, RT-qPCR results demonstrated that MEG3 and ATF4 mRNA expression was upregulated, while miR-214 expression was downregulated, in liver tissues from ob/ob mice. (D) Western blot analysis revealed that ATF4 protein expression in liver tissues from ob/ob mice was upregulated compared with control mice. β-actin served as the loading control in western blots. N=10 for each group. *P<0.05 vs. the HFD or control groups. LFD, low-fat diet; HFD, high-fat diet; miR, microRNA; MEG3, maternally expressed gene 3; ATF4, activating transcription factor 4; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Palmitate time-dependently increases MEG3 and ATF4 but decreases miR-214 expression
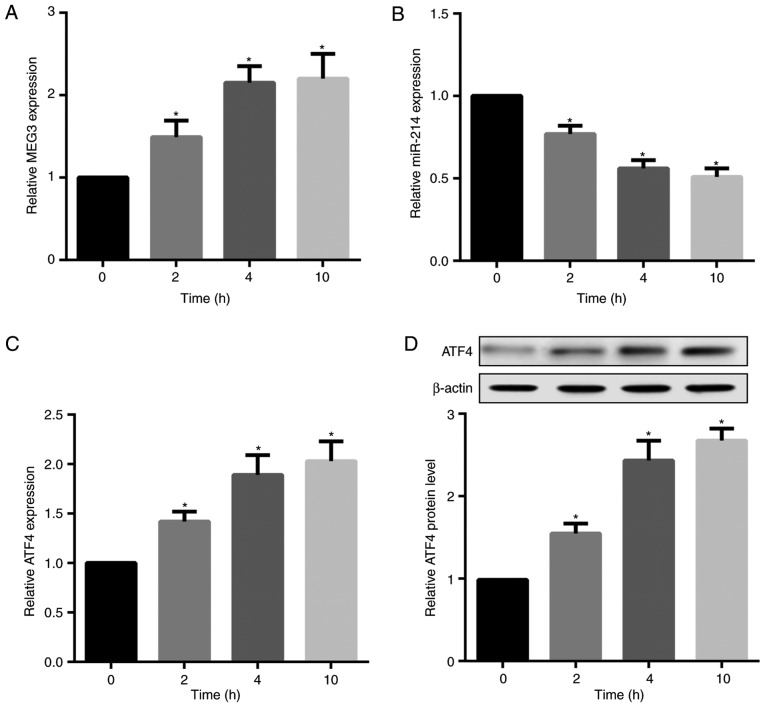
Saturated fatty acid (SFA) has been indicated to induce a proinflammatory response associated with obesity, T2DM, insulin resistance and dyslipidaemia (24,25). Accordingly, the present study investigated the in vitro effects of palmitate, a major SFA in plasma, on MEG3, ATF4 and miR-214 expression in mouse primary hepatocytes. The results revealed that palmitate treatment time-dependently increased MEG3, but decreased miR-214 expression in hepatocytes (Fig. 3A and B). Furthermore, palmitate significantly increased ATF4 mRNA and protein expression levels (Fig. 3C and D).
Figure 3.
Palmitate time-dependently increases MEG3 and ATF4 but decreases miR-214 in hepatocytes. Primary hepatocytes from male C57BL/6 mice were treated with 0.5 mM palmitate for 0, 2, 4 and 10 h. Reverse transcription quantitative polymerase chain reaction results demonstrated that palmitate time-dependently increased (A) MEG3 expression and (B) ATF4 mRNA expression, but decreased (C) miR-214 expression in hepatocytes. (D) Western blot analysis indicated that palmitate time-dependently increased ATF4 protein expression in hepatocytes. β-actin served as the loading control. *P<0.05 vs. 0 h group. miR, microRNA; MEG3, maternally expressed gene 3; ATF4, activating transcription factor 4.
MEG3 serves as a ceRNA of miR-214 to facilitate ATF4 expression
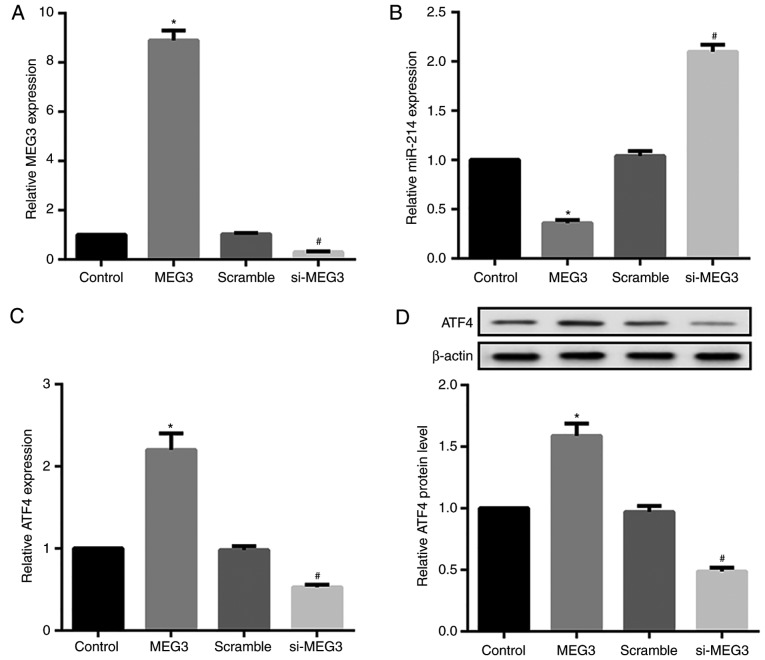
To investigate the effects of MEG3 expression on miR-214 and ATF4 expression, MEG3 was overexpressed or silenced by transfecting hepatocytes with pcDNA3.1-MEG3 or si-MEG3, respectively. The overexpression and knockdown efficiency of MEG3 was confirmed by RT-qPCR (Fig. 4A). MEG3 overexpression significantly decreased relative miR-214 expression, but markedly increased ATF4 mRNA and protein expression. By contrast, MEG3 knockdown exerted the opposite effects on miR-214 and ATF4 expression (Fig. 4B-D). Next, the effects of miR-214 expression on MEG3 and ATF4 expression were assessed. The overexpression and knockdown efficiency of miR-214 was also confirmed by RT-qPCR (Fig. 4E). Compared with their corresponding controls, hepatocytes transfected with the miR-214 mimic demonstrated significantly decreased MEG3 and ATF4 expression, whereas miR-214 inhibitor-transfected hepatocytes exhibited markedly increased MEG3 and ATF4 expression (Fig. 4F–H).
Figure 4.
Effect of MEG3 and miR-214 overexpression and knockdown on ATF4 expression in primary hepatocytes. Mouse primary hepatocytes were transfected with either pcDNA3.1-MEG3, si-MEG3, and corresponding controls, or with miR-214 mimics/inhibitor and their controls. (A) The overexpression and knockdown efficiency of MEG3 was confirmed by RT-qPCR. RT-qPCR and western blot analysis results revealed that MEG3 overexpression significantly decreased (B) relative miR-214 expression, but markedly increased ATF4 (C) mRNA and (D) protein expression. MEG3 knockdown exerted the opposite effects. *P<0.05 vs. NC or control groups. #P<0.05 vs. the scramble group. MEG3, maternally expressed gene 3; ATF4, activating transcription factor 4; si, small interfering RNA. Effect of MEG3 and miR-214 overexpression and knockdown on ATF4 expression in primary hepatocytes. (E) RT-qPCR results indicated that the miR-214 mimic increased miR-214 expression, whereas the miR-214 inhibitor decreased miR-214 expression. RT-qPCR and western blot analysis results revealed that the miR-214 mimic significantly decreased (F) relative MEG3 expression, and ATF4 (G) mRNA and (H) protein expression. The miR-214 inhibitor exerted the opposite effects. β-actin served as the loading control in the western blot analyses. *P<0.05 vs. NC or control groups. #P<0.05 vs. the scramble group. miR, microRNA; NC, negative control.
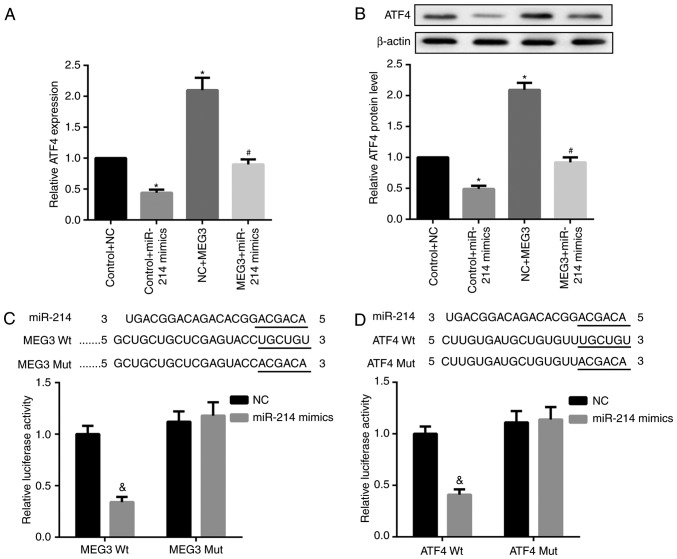
It is well-established that lncRNAs may function as ceRNA by competitively binding miRNAs. Accordingly, whether MEG3 functioned similarly in regulating miR-214 was explored. Compared with the corresponding controls, miR-214 mimics significantly decreased ATF4 mRNA and protein expression, whereas MEG3 overexpression markedly increased ATF4 mRNA and protein expression levels. Furthermore, the simultaneous overexpression of miR-214 and MEG3 significantly decreased MEG3-mediated upregulation of ATF4 mRNA and protein expression (Fig. 5A and B). In addition, the results of the luciferase reporter assay indicated that the miR-214 mimic caused a marked decrease in luciferase activity in the MEG3-Wt reporter group compared with the miR-NC group. By contrast, the miR-214 mimic exhibited no obvious effect on luciferase activity in the MEG3-Mut reporter group, verifying the direct binding between MEG3 and miR-214 (Fig. 5C). In addition, decreased luciferase activity in 293 cells co-transfected with WT ATF4 3′UTR luciferase reporter plasmids and miR-214 mimics (Fig. 5D) was observed, suggesting the 3′UTR of ATF4 is directly targeted by miR-214. Collectively, these results indicate that MEG3 serves as a ceRNA of miR-214 to facilitate ATF4 expression.
Figure 5.
miR-214 binds to MEG3 and ATF4. Primary hepatocytes were co-transfected with pcDNA3.1-MEG or control pcDNA3.1 vectors, and miR-214 mimic or NC sequences. (A) RT-qPCR and (B) western blot analysis results indicated that simultaneous overexpression of miR-214 and MEG3 significantly decreased the MEG3-mediated upregulation of ATF4 mRNA and protein expression. β-actin served as the loading control in the western blot analysis. (C) Results of the luciferase reporter assay demonstrated that the miR-214 mimic led to a marked decrease in luciferase activity in the MEG3-Wt reporter group compared with the miR-NC group. There was no marked effect on luciferase activity in the MEG3-Mut reporter group. (D) Luciferase activity in 293 cells co-transfected with Wt ATF4 3′UTR luciferase reporter plasmids and miR-214 mimics was decreased compared with that in the miR-NC group. *P<0.05 vs. the control + NC group; #P<0.05 vs. the NC+MEG3 group; &P<0.05 vs. the control group. MEG3, maternally expressed gene 3; ATF4, activating transcription factor 4; miR, microRNA; NC, negative control; Wt, wild-type; Mut, mutant.
miR-214 inhibition and ATF4 overexpression reverse the MEG3 knockdown-mediated decrease in FoxO1, G6pc and Pepck expression
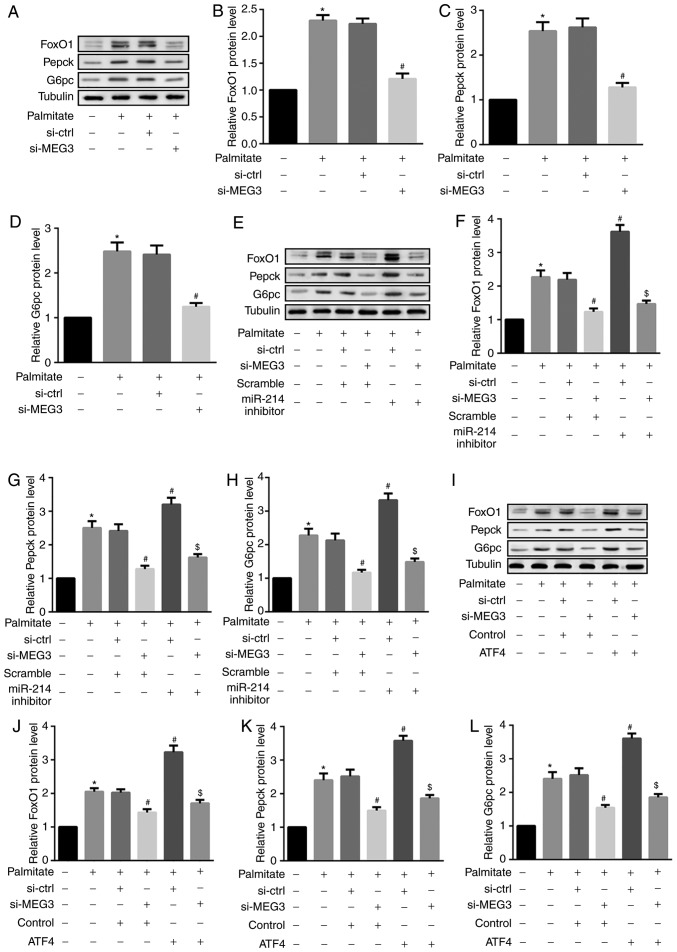
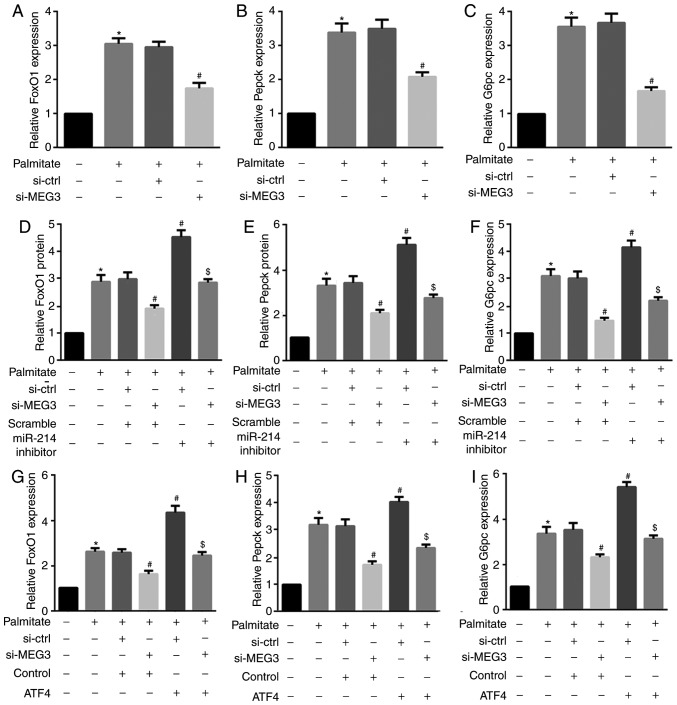
FoxO1 serves an important role in retinoid metabolism within hepatic gluconeogenesis. FoxO1 is also a critical regulator of hepatic glucose and lipid metabolism via its ability to drive the transcription of two key gluconeogenic enzymes, G6pc and Pepck (26). The results from the present study revealed that palmitate significantly increased FoxO1, Pepck and G6pc protein expression in primary hepatocytes (Fig. 6A–D). Furthermore, MEG3 knockdown significantly suppressed the palmitate-mediated upregulation of these 3 proteins (Fig. 6A–D), indicating an attenuation of hepatocyte gluconeogenesis. By contrast, miR-214 inhibitor and ATF4 overexpression additionally induced the palmitate-mediated upregulation of FoxO1, Pepck and G6pc (Fig. 6E–L). Similar results were observed for FoxO1, Pepck, and G6pc mRNA expression (Fig. 7). These results indicated that miR-214 inhibition and ATF4 overexpression significantly reversed the MEG3 knockdown-mediated decrease in palmitate-induced FoxO1, G6pc and Pepck protein expression.
Figure 6.
miR-214 inhibition and ATF4 overexpression reverse MEG3 knockdown-mediated decreases in FoxO1, G6pc and Pepck protein expression. Cells were transfected with the indicated plasmids, followed by treatment with 0.5 mM palmitate for 4 h. (A–D) Western blot analysis revealed that MEG3 knockdown significantly suppressed the palmitate-mediated increase in (B) FoxO1, (C) Pepck, and (D) G6pc protein expression. (E–H) By contrast, the miR-214 inhibitor additionally induced the palmitate-mediated increase in (F) FoxO1, (G) Pepck and (H) G6pc protein expression. (I–L) ATF4 overexpression also induced the palmitate-mediated increase in (J) FoxO1, (K) Pepck and (L) G6pc protein expression. β-tubulin served as the loading control in the western blot analyses. *P<0.05 vs. group 1; #P<0.05 vs. group 3;$P<0.05 vs. group 4. si, small interfering RNA; ctrl, control; MEG3, maternally expressed gene 3; miR, microRNA; FoxO1, forkhead box protein O1; Pepck, phosphoenolpyruvate carboxykinase; G6pc, glucose-6-phosphatase catalytic subunit; ATF4, activating transcription factor 4.
Figure 7.
miR-214 inhibition and ATF4 overexpression reverse the MEG3 knockdown-mediated decrease in FoxO1, G6pc and Pepck mRNA expression. Cells were transfected with the indicated plasmids, followed by treatment with 0.5 mM palmitate for 4 h. (A–C) RT-qPCR results revealed that MEG3 knockdown significantly suppressed the palmitate-mediated increase in (A) FoxO1, (B) Pepck, and (C) G6pc mRNA expression. (D–F) By contrast, the miR-214 inhibitor induced the palmitate-mediated increase in (D) FoxO1, (E) Pepck, and (F) G6pc mRNA expression. (G–I) ATF4 overexpression also induced the palmitate-mediated increase in (G) FoxO1, (H) Pepck, and (I) G6pc mRNA expression. *P<0.05 vs. group 1; #P<0.05 vs. group 3; $P<0.05 vs. group 4. miR, microRNA; ATF, activating transcription factor 4; MEG3, maternally expressed gene 3; FoxO1, forkhead box protein O1; Pepck, phosphoenolpyruvate carboxykinase; G6pc, glucose-6-phosphatase catalytic subunit; si, small interfering RNA; ctrl, control; MEG3.
MEG3 knockdown improves glucose and insulin tolerance in HFD-fed mice
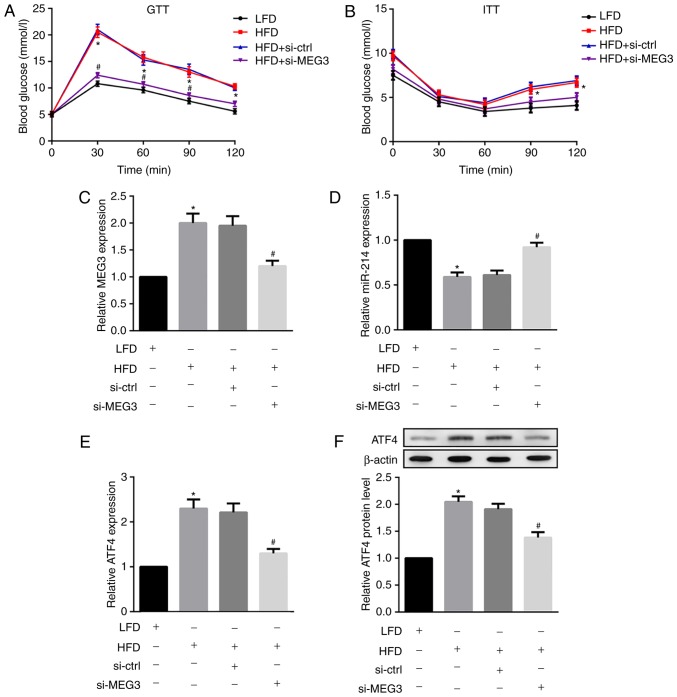
Next, HFD-fed male C57BL/6 mice were injected with si-MEG3 fragments via the tail vein to examine the effects of MEG3 knockdown on glucose and insulin tolerance. As indicated in Fig. 8A, HFD-fed mice demonstrated significantly increased fasting blood glucose levels compared with the control LFD-fed mice. However, the treatment of HFD-fed mice with si-MEG3 substantially improved impaired glucose tolerance. Additionally, HFD-fed mice treated with si-MEG3 also improved impaired insulin tolerance (Fig. 8B).
Figure 8.
Effects of MEG3 knockdown on miR-214 and ATF4 expression, glucose tolerance and insulin tolerance in HFD-fed mice. HFD-fed male C57BL/6 received a si-MEG3 fragment injection via the tail vein twice a week for 10 weeks. At week 10, following overnight fasting, (A) GTTs and (B) ITTs were performed to analyse glucose and insulin tolerance, respectively. MEG3 knockdown improved glucose and insulin tolerance in HFD-fed mice. Liver tissues were separated and the relative mRNA expression of (C) MEG3, (D) miR-214 and (E) expression of ATF4 were examined by reverse transcription polymerase chain reaction. (F) Protein expression of ATF4 was examined by western blot analysis. MEG3 knockdown upregulated miR-214 but downregulated ATF4 expression in HFD-fed mice. β-actin served as the loading control. N=10 for each group.*P<0.05 vs. the LFD group; #P<0.05 vs. the HFD + si-Ctrl group. MEG3, maternally expressed gene 3; miR, microRNA; ATF, activating transcription factor 4; HFD, high-fat diet; LFD, low-fat diet; si, small interfering RNA; ctrl, control; GTTs, glucose tolerance tests; ITTs, insulin tolerance tests.
MEG3 knockdown upregulates miR-214 but downregulates ATF4 expression in HFD-fed mice
Finally, the in vivo effects of MEG3 knockdown on miR-214 and ATF4 expression in liver tissues from HFD-fed mice were investigated. MEG3 knockdown was demonstrated to significantly down-regulate the HFD-induced expression of MEG3 (Fig. 8C) and ATF4 (Fig. 8E and F) in mouse liver tissues. By contrast, MEG3 knockdown fragments significantly upregulated HFD-suppressed miR-214 expression in mouse liver tissues (Fig. 8D). In agreement with in vitro results, the in vivo results also indicated that MEG3 knockdown upregulated miR-214 but downregulated ATF4 expression in HFD-fed mice.
Discussion
The aim of the present study was to evaluate the expression of MEG3, miR-214 and ATF4 and their interaction and effects on insulin resistance in T2DM. Leptin-deficient ob/ob mice are overweight, develop insulin resistance, and serve as a model for T2DM. Insulin resistance is a characteristic feature of T2DM. The results of the present study provided evidence that MEG-3 and ATF4 were upregulated, while miR-214 was downregulated, in the livers of HFD-fed and ob/ob mice. Furthermore, in mouse primary hepatocytes, palmitate time-dependently increased MEG3 and ATF4 expression, but decreased miR-214 expression. It was also identified that MEG3 served as a ceRNA of miR-214 to facilitate ATF4 expression. In addition, miR-214 inhibition and ATF4 overexpression reversed the MEG3 knockdown-mediated decrease in the expression of FoxO1 and FoxO1-downstream targets Pepck and G6pc. Finally, in HFD-fed mice, MEG3 knockdown substantially improved impaired glucose and insulin tolerance, while downregulating induced ATF4 expression and upregulating suppressed miR-214 expression.
MEG3, an imprinted lncRNA within the DLK1-MEG3 locus on chromosome 14q32 in humans, is expressed in a number of normal tissues (27). MEG3 has been demonstrated to serve as a tumour suppressor in several types of cancer, including glioma (28), bladder cancer (29) and colorectal cancer (30). However, the functional role of MEG3 genes in hepatic insulin resistance in T2DM is poorly understood. In our previous study, it was identified that lncRNA MEG3 upregulation enhanced hepatic insulin resistance via increased FoxO1 expression (5). In the present study, the specific mechanism by which MEG3 increased FoxO1 expression was explored.
Previous evidence has confirmed that lncRNAs may serve as ceRNA to absorb miRNAs away from target genes, and thereby alter the expression of miRNA target genes (31). Notably, MEG3 was recently demonstrated to serve as a ceRNA of certain miRNAs during the tumorigenesis of several types of cancer: For example, Qin et al (28) stated that MEG3 functioned as a ceRNA of miR-19a that repressed phosphatase and tensin homolog expression to inhibit glioma cell proliferation, migration and invasion. Peng et al (32) demonstrated that MEG3 may upregulate B-cell lymphoma-2 via its ceRNA activity on miR-181 to regulate gastric carcinogenesis. Yan et al (33) identified that MEG3 served as a ceRNA and competed with programmed cell death 4 mRNA to directly bind miR-21 to regulate ischemic neuronal death. MEG3 also positively regulated the expression of sex-determining region Y-box7 by functioning as a ceRNA of miR-21-5p to regulate cisplatin resistance in non-small cell lung cancer (13). The present study attempted to investigate whether MEG3 may also function as a ceRNA to regulate FoxO1 expression.
miR-214 was recently identified as a direct target of MEG3 (15). Li et al (16) revealed that the expression of miR-214 was downregulated in the livers of fasting and HFD-induced diabetic mice, which was consistent with the results of the present study. That study also suggested that miR-214 suppressed gluconeogenesis by targeting ATF4, which regulates gluconeogenesis by affecting FoxO1 transcriptional activity (16). ATF4 serves a role in high-carbohydrate diet-induced insulin resistance (17). Notably, the present study provided evidence of a novel MEG3/miR-214/ATF4 axis: MEG3 was identified to serve as a ceRNA of miR-214 to facilitate ATF4 expression.
Abnormally elevated hepatic gluconeogenesis is largely responsible for the overproduction of glucose in patients with T2DM (16). Insulin represses hepatic gluconeogenesis by suppressing the transcriptional activity of FoxO1 via protein kinase B phosphorylation-mediated protein degradation (34). Increased FoxO1 activity leads to hyperglycaemia and increased cellular production of hepatic glucose, which is associated with insulin resistance (18). FoxO1 is a key regulator of hepatic gluconeogenesis that drives G6pc and Pepck mRNA transcription (26). The results from the present study from mouse primary hepatocytes indicated that MEG3 directly bound to miR-214 and suppressed its expression. ATF4 was a direct target of miR-214 and MEG3 positively regulated ATF4 expression by inhibiting miR-214. In addition, miR-214 inhibition and ATF4 overexpression reversed the MEG3 knockdown-mediated decrease in the expression of FoxO1 and FoxO1-downstream targets Pepck and G6pc. Furthermore, in HFD-fed mice, MEG3 knockdown led to substantial improvements in impaired glucose and insulin tolerance, while downregulating HFD-induced ATF4 expression and upregulating HFD-suppressed miR-214 expression. Collectively, the results from the present study indicated that MEG3 functioned as a ceRNA of miR-214 to upregulate ATF4 expression, leading to the promotion of FoxO1 expression and its downstream gluconeogenic enzymes, G6pc and Pepck. This, in turn, increased gluconeogenesis and promoted insulin resistance (Fig. 9).
Figure 9.
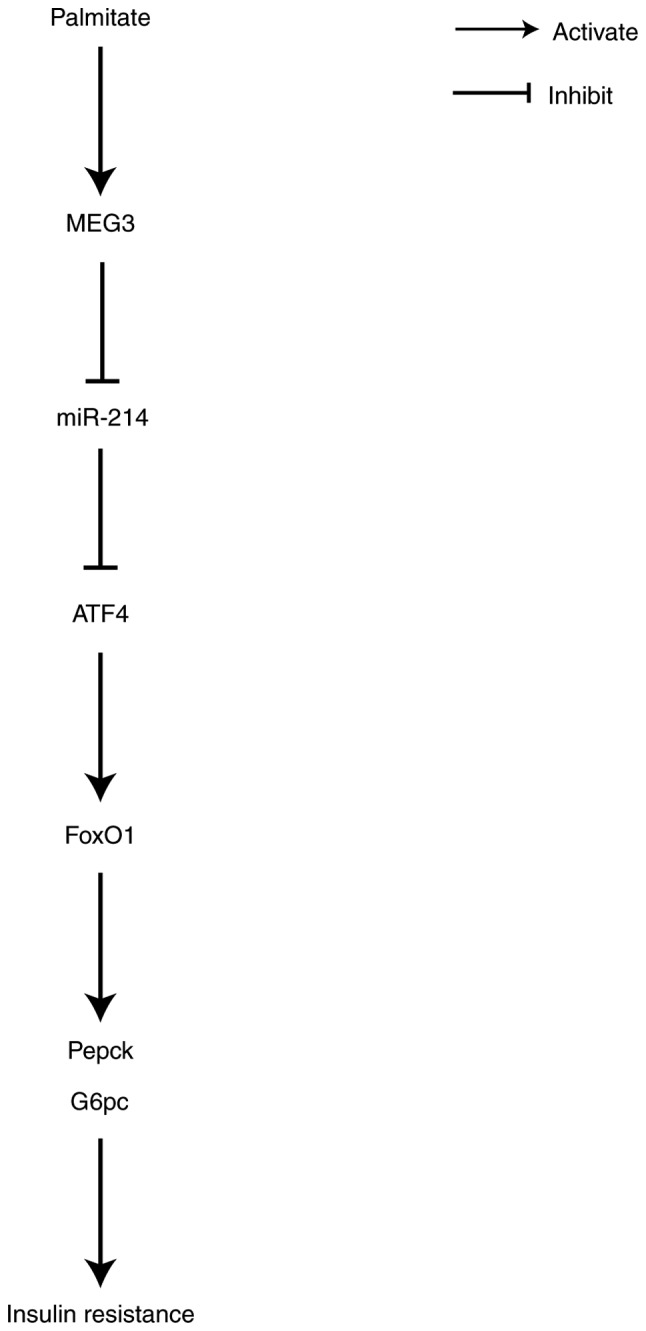
Proposed associations between target genes. Palmitate time-dependently increased MEG3 and ATF4 expression, but decreased miR-214 expression. MEG3 functioned as a competing endogenous RNA of miR-214 to upregulate ATF4 expression, leading to the promotion of FoxO1 and its downstream gluconeogenic enzymes G6pc and Pepck. This thereby increases gluconeogenesis and promotes insulin resistance. MEG3, maternally expressed gene 3; miR, microRNA; ATF4, activating transcription factor 4; FoxO1, Forkhead box protein O1; O1; Pepck, phosphoenolpyruvate carboxykinase; G6pc, glucose-6-phosphatase catalytic subunit.
In conclusion, the data from the present study demonstrate that MEG3 promoted hepatic insulin resistance by serving as a ceRNA of miR-214 to facilitate ATF4 expression. These results provide insight into the molecular mechanism of MEG3 involvement in the development of T2DM.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
XZ was responsible for the literature review, development of the intellectual content of the manuscript and data analysis. XZ and HL performed the experiments. XZ prepared the manuscript, and XZ and HL were involved in editing the manuscript. YW and JZ were responsible for data acquisition. XZ and GY performed the statistical analysis. WW was involved in developing the concept and design of the study, and is guarantor of integrity of the entire study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal experiments performed in the present study were approved by the Animal Ethics Committee of Anhui Provincial Hospital, The First Affiliated Hospital of University of Science and Technology of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Garabadu D, Krishnamurthy S. Metformin attenuates hepatic insulin resistance in type-2 diabetic rats through PI3K/Akt/GLUT-4 signalling independent to bicuculline-sensitive GABAA receptor stimulation. Pharm Biol. 2017;55:722–728. doi: 10.1080/13880209.2016.1268635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y, Thakran S, Bheemreddy R, Coppess W, Walker RJ, Steinle JJ. Sodium salicylate reduced insulin resistance in the retina of a type 2 diabetic rat model. PLoS One. 2015;10:e0125505. doi: 10.1371/journal.pone.0125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Wu YB, Zhou J, Kang DM. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun. 2016;469:319–325. doi: 10.1016/j.bbrc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Burchell A. Glycogen storage diseases and the liver. Baillieres Clin Gastroenterol. 1998;12:337–354. doi: 10.1016/S0950-3528(98)90138-5. [DOI] [PubMed] [Google Scholar]
- 7.Pan T, Guo JH, Ling L, Qian Y, Dong YH, Yin HQ, Zhu HD, Teng GJ. Effects of Multi-electrode renal denervation on insulin sensitivity and glucose metabolism in a canine model of type 2 diabetes mellitus. J Vasc Interv Radiol. 2018;29:731–738.e2. doi: 10.1016/j.jvir.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Ruan X, Yang L, Kiesewetter K, Zhao Y, Luo H, Chen Y, Gucek M, Zhu J, Cao H. A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab. 2015;21:455–467. doi: 10.1016/j.cmet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morá I, Akerman İ, van de Bunt M, Xie R, Benazra M, Nammo T, Arnes L, Nakić N, García-Hurtado J, Rodríguez-Seguí S, et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16:435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: A tumor suppressor. J Mol Endocrinol. 2012;48:R45–R53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: Two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46:567–574. doi: 10.5483/BMBRep.2013.46.12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Chen D, Ma H, Li Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco Targets Ther. 2017;10:5137–5149. doi: 10.2147/OTT.S146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 15.Fan FY, Deng R, Yi H, Sun HP, Zeng Y, He GC, Su Y. The inhibitory effect of MEG3/miR-214/AIFM2 axis on the growth of T-cell lymphoblastic lymphoma. Int J Oncol. 2017;51:316–326. doi: 10.3892/ijo.2017.4006. [DOI] [PubMed] [Google Scholar]
- 16.Li K, Zhang J, Yu J, Liu B, Guo Y, Deng J, Chen S, Wang C, Guo F. MicroRNA-214 suppresses gluconeogenesis by targeting activating transcriptional factor 4. J Biol Chem. 2015;290:8185–8195. doi: 10.1074/jbc.M114.633990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Meng Q, Xiao F, Chen S, Du Y, Yu J, Wang C, Guo F. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem J. 2011;438:283–289. doi: 10.1042/BJ20110263. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metabolism. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Yu J, Jia X, Xiong L, Li N, Wen X. Zhenqing recipe improves glucose metabolism and insulin sensitivity by repressing hepatic FOXO1 in type 2 diabetic rats. Am J Chin Med. 2012;40:721–733. doi: 10.1142/S0192415X12500541. [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Liu C, Qiao A, Cui Y, Zhang H, Cui A, Zhang S, Yang Y, Xiao X, Chen Y, et al. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. J Hepatol. 2013;58:535–542. doi: 10.1016/j.jhep.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303:G979–G992. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbo T, Perry RJ, Nishimura E, Samuel VT, Quistorff B, Shulman GI. PP2A inhibition results in hepatic insulin resistance despite Akt2 activation. Aging (Albany NY) 2013;5:770–781. doi: 10.18632/aging.100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Bunn RC, Cockrell GE, Ou Y, Thrailkill KM, Lumpkin CK, Jr, Fowlkes JL. Palmitate and insulin synergistically induce IL-6 expression in human monocytes. Cardiovasc Diabetol. 2010;9:73. doi: 10.1186/1475-2840-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volpe CM, Abreu LF, Gomes PS, Gonzaga RM, Veloso CA, Nogueiramachado JA. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid Med Cell Longev. 2014;2014;479587 doi: 10.1155/2014/479587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, Ishino F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5:211–220. doi: 10.1046/j.1365-2443.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- 28.Qin N, Tong GF, Sun LW, Xu XL. Long noncoding RNA MEG3 suppresses glioma cell proliferation, migration, and invasion by acting as a competing endogenous RNA of miR-19a. Oncol Res. 2017;25:1471–1478. doi: 10.3727/096504017X14886689179993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying L, Huang Y, Chen H, Wang Y, Xia L, Chen Y, Liu Y, Qiu F. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol Biosyst. 2013;9:407–411. doi: 10.1039/c2mb25386k. [DOI] [PubMed] [Google Scholar]
- 30.Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biol. 2015;36:4851–4859. doi: 10.1007/s13277-015-3139-2. [DOI] [PubMed] [Google Scholar]
- 31.Lu MH, Tang B, Zeng S, Hu CJ, Xie R, Wu YY, Wang SM, He FT, Yang SM. Long noncoding RNA BC032469, a novel competing endogenous RNA, upregulates hTERT expression by sponging miR-1207-5p and promotes proliferation in gastric cancer. Oncogene. 2016;35:3524–3534. doi: 10.1038/onc.2015.413. [DOI] [PubMed] [Google Scholar]
- 32.Peng W, Si S, Zhang Q, Zhao F, Wang F, Yu J, Ma R. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res. 2015;34:79. doi: 10.1186/s13046-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan H, Rao J, Yuan J, Gao L, Huang W, Zhao L, Ren J. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis. 2017;8:3211. doi: 10.1038/s41419-017-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.