Abstract
Objective
Hepatitis B virus (HBV) is not uncommon among persons infected with human immunodeficiency virus (HIV). Severity of HBV infection and treatment outcome are associated with specific HBV genotypes. No study has reported the types of HBV genotypes circulating among HIV-infected subjects in Nigeria. This study was designed to determine the prevalence of HBV, as well as its genotypic distribution among HIV-infected subjects in Benin City, Nigeria.
Methods
Whole blood was collected from a total of 564 HIV-infected and 250 apparently healthy HIV-negative subjects. Serodiagnosis of HBV infection was done using an immunochromatographic kit. Detection of HBV-DNA and sequencing of amplicons were done using standard molecular techniques.
Results
HIV status was not significantly associated with HBV seroinfection (HIV vs. non-HIV: 4.6% vs. 4.0%; odds ratio = 1.168, 95% confidence interval = 0.550, 2.444, and P = 0.854). HIV-infected subjects were observed to have an insignificantly (P = 0.645) higher prevalence of true HBV infection than their non-HIV-infected counterparts (HIV positive vs. HIV negative: 23.1% vs. 10.0%). All patients with true HBV infection were found to harbor HBV genotype E, which did not cluster around other HBV genotype E.
Conclusion
This study reports novel strains of HBV genotype E circulating in Nigeria.
Keywords: Hepatitis B virus genotypes, human immunodeficiency virus infected patients, Nigeria
Introduction
Hepatitis B virus (HBV) is a leading cause of liver diseases worldwide. It is reported to be responsible for over 360 million cases of chronic hepatitis and 620,000 deaths each year.[1] HBV infection is endemic in Sub-Saharan Africa.[2] So far, eight genotypes of HBV have been reported based on sequence diversity.[3] Factors such as genotypes, viral load, presence of Hepatitis B E antigens, and escape mutations have been reported to affect the outcome of HBV infection.[3] Seven drugs have been approved for the management of patients with chronic HBV infection, namely standard interferon (IFN)-α, pegylated IFN-α (PEG-IFN-α), lamivudine, telbivudine, entecavir, adefovir dipivoxil, and tenofovir disoproxil fumarate.[4] Patients infected with HBV genotype D or E have been reported to have a lower response rate to PEG IFN than those infected with genotype A.[5] Furthermore, some studies have documented that patients infected with HBV genotype B responded much more favorably to treatment with PEG IFN than those infected with genotype C.[6,7] HBV genotype B has been reported to be independently associated with the earlier emergence of lamivudine-resistant strains than genotype C.[8]
HBV is common among human immunodeficiency virus (HIV)-infected patients. This is because HIV and HBV share similar mode of transmission.[9] HIV/HBV coinfection is associated with a poorer disease outcome.[10] Spontaneous clearance of HBV is poorer in HIV infected than non-HIV infected ones. This often results in higher HBV-DNA levels and HBV antigenemia among HIV infected patients, increasing the risk for development of liver disease, cirrhosis and hepatocellular carcinoma.[11,12]
In Nigeria, the routine screening of HIV-infected patients for HBV is not done in most hospitals. At present, there is a gap in knowledge on the genotypic distribution of HBV among HIV patients in Nigeria. In the light of the above, this study aimed at determining the prevalence of true HBV infection, as well as the genotypic and subtype distribution of HBV among HIV-infected subjects in Nigeria.
Methods
Study population
This study was conducted among patients registered at the University of Benin Teaching Hospital (UBTH), Benin City, a public tertiary hospital with referral status located in the Southern part of Nigeria.
A total of 814 subjects comprising of 564 HIV-infected and 250 apparently healthy non-HIV-infected subjects were recruited for this study. Whole blood was collected from all 814 participants, after receiving written informed consent from them. Minor consent was obtained from their parents or guardians. Exclusion criterion in the study was age <18 months. The study protocol was approved by the Ethics and Research Committee of the UBTH, Benin City, Nigeria.
Specimen processing
Specimen (serum) obtained from each patient was examined for the presence of hepatitis B surface antigen (HBsAg) using Determine™ HBsAg immunochromatographic test kit (Abbott Laboratories, USA). Test kit manufacturer instruction was strictly followed. 2 ml of patients’ sera that tested positive to HBsAg were put in cryovers and kept at −80°C for further analysis.
Detection of HBV-DNA
HBV-DNA was harvested from 140 ul of patients’ serum, with the aid of Zymo ZR Viral DNA/RNA kit, following the manufacturer’s instruction. Detection of HBV was achieved by amplification of the S gene of HBV using OneTaq Quick-Load 2X Master Mix Kit (New England BioLabs). The primer set (forward primer 5`- TCACCATATTCTTGGGAACAAGA-3` and reverse primer 5`-CGAACCACTGAACAAATGGC-3`) used for the amplification of target gene has been described in a previous study (Rashid et al., 2014). The total volume of reaction mixture was 25 ul and consisted of the following components: 12.5 ul of OneTag Quick-Load 2X Master Mix, 0.5 ul each of forward and reverse primers, 2.5 ul of DNA extract, and 9 ul of nuclease-free water. The thermal profile for amplification used had been previously adopted by an earlier study (Rashid et al., 2014) and is as follows: An initial cycle of 94°C for 5 min, followed by 40 cycles consisting of 94°C for 30 s, 55°C for 1 min, and 72°C for 1.5 min. The final elongation was 72°C for 5 min. Amplified polymerase chain reaction products were subsequently detected on 1.5% agarose gel following staining with ethidium bromide.
Characterization of HBV isolates
All HBV-DNA-positive amplicons were sequenced at Inqaba Biotec, South Africa. Resulting sequence output was opened with the software FinchTV. After trimming, editing, and alignment of forward and reverse sequences, the identity of the sequence was confirmed with the use of Basic Local Alignment Search Tool. Confirmed HBV genotypes (A-H) retrieved from GeneBank database were used to determine the genotype of study isolates. Phylogenetic tree was created by the neighbor-joining method using study isolates and 29 HBV isolates from different countries as previously described.[13,14]
Statistical analysis
Chi-square or Fischer’s exact tests (INSTAT®) as appropriate were used to analyze data from this study. P < 0.05 was considered as statistically significant.
Results
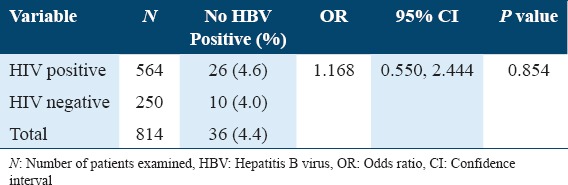
The overall seroprevalence of HBV infection in this study was 4.4%. HIV-infected patients were observed to have a higher seroprevalence of HBV infection than non-HIV-infected subjects (HIV vs. non-HIV: 4.6% vs. 4.0%). HIV status was not significantly associated with HBV seroinfection in this study (HIV vs. non-HIV: odds ratio [OR] = 1,168, 95% confidence interval = 0.550, 2.444, and P = 0.854) [Table 1].
Table 1.
Seroprevalence of hepatitis B virus infection among study subjects
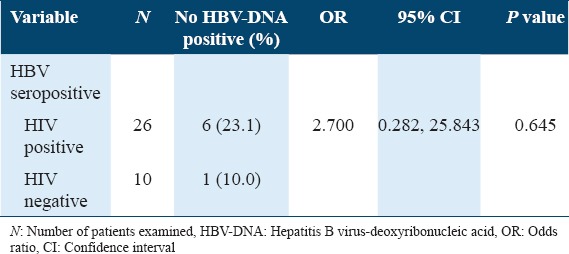
Of the 26 HIV patients’ seropositive for HBV, 6 (23.3%) were observed to have detectable HBV-DNA, while 1 (10%) of 10 non-HIV subjects’ seropositive for HBV infection had detectable HBV DNA. HIV-infected patients were observed to have about 3 times higher risk (OR = 2.700) of acquiring true HBV infection than their non-HIV-infected counterparts. However, HIV positivity was not identified as a risk factor for true HBV infection (P = 0.645) [Table 2].
Table 2.
Prevalence of true HBV infection among HIV-infected and non-HIV-infected subjects
All HBV isolates were found to be genotype E [Figure 1]. Phylogenic analysis revealed that none of the HBV isolates clustered around already known genotype E recovered from the GenBank, indicating new strains or variants [Figure 2].
Figure 1.
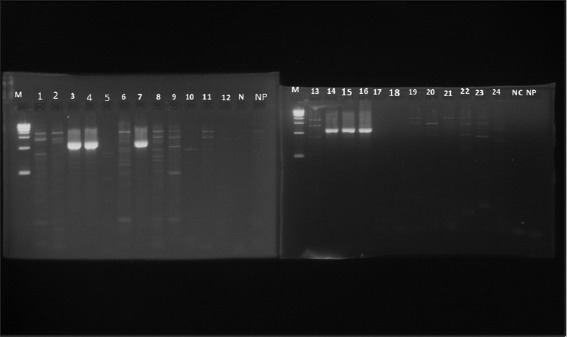
Hepatitis B virus-positive samples (1063 bp) detected by polymerase chain reaction (PCR) among HIV-infected patients after staining with ethidium bromide. Lane M - 10 kb DNA ladder, Lanes 3, 4.7, 14-16: HBV-positive samples (1063 bp), Lane 1, 2, 5, 6, 8-13, 17-25: HBV-negative samples, Lane NC: Negative control, NP: Negative PCR
Figure 2.
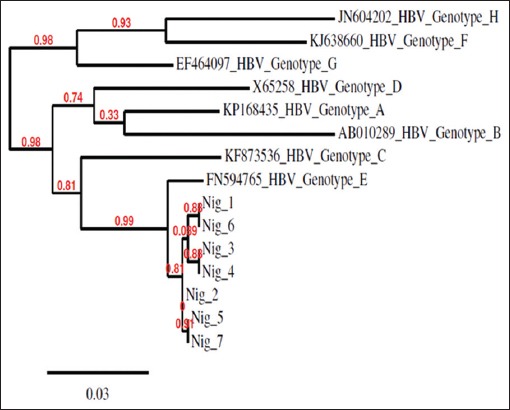
Genotypic characterization of hepatitis B virus isolates from the study
Discussion
Reports indicate that HIV facilitates HBV replication leading to an increased risk for the development of liver diseases.[11,12] Treatment outcome of HBV infections is associated with specific genotypes.[15] Data on the genotypic prevalence of HBV in the Nigeria are sparse. Indeed, there is no report on the genotypic prevalence of HBV among HIV patients in Nigeria. This informed our study.
A total of 26 (4.6%) of the 564 HIV-infected patients studied were found to be seropositive to HBV. This is lower than the values reported in some African studies[16-19] but higher than others.[20,21] One Greek study[9] and another Brazilian one[22] have, however, documented higher seroprevalence of HBV, while two others from Brazil[23,24] reported lower values than that recorded in this study. The prevalence of HBV is reported to have geographical variation.[25] This may explain the pattern of the result obtained in this study. Again, differences in serological diagnostic technique used may also account for the observed variation in these studies.
Among non-HIV-infected subjects, an HBV seroprevalence of 4.0% was recorded. This is higher than the value reported by a previous Nigerian study.[26] Other Nigerian studies have, however, reported lower prevalence rate.[27,28] The observed variation may be due to differences in nature of study population, as the studies conducted by Oladeinde et al., 2013[26,27] were carried out on pregnant women in contrast to our study population in which pregnant women accounted for <3%. Although a higher seroprevalence of HBV was recorded among HIV-infected patients, HIV was not identified as a risk factor for HBV seroinfection in this study. Similar findings have been reported by some Nigerian studies[28,29] and a Ugandan one.[30]
Molecular analysis of the sera of all 26 HIV-infected patients that were seropositive for HBV showed that 6 (23.1%) of them had a true HBV infection. Much higher prevalence of true HBV infection has been reported by other African studies.[31-34] Much higher prevalence of true HBV infection has been reported by other African studies,[31-34] with a lower value documented in one study conducted outside Africa.[35] The role of CD4+ and CD8+ T-cell in the control of HBV infection is critical.[36] The outcome of HBV infection is associated with specific HBV genotype.[3] Thus, the observed variation in prevalence of true HBV infection may be related to differences in the immune status of the study population and circulating HBV genotypes. The disparity could also be attributed to differences in severity of HBV infection among study populations, variation in molecular diagnostic technique employed, and specific HBV gene targeted in these studies.
Of the 10 non-HIV-infected subjects positive for HBsAg, only 1 (10.0%) was found to have a true HBV infection. This is lower than 83.3% recorded among blood donors in one Iranian study.[37] The risk of acquiring true HBV infection was observed to be about 3 times (OR = 2.700) higher among HIV-infected than non-infected subjects. HBV disease progression is affected by the degree of immunodeficiency of a patient.[38] HIV infection results in an immunedeficient state.[39] Studies have shown that HIV-infected persons have a lower rate of clearance of Hepatitis B E antigen and a much more prolonged acute illness after infection with HBV than those without HIV.[40] This may explain the pattern of result observed in this study. In general, the prevalence of true HBV infection did not differ significantly with respect to HIV status of subjects in this study.
All HIV-infected patients with true HBV infection in this study were found to harbor HBV genotype E. A preponderance of HBV genotype E has been reported among HIV-infected patients in several African studies.[31,33,41] However, in one South African study, no HBV genotype E was detected among HIV-infected patients.[34] Other studies conducted outside Africa also failed to detect HBV genotype E among HIV-infected patients.[35,42,43] HBV genotype E has been reported to account for a majority of HBV infection in West Africa.[44,45] Reports show that HBV genotype E is essentially not found outside of Africa, even among African-American populations, many of whom are of West African origin, which suggests that it is probably a recently evolved genotype.[44]
Studies have reported an association between the spread of specific HBV genotypes and HIV. In one French study, an association was reported to exist between HBV genotype B and HIV seropositivity,[46] while another conducted in the United States of America[47] reported a preponderance of HBV genotype C among non-HIV-infected patients. Furthermore, marked differences in the genotypic distribution of HBV have been reported among HIV infected and non-infected patients in one Venezuelan study.[48] In this study, no such association was found as all HBV isolates from HIV- and non-HIV-infected subjects were genotype E. The observed variation in report may be due to differences in geographical location. A genetic factor may also play a role in the pattern of result observed. This definitely will require further investigations to verify.
Phylogenetic analysis of all HBV isolates obtained in this study with 29 other reference HBV genotype E strains Figure 3 showed that they did not cluster around other HBV genotype E isolates. This indicates that these HBV isolates are new strains of HBV genotype E. To the best of our knowledge, this is the first Nigerian study to determine the HBV genotypic prevalence among HIV-infected patients. The sequences of HBV isolate from all six HIV-infected patients in this study have been deposited in the GenBank database with the accession numbers - KY886211, KY886212, KY886213, KY886214, KY886216, and KY886217. The HBV isolate from non-HIV-infected subject has also been assigned the accession number - KY886215 in GenBank database.
Figure 3.
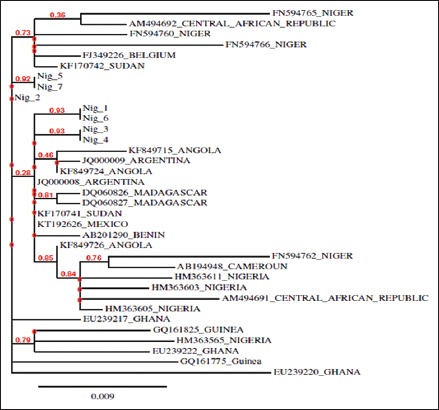
Phylogenetic analysis of study hepatitis B virus (HBV) isolates and other HBV genotype E isolates from GenBank database
Conclusion
The sero- and true-prevalence of HBV infection did not differ significantly between HIV-infected and non-infected patients. All HIV-infected and non-infected patients with true HBV infection were found to harbor HBV genotype E, which did not cluster around previous HBV genotype E isolates. The study highlights the presence of new variants of the HBV genotype E in Nigeria.
Acknowledgment
Authors acknowledge with thanks all those who participated in this study.
References
- 1.Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria 2000-2013: A systematic review and meta-analysis. Niger J Clin Pract. 2015;18:163–72. doi: 10.4103/1119-3077.151035. [DOI] [PubMed] [Google Scholar]
- 2.Ola SO, Odaibo GN. Alfafeto protein, HCV and HBV infections in Nigerian patients with primary hepatocellular carcinoma. Nig Med Pract. 2007;51:335. [Google Scholar]
- 3.Bihl F, Martinetti G, Wandeler G, Weber R, Ledergeber B, Calmy A, et al. HBV genotypes and response to tenofovir disoproxil fumarate in HIV/HBV-coinfected persons. BMC Gastroenterol. 2015;15:79. doi: 10.1186/s12876-015-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, Kao J. Hepatitis B viral factors and treatment responses in hepatitis B. J Form Med Assoc. 2013;112:302–11. doi: 10.1016/j.jfma.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zoulim F, Habersetzer F, Xiong S, Trépo C. Analysis of hepatitis B virus genotypes and pre-core region variability during interferon treatment of HBe antigen negative chronic hepatitis B. J Med Virol. 1996;48:8–16. doi: 10.1002/(SICI)1096-9071(199601)48:1<8::AID-JMV2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998–1002. doi: 10.1016/s0168-8278(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 7.Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425–30. doi: 10.1053/jhep.2002.37139. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh TH, Tseng TC, Liu CJ, Lai MY, Chen PJ, Hsieh HL, et al. Hepatitis B virus genotype B has an earlier emergence of lamivudine resistance than genotype C. Antivir Ther. 2009;14:1157–63. doi: 10.3851/IMP1454. [DOI] [PubMed] [Google Scholar]
- 9.Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, Zavitsanos X, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: A cohort study and meta-analysis. Clin Infect Dis. 2009;48:1763–71. doi: 10.1086/599110. [DOI] [PubMed] [Google Scholar]
- 10.Andersson MI, Preiser W, Van Rensburg C, Taljaard J, Hoffmann CJ. The HIV/HBV co-infected patient: Time for proactive management. S Afr Med J. 2015;105:281–2. doi: 10.7196/samj.8907. [DOI] [PubMed] [Google Scholar]
- 11.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter cohort study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 12.Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national mortalité2005 study. J Hepatol. 2009;50:736–45. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dereeper A, Audic S, Claverie JM, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol. 2010;10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akuta N, Kumada H. Influence of hepatitis B virus genotypes on the response to antiviral therapies. J Antimicrob Chemother. 2005;55:139–42. doi: 10.1093/jac/dkh533. [DOI] [PubMed] [Google Scholar]
- 16.Sirisena ND, Njoku MO, Idoko JA, Isamade E, Barau C, Jelpe D, et al. Carriage rate of hepatitis-B surface antigen (HBsAg) in an urban community in Jos, Plateau state, Nigeria. Niger Postgrad Med J. 2002;9:7–10. [PubMed] [Google Scholar]
- 17.Forbi JC, Gabadi S, Alabi R, Iperepolu HO, Pam CR, Entonu PE, et al. The role of triple infection with hepatitis B virus, hepatitis C virus, and human immunodeficiency virus (HIV) type-1 on CD4+lymphocyte levels in the highly HIV infected population of north-central Nigeria. Mem Inst Oswaldo Cruz. 2007;102:535–7. doi: 10.1590/s0074-02762007005000025. [DOI] [PubMed] [Google Scholar]
- 18.Hamza M, Samaila AA, Yakasai AM, Babashani M, Borodo MM, Habib AG. Prevalence of Hepatitis B and C virus infections among HIV infected patients in atertiary hospital in North-Western Nigeria. Niger J Bas Clin Sci. 2013;10:76–80. [Google Scholar]
- 19.Onwuliri EA, Ndako JA, Dimlong MY. Seroprevalence of hepatitis B surface antigen [HBsAg] co-infections among HIV positive individuals. Res. 2014;6(8):74–8. [Google Scholar]
- 20.Egah DZ, Banwat EB, Audu ES, Iya D, Mandong BM, Anele AA, et al. Hepatitis B surface antigen, hepatitis C and HIV antibodies in a low-risk blood donor group, Nigeria. East Mediterr Health J. 2007;13:961–6. [PubMed] [Google Scholar]
- 21.Diwe CK, Okwara EC, Enwere OO, Azike JE, Nwaimo NC. Sero-prevalence of hepatitis B virus and hepatitis C virus among HIV patients in a suburban university teaching hospital in South-East Nigeria. Pan Afr Med J. 2013;16:7. doi: 10.11604/pamj.2013.16.7.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souza MG, Passos AD, Machado AA, Figueiredo JF, Esmeraldino LE. HIV and hepatitis B virus co-infection: Prevalence and risk factors. Rev Soc Bras Med Trop. 2004;37:391–5. doi: 10.1590/s0037-86822004000500004. [DOI] [PubMed] [Google Scholar]
- 23.Mendes-Corrêa MC, Barone AA, Cavalheiro N, Tengan FM, Guastini C. Prevalence of hepatitis B and C in the sera of patients with HIV infection in São Paulo, Brazil. Revis Inst Med Trop de Sao Paulo. 2000;42:81–5. doi: 10.1590/s0036-46652000000200004. [DOI] [PubMed] [Google Scholar]
- 24.Pereira LM, Martelli CM, Merchán-Hamann E, Montarroyos UR, Braga MC, de Lima ML, et al. Population-based multicentric survey of hepatitis B infection and risk factor differences among three regions in Brazil. Am J Trop Med Hyg. 2009;81:240–7. [PubMed] [Google Scholar]
- 25.Pantazis KD, Elefsiniotis IS, Brokalaki H. New data concerning the epidemiology of hepatitis B virus infection in Greece. Gastroenterol Res Pract. 2008;2008:580341. doi: 10.1155/2008/580341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oladeinde BH, Omoregie R, Oladeinde OB. Prevalence of HIV, HBV, and HCV infections among pregnant women receiving antenatal carein a traditional birth home in Benin City, Nigeria. Saudi J Health Sci. 2013;2:113–7. [Google Scholar]
- 27.Oladeinde BH, Omoregie R, Olley M, Anunibe JA, Oladeinde OB. Hepatitis B and C viral infections among pregnant women in a rural community of Nigeria. Int J Bas Appl Virol. 2013;1:1–5. [Google Scholar]
- 28.Okocha EC, Oguejiofor OC, Odenigbo CU, Okonkwo UC, Asomugha L. Prevalence of hepatitis B surface antigen seropositivity among HIV-infected and non-infected individuals in Nnewi, Nigeria. Niger Med J. 2012;53:249–53. doi: 10.4103/0300-1652.107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eze EU, Ofili AN, Onunu AN. Prevalence of hepatitis C virus in HIV infected persons in a tertiary hospital in Nigeria. Niger J Clin Pract. 2010;13:41–6. [Google Scholar]
- 30.Nakwagala FN, Kagimu MM. Hepatitis B virus and HIV infections among patients in Mulago hospital. East Afr Med J. 2002;79:68–72. doi: 10.4314/eamj.v79i2.8903. [DOI] [PubMed] [Google Scholar]
- 31.Moussa D, Bengue AK, Sevede D, Gazoa SK, Dosso M. Molecular characterization of hepatitis B virus circulating in asymptomatic and symptomatic carriers in côte d'ivoire from 2010 to 2013. Brit Microbiol Res J. 2014;4:831–40. [Google Scholar]
- 32.Geretti AM, Patel M, Sarfo FS, Chadwick D, Verheyen J, Fraune M, et al. Detection of highly prevalent hepatitis B virus coinfection among HIV-seropositive persons in Ghana. J Clin Microbiol. 2010;48:3223–30. doi: 10.1128/JCM.02231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hønge BL, Jespersen S, Medina C, da Silva TéD, da Silva ZJ, Lewin SR, et al. Hepatitis C prevalence among HIV-infected patients in Guinea-Bissau: A descriptive cross-sectional study. Int J Infect Dis. 2014;28:35–40. doi: 10.1016/j.ijid.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Andersson MI, Maponga TG, Ijaz S, Barnes J, Theron GB, Meredith SA, et al. The epidemiology of hepatitis B virus infection in HIV-infected and HIV-uninfected pregnant women in the western cape, South Africa. Vaccine. 2013;31:5579–84. doi: 10.1016/j.vaccine.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bautista-Amorocho H, Castellanos-Domínguez YZ, Rodríguez-Villamizar LA, Velandia-Cruz SA, Becerra-Peña JA, Farfán-García AE, et al. Epidemiology, risk factors and genotypes of HBV in HIV-infected patients in the Northeast region of Colombia: High prevalence of occult hepatitis B and F3 subgenotype dominance. PLoS One. 2014;9:e114272. doi: 10.1371/journal.pone.0114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer T, Sprinzl M, Protzer U. Immune control of hepatitis B virus. Dig Dis. 2011;29:423–33. doi: 10.1159/000329809. [DOI] [PubMed] [Google Scholar]
- 37.Tajbakhsh E, Momtaz H, Momeni M, Hamedi S. Molecular detection of hepatitis B virus (HBV) among voluntary blood donor's HBsAg positive in Shahrekord, Iran. Afri J Microbiol Res. 2010;4:1419–23. [Google Scholar]
- 38.Gaeta GB, Stornaiuolo G, Precone DF, Lobello S, Chiaramonte M, Stroffolini T, et al. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J Hepatol. 2003;39:1036–41. doi: 10.1016/s0168-8278(03)00470-7. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, Yotsuyanagi H, Yatsuhashi H, Karino Y, Takikawa Y, Saito T, et al. Risk factors for long-term persistence of serum hepatitis B surface antigen following acute hepatitis B virus infection in Japanese adults. Hepatology. 2014;59:89–97. doi: 10.1002/hep.26635. [DOI] [PubMed] [Google Scholar]
- 40.Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, et al. Interactions between HIV and hepatitis B virus in homosexual men: Effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Magoro T, Gachara G, Mavhandu L, Lum E, Kimbi HK, Ndip RN, et al. Serologic and genotypic characterization of hepatitis B virus in HIV-1 infected patients from south west and littoral regions of Cameroon. Virol J. 2016;13:178. doi: 10.1186/s12985-016-0636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awan ZR, Abdul H, Khan S, Rahma SU, Rahman HM. Molecular prevalence of Hepatitis B virus infection in Khyber Pathtunkhya Pakistan. Int J Med Med Sci. 2012;4:123–7. [Google Scholar]
- 43.Liu CJ, Kao JH. Global perspective on the natural history of chronic hepatitis B: Role of hepatitis B virus genotypes A to J. Semin Liver Dis. 2013;33:97–102. doi: 10.1055/s-0033-1345716. [DOI] [PubMed] [Google Scholar]
- 44.Mulders MN, Venard V, Njayou M, Edorh AP, Bola Oyefolu AO, Kehinde MO, et al. Low genetic diversity despite hyperendemicity of hepatitis B virus genotype E throughout West Africa. J Infect Dis. 2004;190:400–8. doi: 10.1086/421502. [DOI] [PubMed] [Google Scholar]
- 45.Kramvis A, Restorp K, Norder H, Botha JF, Magnius LO, Kew MC, et al. Full genome analysis of hepatitis B virus genotype E strains from South-Western Africa and Madagascar reveals low genetic variability. J Med Virol. 2005;77:47–52. doi: 10.1002/jmv.20412. [DOI] [PubMed] [Google Scholar]
- 46.Lacombe K, Massari V, Girard P, Lawrence S, Joël G, Gilles P, et al. Major role of hepatitis B genotypes in liver fibrosis in HIV–HBV co-infection. AIDS. 2005;20:419–27. doi: 10.1097/01.aids.0000200537.86984.0e. [DOI] [PubMed] [Google Scholar]
- 47.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–60. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 48.Jaspe RC, Sulbarán YF, Loureiro CL, Martínez N, Devesa M, Rodríguez Y, et al. Genetic diversity of hepatitis B virus and hepatitis C virus in human immunodeficiency virus type 1-co-infected patients from Venezuela. J Med Microbiol. 2014;63:1099–104. doi: 10.1099/jmm.0.067496-0. [DOI] [PubMed] [Google Scholar]


