Highlights
-
•
Determining normal tissue tolerance of organs at risk is of utmost importance for the success of carbon ion radiotherapy (CIRT).
-
•
This manuscript is the first report on experimental RBE for development of lung fibrosis assessed by quantitative CT after fractionated CIRT.
-
•
We further propose RBE estimation by integrating RBEmax and α/βL based modified high-LET BED model.
-
•
This model was found to well predict RBE but is sensitive to the uncertainties of α/βL estimates from the reference photon irradiation.
-
•
Our findings will contribute to a more precise knowledge of RBE as well as the fractionated dose effects of carbon ion therapy on normal lung tissue.
Abbreviations: RBE, relative biological effectiveness; LET, linear energy transfer; BED, biologically effective dose; CT, computed tomography; RILF, Radiation-induced lung fibrosis; RP, radiation pneumonitis; NSCLC, non-small cell lung cancer; SBRT or SABR, hypofractionated stereotactic body or ablative radiation therapy; V5, volume of lung receiving ≥5 Gy (RBE); PMMA, Polymethylmethacrylat; FI, fibrosis index; HU, Hounsfield unit; CPFE, combined pulmonary fibrosis and emphysema syndrome; α/β, alpha/beta ratio; LQ model, linear quadratic model
Keywords: Relative biological effectiveness (RBE), Carbon ion radiotherapy (CIRT), Lung fibrosis, Fractionation, High-linear energy transfer (high-LET), Biologically effective dose (BED), Normal tissue response
Abstract
Background and purposes
Carbon ion radiotherapy (CIRT) with raster scanning technology is a promising treatment for lung cancer and thoracic malignancies. Determining normal tissue tolerance of organs at risk is of utmost importance for the success of CIRT. Here we report the relative biological effectiveness (RBE) of CIRT as a function of dose and fractionation for development of pulmonary fibrosis using well established fibrosis index (FI) model.
Materials and Methods
Dose series of fractionated clinical quality CIRT versus conventional photon irradiation to the whole thorax were compared in C57BL6 mice. Quantitative assessment of pulmonary fibrosis was performed by applying the FI to computed tomography (CT) data acquired 24-weeks post irradiation. RBE was calculated as the ratio of photon to CIRT dose required for the same level of FI. Further RBE predictions were performed using the derived equation from high-linear energy transfer biologically effective dose (high-LET BED) model.
Results
The averaged lung fibrosis RBE of 5-fraction CIRT schedule was determined as 2.75 ± 0.55. The RBE estimate at the half maximum effective dose (RBEED50) was estimated at 2.82 for clinically relevant fractional sizes of 1–6 Gy. At the same dose range, an RBE value of 2.81 ± 0.40 was predicted by the high-LET BED model. The converted biologically effective dose (BED) of CIRT for induction of half maximum FI (BEDED50) was identified to be 58.12 Gy3.95. In accordance, an estimated RBE of 2.88 was obtained at the BEDED50 level. The LQ model radiosensitivity parameters for 5-fraction was obtained as αH = 0.3030 ± 0.0037 Gy−1 and βH = 0.0056 ± 0.0007 Gy−2.
Conclusion
This is the first report of RBE estimation for CIRT with the endpoint of pulmonary fibrosis in-vivo. We proposed in present study a novel way to mathematically modeling RBE by integrating RBEmax and α/βL based on conventional high-LET BED conception. This model well predicted RBE in the clinically relevant dose range but is sensitive to the uncertainties of α/β estimates from the reference photon irradiation (α/βL). These findings will assist to eliminate current uncertainties in prediction of CIRT induced normal tissue complications and builds a solid foundation for development of more accurate in-vivo data driven RBE estimates.
1. Introduction
Charged particle therapy has emerged as a promising treatment for a growing number of malignancies [1], [2], [3]. This field is rapidly growing and a number of heavy ions therapy facilities are expected to be installed worldwide [4]. Owing to the physical and radiobiological advantageous, carbon ion radiotherapy (CIRT) is particularly appealing for treating radio-resistant, hypoxic cancers [5], i.e., non-small cell lung cancer (NSCLC) [6], [7]. For early stage NSCLC, Miyamoto et al. reported a 5-year local control (LC) rate and overall survival (OS) of 50.0% and 94.7% following a hypofractionated regimen of CIRT [8]; a 5-year LC and OS of 90% and 45% by a 1-week regimen [9], and of 95.8% and 30.7% for patients aged 80 + year-old [10]. A recent study showed also the promise of CIRT for locally advanced NSCLC (stage II-III), with the 2-year LC and OS were 93.1%, and 51.9%, respectively [11].
The enhanced relative biological effectiveness (RBE) for tumor eradication by high linear energy transfer (LET) particle beams may be also accompanied with an increased risk for normal tissue complications. The high radiosensitivity of the lung constitutes a critical dose-limiting factor in radiation treatment of thoracic tumors [12], [13]. A variety of pulmonary reactions can be observed after CIRT, e.g., radiographic lung damage, pleural reactions, pneumonitis or fibrosis [14], [15]. This was also evident in preclinical models after whole body irradiation with plateau level LETs (∼14.55 keV/μm) [16]. Studies by Hayashi et al. reported that lung volume received a dose as low as 5 Gy (V5) was able to predict ≥ grade 2 radiation pneumonitis (RP) in patients treated with CIRT for locally advanced NSCLC [14]. The conception of RBE allows the comparison of CIRT with conventional photon (X-ray) irradiation at an iso-effective endpoint [17]. In order to improve the clinical safety and effectiveness, a better understanding of RBE for CIRT effects on normal tissue is an important area of research [18], [19].
The biological effects of high precision raster scanning particle therapy with carbon-ions (12C-ions) in the lung are not well characterized. Especially, considering that RBE for heavy ions depends on a variety of physical (i.e., LETs, dose level) and biological (i.e., tissue types, different endpoints) properties [20]. No data are available on RBE of clinical quality CIRT with respect to normal lung tissue response in vivo. Current uncertainties in RBE values for CIRT need to be addressed urgently by preclinical models [21]. On the basis of our recently established non-invasive CT based fibrosis index (FI) model [22], the present study aimed to determine the RBE of fractionated carbon-ions in development of late lung toxicity, within a clinically relevant fraction size of 1–6 Gy. The capacity and reliability of high-LET biologically effective dose (BED) model in prediction of RBE was further investigated. This work was conducted in frame of German Research Foundation (DFG) “clinical research group heavy ion therapy (KFO-214)” in collaboration between the project TP5 and the central platform (ZP1).
2. Materials and methods
2.1. Irradiation and animals
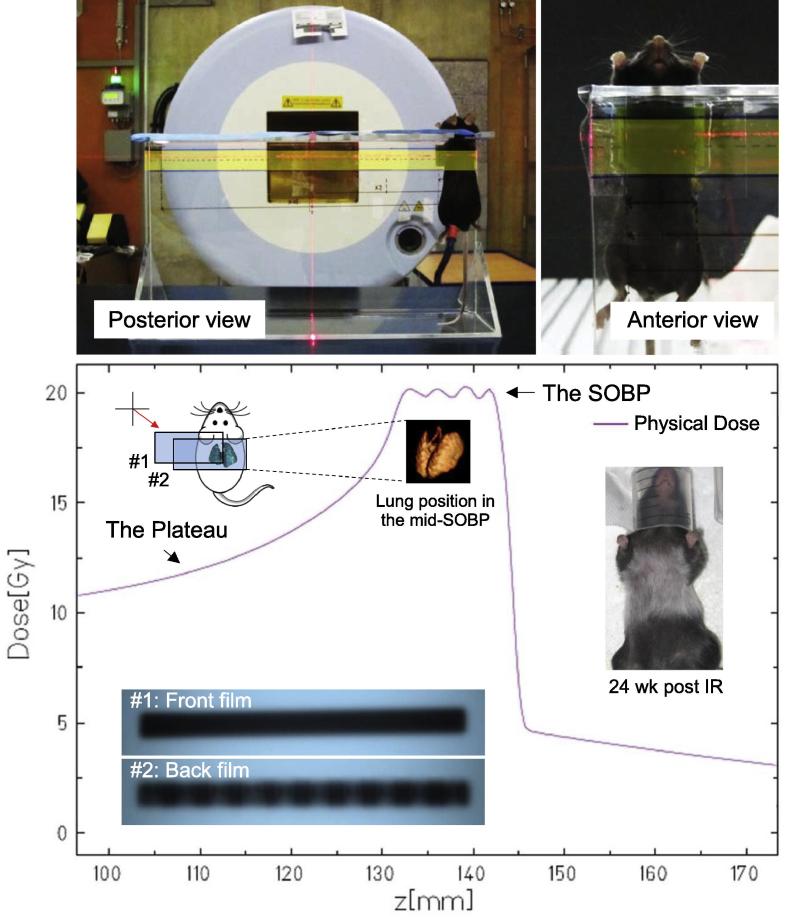
Female C57BL/6 mice (Charles River Breeding Laboratories, MA) aged between 8 and 10 weeks were irradiated with carbon-ions or photons beams in the region of the thorax. Particle irradiations were performed at the Heidelberg Ion-Beam Therapy Center (HIT). Carbon-ions (12C-ions) were applied at the spread-out Bragg peak (SOBP, 252.400–270.550 MeV/u, the width of the SOBP = 23 mm) with linear energy transfer (LET) = 70–157 keV/µm (mean at 86 keV/µm) to the thorax of mice. The detailed setup for particle irradiation is provided (Fig. 1). Briefly, ten anesthetized mice were placed in a specially constructed Polymethylmethacrylat (PMMA) holder for immobilization and irradiated simultaneously. The particle dosing in lung was homogenous as verified by the entrance and exit filmes (Kodak EDR2). There were two independent irradiation arms: the five fractions (5-fx) arm of: 0, 1, 2, 3 and 4 Gy per fraction, for consecutive 5 days; the single fraction (1-fx) of: 0, 10.5, 12.5, 14.5, 17.5 and 20 Gy. Each dose group contained 12 randomly grouped mice.
Fig. 1.
The setup for precision mice thorax irradiation by high-LET carbon-ions irradiation. Mouse was immobilized in a specially designed polymethylmethacrylate (PMMA) holder for whole thoracic irradiation. The beam field was shown as highlighted rectangle in yellow color. The delivered dose to the lung was at the spread-out Bragg peak (SOBP) of carbon-ions as demonstrated by the experimental sketch. The homogenous and conformal particle dosing in the lung was verified by the entrance and exit films with rare scattering doses. The back film (#2) also evidenced that the lung was entirely covered with carbon-ions independent of breath motions. The irradiated region was eventually evidenced with white hair at the endpoint of 24 weeks, which was in consistent to the treatment plan.
The reference photon irradiation data was utilized from our previous report [22]. In brief, each mouse received whole thoracic X-ray irradiation delivered by a 6 MeV Artist Linac (Siemens, Germany) at a dose rate of 3 Gy/min. Dosimetry was used to confirm the dose uniformity in advance. Prior to thoracic irradiation, mice were anaesthetized by an intraperitoneal application of 0.36 ml/kg Rompun 2% (Bayer HealthCare) and 0.54 ml/kg ketamine 10% (Pfizer). The doses for photons irradiation arm were 0, 2, 4, 6, 7, 8.5 Gy per fraction for a total of 5 fractions. All animal work was approved and performed in compliance with rules outlined by the local and governmental animal care committee instituted by the German government (Regierungspraesidium, Karlsruhe).
2.2. Assessment of lung fibrosis by computed tomography (CT)
Lung fibrosis was measured by quantitative CT imaging at the endpoint of 24 weeks post irradiation. The detailed parameters for low-dose CT scanning has been reported previously [22]. CT images were reconstructed and analyzed with Medical Imaging Interaction Toolkit software (MITK, Heidelberg) and OsiriX Imaging Software (OsiriX v.3.9.4, Switzerland). The three-dimensional (3D) analysis of CT data as well as the differential diagnosis of combined pulmonary fibrosis and emphysema syndrome (CPFE) was also provided elsewhere [22]. In brief, the fibrosis index was employed to assess the extent of fibrosis as the major endpoint. The FI model is based on two critical parameters derived from CT segmented data: the relative increase in mean lung density (ΔHU) and decreased lung volume (ΔV) when compared to the mean of an age-matched reference mice cohort. Biologically, the augmented ΔHU is an overall representation of collagen deposition and increased cellularity; whereas ΔV reflects the nature of fibrosis as a constrictive lung disease. The extent of fibrosis was determined quantitatively by a fibrosis index based on averaged quantities of ΔHU and ΔV from the cohort as:
| (1) |
2.3. Dose-response and RBE modeling
The dose-response relationship between fibrosis development (fibrosis index) and irradiated doses were obtained based on FI-model as previously reported [22]. In brief, both carbon ions and photons irradiated arms were fitted by a modified probit model derived from Kallman et al. [23] using OriginPro 8.0 and Mathematica Software 9.0.
| (2) |
where A is the saturation constant for maximal development of fibrosis measured experimentally to be 7.20 (equal to 100% fibrosis), serving to quantize all FIs. γ is the maximum value of the normalized dose-response gradient. In this deterministic model, ED50 is interpreted as the dose where the whole population experiences an average 50% increase of the FI (FI = 3.60) relative to maximum possible effect (FI = 7.20).
Charged particle beams deposit intensified energy in a more localized form compared to megavoltage X-rays. The biological consequences of this are determined in terms of relative biological effectiveness (RBE). RBE is defined as the ratio of absorbed dose of a reference beam of photons to the absorbed dose of any other high-LET radiation, resulting in an identical effect [17]. In present study, the iso-effect is considered as the same level of fibrosis index achieved by paired fractionated doses from photons (dL) versus carbon-ions (dH) for RBE estimation (the subscripts L and H refer to low- and high- LET radiations respectively).
2.4. RBE prediction by high-LET BED model
According to the linear-quadratic (LQ) model, radiation effect (E) from low-LET radiation can be expressed as:
| (3) |
where is the number of fractions and is the fractionated dose. In high-LET radiation the resultant biological effect (E) is dominated by the linear term (α-coefficient of cell kill, ) rather than the quadratic compartment () [24]. Thus the value might be considered as a negligible level (β-coefficient does not alter very much) and the expression for EL becomes:
| (4) |
To achieve iso-effective biological effects Eqs. (3) and (4) are equated as:
| (5) |
leading to:
| (6) |
The maximized RBE () can be achieved at very low dose level as [25]:
| (7) |
Giving the definition of RBE, using Eqs. (6) and (7) we have the predicted RBE as:
| (8) |
Provided that the low-LET α/β values for both single- and five- fractions photons irradiation on normal lung tissue are calculated [22], RBE may be therefore determined based on and .
2.5. Fibrosis development and BEDH
An asymptotic minimum value of RBE () can be approached at extremely high fractional doses [25]. Refer to this dose range, the biological effect (E) for individual low- or high-LET fractions is determined by the quadratic compartment (β-coefficient alters with changing LET). Hence, for an iso-effect between high-LET carbon-ions and low-LET photons, and are considered to be equal. Herein, it gives rise to the as [26]:
| (9) |
According to the conception of biologically effective dose (BED) [27], we have:
| (10) |
By substitutions from Eqs. (7) and (9) into Eq. (10), the BED for high-LET radiation can be derived as [25]:
| (11) |
The fibrosis index was also simulated as a function of BEDH doses by sigmoidal fitting as:
| (12) |
where ED50 of BEDH doses (BEDED50) was derived as the parameter .
2.6. LQ modeling of fibrosis dose-response data
To estimating the radiosensitivity coefficients of fractionated carbon ions, the FI data was transformed with the natural logarithm (exp(-FI)) and fitted into the linear-quadratic (LQ) model [28]. Briefly, the fibrosis index (FI) representing delta degree of fibrosis vs. non-irradiated control lung and was inverted to represent the relative fraction of “healthy lung tissue” in analogy to the survival fraction. The curves were generated by an exponential function where the inverted fibrosis index (-FI) values were plotted against “total dose” and fitted into the equation of LQ model as:
| (13) |
where the “survival fraction” was replaced by “healthy lung tissue fraction”. Lungs irradiated with 0 Gy C-ions or photons developed no pulmonary fibrosis (FI = 0), corresponding to the coordinate (0, 0) in Fig. 2. Accordingly, in the context of LQ modeling, applying natural logarithm of 0 results to 1 (exp(0) = 1). A coordinate at (0, 1) thus stands for no fibrosis was measured at the 0 Gy irradiated lung. The Y-value (exp(-FI)) decreases corresponding to the loss of functional lung tissue with dose-dependent induction of fibrosis (X-value increase) with the exponential decay function of LQ model. The reference photon data (1- and 5- fractions) was published in our previous report as the basis for dose-response modeling of carbon ion effects [22]. All data in the present study is presented as mean ± SD.
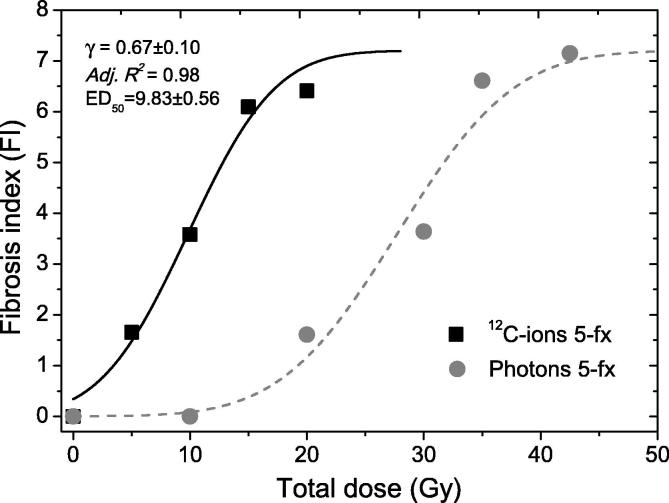
Fig. 2.
Dose-response curves for induction of pulmonary fibrosis surrogated by fibrosis index (FI) after five fractions of carbon-ions (12C-ions) versus photons irradiation. The sigmoidal relationships were plotted based on FI-model (for 5-fractionated carbon-ions: γ = 0.67 ± 0.10, Adjusted R2 = 0.98, ED50 = 9.83 ± 0.56). C-ions is shown as black squares and photons beam is in gray circles.
3. Result
3.1. Dose-response curves and fibrosis ED50
The dose-response curves of 5-fractionated carbon-ions versus photons using FI-model is shown (Fig. 2). The radiation effect curve of carbon-ions (γ = 0.67 ± 0.1, Adj. r2 = 0.98) was clearly left-shifted compared to photon fractionation. The fibrosis ED50 (effective dose for 50% fibrosis or FI = 3.60) for fractionated carbon-ions was as low as 9.83 ± 0.34 Gy, in comparison with the previously reported 27.7 ± 1.22 Gy for photons [22]. This indicated a substantial enhancement of normal lung tissue radiosensitivity to carbon-ions.
3.2. Experimentally derived RBEs
To investigate the variation of RBE values with different fractional carbon-ions dose, experimentally derived RBE was estimated (Fig. 3). Within the clinically relevant dose range of 1–6 Gy per fraction (in total of 5–30 Gy), the RBE was determined as 2.75 ± 0.55. Further, the estimated value of RBE at the ED50 level was 2.82; whereas the RBE after 2 Gy fractionated dose was 2.80.
Fig. 3.
Comparison of high-LET BED based RBE simulations with experimental data. RBE values derived by High-LET BED Model using α/βL = 3.95 from fractionated photon irradiation (5-fx, green curve) correlated particularly well with experimental data (black curve) at dose range 1–6 Gy. Experimentally derived RBE is shown as black squares, high-LET BED predicted RBE using α/βL from 1- and 5- fractionated low-LET photons shown as blue circles and green triangles, respectively.
3.3. Comparison of RBE prediction based on High-LET BED model versus experimental data
Predictions of RBE using derived equation from high-LET biologically effective dose (BED) model were also carried out (Fig. 3). First, α/β ratio from single fraction photon reference irradiation (α/βL = 4.49 [22]) was utilized for simulation of RBE based on the High-LET BED model (Fig. 3, blue curve). The averaged RBE was hence obtained as 2.94 ± 0.41. Next, an α/βL of 3.95 from five-fraction photon irradiations [22] was employed (Fig. 3, green curve) and the averaged RBE was determined as 2.81 ± 0.40. Within the fractional dose range of 1–6 Gy the estimated RBE profiles by high-LET BED model correlated well with experimentally obtained RBEs (Fig. 3, black curve). RBE simulations using 5-fx specific α/βL were closer to the experimental RBE profiles, indicating that high-LET BED based RBE prediction is sensitive to precise estimation of the α/βL value.
3.4. BEDH dose-response of fibrosis development
In order to study dosimetric dependency for fibrotic responses, the fractionated carbon ion doses were converted to biologically effective doses (BEDH) using α/βL of 3.95. The dose-response relationship between fibrosis development and BEDH is shown (Fig. 4). The BED dose at ED50 (BEDED50) was identified to be 58.12 Gy3.95. On the other hand, the value of RBE tended to reduce with BEDH. In accordance with previous results, an estimated RBE of 2.88 was obtained at the BEDED50 level. The dependency of RBEs on BEDH was also provided (Table 1).
Fig. 4.
The dose-response curve of biologically effective dose (BEDH) converted from high-LET carbon-ions in development of pulmonary fibrosis (shown as black squares). The dose-dependency of RBE with reference to increasing BEDH was also revealed (shown as red circles). BEDH for carbon-ions resulting in half maximum fibrosis (BEDED50) was estimated as 58.12 Gy; whereas RBE at this BEDED50 level was estimated as 2.88.
Table 1.
The dose-dependency of RBEs with reference to BEDH. Estimated RBEs from different methods, i.e., experimental derived, high-LET predictions based on a 1-fx α/βL = 4.49 Gy−1 or 5-fx α/βL = 3.95 Gy−1 or are listed.
| BEDH | Dose per fraction | Total dose | RBE |
||
|---|---|---|---|---|---|
| (Gy) | (dH, Gy/fx) | (D, Gy) | Exp. derived | α/βL = 3.95 Gy−1 | α/βL = 4.49 Gy−1 |
| 25.50 | 1.00 | 5.00 | 4.26 | 3.70 | 3.86 |
| 33.19 | 1.25 | 6.25 | 3.67 | 3.43 | 3.59 |
| 41.38 | 1.50 | 7.50 | 3.28 | 3.22 | 3.37 |
| 50.12 | 1.75 | 8.75 | 3.01 | 3.05 | 3.19 |
| 59.38 | 2.00 | 10.00 | 2.80 | 2.90 | 3.04 |
| 69.17 | 2.25 | 11.25 | 2.63 | 2.78 | 2.91 |
| 79.48 | 2.50 | 12.50 | 2.50 | 2.67 | 2.80 |
| 90.31 | 2.75 | 13.75 | 2.40 | 2.57 | 2.70 |
| 101.68 | 3.00 | 15.00 | 2.31 | 2.48 | 2.61 |
| 113.58 | 3.25 | 16.25 | 2.23 | 2.41 | 2.53 |
| 126.00 | 3.50 | 17.50 | 2.17 | 2.33 | 2.46 |
| 138.93 | 3.75 | 18.75 | 2.11 | 2.27 | 2.39 |
| 152.40 | 4.00 | 20.00 | 2.07 | 2.21 | 2.33 |
| 166.38 | 4.25 | 21.25 | 2.02 | 2.16 | 2.27 |
| 181.03 | 4.50 | 22.50 | 1.99 | 2.11 | 2.22 |
| 196.12 | 4.75 | 23.75 | 1.95 | 2.06 | 2.17 |
| 211.94 | 5.00 | 25.00 | 1.92 | 2.02 | 2.13 |
| 227.97 | 5.26 | 26.30 | 1.89 | 1.98 | 2.08 |
| 246.14 | 5.53 | 27.65 | 1.86 | 1.93 | 2.04 |
| 263.05 | 5.77 | 28.85 | 1.84 | 1.90 | 2.00 |
| 280.04 | 6.01 | 30.05 | 1.82 | 1.87 | 1.97 |
3.5. Estimation of α-, β-coefficients from LQ model
To study carbon-ions dose and fractionation effects on the normal lung tissue, fibrosis index data was further fitted into linear-quadratic (LQ) model (Fig. 5). Substantial difference in lung tissue radiosensitivity was demonstrated in 5-fractionated carbon-ions in comparison to 5-fractionated photons. The estimated values for 5-fraction carbon-ions were determined as of αH = 0.3030 ± 0.0037 Gy−1 and βH = 0.0056 ± 0.0007 Gy−2.
Fig. 5.
The linear-quadratic (LQ) views of five fractions of carbon-ions effect on normal lung tissue compared to five-fractions photons as reported previously. The negative natural logarithm transformed FI values are plotted against a function of the total prescribed doses. The observed biological effects of carbon-ions differed prominently from photons, indicating a significantly intensified role on normal lung tissue. Five-fraction carbon-ions doses are shown as solid squares; the gray solid circles for five fractionated photons as a reference. The reference photons 5-fraction data were from the previous report [22] and therefore shown in in gray dash lines.
4. Discussion
Carbon ion radiotherapy (CIRT) is an emerging treatment for many malignancies [4], [29]. Current clinical evidence indicates that CIRT has possible advantages over state-of-the-art photon or proton therapy in radio-resistant tumors [4]. Nevertheless, the potential advantageous of the high relative biological effectiveness (RBE) of CIRT to ablate tumors must be weighed against the potential risk of increased late normal tissue damage [21]. The prescribed CIRT dose to patients is converted from the photon physical dose by using an RBE weighting factor leading to GyRBE or Gy Equivalent dose estimates [30]. If RBE is inaccurately assigned to different tissues and tumors, there will be an inevitable under- or over- dosage to the patients [31]. Current uncertainties in RBE values for CIRT need to be urgently addressed by comparing dose effects on normal tissues treated with particle beams versus photons. By integration into treatment planning algorithms, these in-vivo data driven RBE can be further tested in prospective clinical trials and eventually facilitate precision CIRT [21].
Lung is widely considered as a dose-limiting normal tissue in radiotherapy [12], [32]. In particular, breath motions during the treatment may expose normal tissue to the high-LET therapeutic dose component. A better understanding of the effects of high-LET radiation on this tissue type is therefore essential for the optimized application of carbon ion in thoracic radiotherapy. However, studies in lung RBE so far are based mainly on in vitro assays of mammalian cell lines, with a focus on clonogenic survival capacity or cell inactivation. RBE derived from those cellular parameters should be used with caution for clinical endpoints, since in vitro readouts may not fully represent the tissue response and additional uncertainties may therefore occur [30]. To our knowledge, the present study is among the first to determine RBE of carbon-ions on the lung tissue in vivo. The biological fibrosis endpoint fibrosis is of clinical interest and was obtained at the late timepoint of 24 weeks following fractionated irradiation. It mimicked the inflammatory development and progressive tissue remodeling as observed in patients with chronic normal tissue response to radiotherapy. The investigated dose range in the present study is also relevant to clinical routine with doses per fraction in the range of 1–6 Gy.
The lung tissue tolerance is elevated when exposed to fractionated megavoltage radiotherapy as evidenced preclinically e.g. by our previous study [22] or clinically from a series of important reports [33], [34], [35], [36], [37]. The radiobiological rationales behind this phenomenon are well recognized as a substantial amount of dose recovery (e.g., due to repair of sub-lethal damage and repopulation) occurs during a protracted treatment period, such as photon based treatments over 6–7 weeks [32]. However, the conditions for high-LET radiation carbon ions are quite different due to the higher radiosensitivity and reduced repair capacity, as well as the shorter treatment times. Our data implies that the lung recovery capacity to carbon-ions is remarkably lower and little to no normal tissue sparing effects are found after fractionated doses. These findings, at least to some extent, support the use of hypofractionated carbon ion to non-small cell lung cancer (NSCLC) patients as administered in NIRS [4], [38], [39] and HIT [40].
The precise prediction of RBE for carbon ion by a reliable biological model is yet to be realized. The situation is complex since RBE varies with LET, tissues type, dose per fraction, different endpoints, etc. A direct comparison of RBE between experimentally derived and high-LET BED model predicted values was performed in this study. Our data reveal that high-LET BED model underestimates the RBE effect at the very low dose range (<1 Gy per fraction). Good prediction of RBE is found within the relevant dose range (1–6 Gy per fraction). Using the 5-fraction specific α/β ratio, an estimated RBE of 2.81 ± 0.40 was found in an agreement with the experimental readout (2.75 ± 0.55). While noticeable, the accuracy of high-LET BED based RBE prediction tends to rely considerably on the precise value of α/β ratio. Variations may occur if incorrect allocation of an α/β ratio are used in the calculation. Instead of using absorbed dose, it is of practical expediency to specify the carbon-ion radiation tolerances of normal tissues [41], for example, in terms of BEDs. For this purpose, the converted BEDH dose dependency of fibrosis development was investigated in this study, indicating a marginally smaller BEDED50 of 58.12 Gy compared to 61.63 Gy from conventional photon irradiation [22]. To facilitate these findings for further preclinical or clinical evaluation, all estimated values of RBEs with reference to carbon-ion BEDH are provided (Table 1).
Potential limitations have to be appreciated in the present study. The modeling of RBE was based on the implementation of non-invasive CT scanning and proposed fibrosis index algorithm. A comprehensive scoring criterion integrating not only radiologic, but also functional, histopathological and molecular assessments to define a sophisticated tissue response may lead to a more accurate and reliable RBE calculation. It is also of particular attention that the organ interactions after concomitant irradiation of the heart might reduce the tolerance of the lung to carbon-ions [42], [43]. The dose-fractionation schemes for both carbon-ions and photon irradiation included in this study are limited. Uncertainties may lie in the very low- and/or high- dose range for RBE estimation. The prediction range and accuracy can be further improved if more fractionated regimens are applied in the future studies. Finally, investigations in different animal models varying in sensitivity for development of fibrosis and most ideally comparison of data driven RBE models with prospective clinical data would be of high relevance.
With the increasing availability of heavy ion therapy for a wide range of malignancies, the biological characterization of cellular and tissue responses to carbon-ions and conventional photon irradiation is urgently needed [44], [45], [46], [47]. The current study utilized a well-established preclinical radiation induced lung fibrosis model combined with a quantitative CT-based Fibrosis Index (FI) as the endpoint for estimation of RBE. The averaged RBE was determined experimentally as 2.75 ± 0.55, whereas the RBEED50 was estimated at 2.82 within a clinically relevant fraction size of 1–6 Gy. Taking the advantage of the high-LET BED conception, we proposed in present study a novel way to modeling RBE by integrating RBEmax and α/βL (refer to Eq. (9)). Based on this model, averaged RBE was predicted as 2.81 ± 0.40, which is in a good agreement with experimental observations. The high-LET BED model is evidenced to predict robustly the RBEs in the same dose range. However, uncertainties in estimation of the α/βL impacts the precision of high-LET BED based RBE prediction. Together, our findings will contribute to the precise knowledge of RBE as well as the fractionated dose effects of carbon ion therapy on normal lung tissue.
5. The conflict of interest statement
The authors declare no conflict of interests.
Funding
This work was supported by German Research Council (DFG-KFO214, TP5 and ZP1). It was in part also supported by the Deutsche Krebshilfe (Max-Eder 108876), intramural grants from National Center for Tumor diseases (NCT 3.0–2015.22 BioDose and DKTK/DKFZ) and the NSFC (No. 81703166/H2201). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Ethics approval and consent to participate
All animal work was approved and performed in compliance with rules outlined by the local and governmental animal care committee instituted by the German government (Regierungspraesidium, Karlsruhe).
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CZ and AA designed the study and wrote the manuscript. CZ and MM performed the animal experiments. CZ, MM, CS and YD collected, analyzed and interpreted the experimental data. CZ and LJC performed CT data analysis. SB did carbon-ions treatment planning. CZ, BY and BJ did the mathematical modeling. BJ, AM, MC, OJ, LHC and JD gave important intellectual input and carefully revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Barbara Schwager, Claudia Rittmüller and Christine Schmidt for their excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2018.10.005.
Contributor Information
Cheng Zhou, Email: c.zhou@dkfz.de.
Amir Abdollahi, Email: a.amir@dkfz.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Suit H., DeLaney T., Goldberg S., Paganetti H., Clasie B., Gerweck L. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiotherapy Oncol J Eur Soc Therap Radiol Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Schulz-Ertner D., Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol Official J Am Soc Clin Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 3.Tsujii H., Mizoe J., Kamada T., Baba M., Tsuji H., Kato H. Clinical results of carbon ion radiotherapy at NIRS. J Radiation Res. 2007;48:A1–A13. doi: 10.1269/jrr.48.a1. [DOI] [PubMed] [Google Scholar]
- 4.Kamada T., Tsujii H., Blakely E.A., Debus J., De Neve W., Durante M. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 5.Klein C., Dokic I., Mairani A., Mein S., Brons S., Haring P. Overcoming hypoxia-induced tumor radioresistance in non-small cell lung cancer by targeting DNA-dependent protein kinase in combination with carbon ion irradiation. Radiat Oncol. 2017;12:208. doi: 10.1186/s13014-017-0939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pijls-Johannesma M., Grutters J.P., Lambin P., Ruysscher D.D. Particle therapy in lung cancer: where do we stand? Cancer Treat Rev. 2008;34:259–267. doi: 10.1016/j.ctrv.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Dai Y., Wei Q., Schwager C., Hanne J., Zhou C., Herfarth K. Oncogene addiction and radiation oncology: effect of radiotherapy with photons and carbon ions in ALK-EML4 translocated NSCLC. Radiat Oncol. 2018;13:1. doi: 10.1186/s13014-017-0947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto T., Baba M., Yamamoto N., Koto M., Sugawara T., Yashiro T. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–758. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto T., Baba M., Sugane T., Nakajima M., Yashiro T., Kagei K. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–926. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]
- 10.Sugane T., Baba M., Imai R., Nakajima M., Yamamoto N., Miyamoto T. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer. 2009;64:45–50. doi: 10.1016/j.lungcan.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi W., Nakajima M., Yamamoto N., Yamashita H., Nakagawa K., Miyamoto T. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC) Cancer. 2015;121:1321–1327. doi: 10.1002/cncr.29195. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi A., Li M., Ping G., Plathow C., Domhan S., Kiessling F. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graves P.R., Siddiqui F., Anscher M.S., Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Seminars Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K., Yamamoto N., Karube M., Nakajima M., Matsufuji N., Tsuji H. Prognostic analysis of radiation pneumonitis: carbon-ion radiotherapy in patients with locally advanced lung cancer. Radiat Oncol. 2017;12:91. doi: 10.1186/s13014-017-0830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura H., Miyamoto T., Yamamoto N., Koto M., Sugimura K., Tsujii H. Radiographic pulmonary and pleural changes after carbon ion irradiation. Int J Radiat Oncol*Biol*Phys. 2003;55:861–866. doi: 10.1016/s0360-3016(02)04495-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z., Wang X., Yang R., Liu Y., Zhao W., Si J. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Exp Ther Med. 2013;5:771–776. doi: 10.3892/etm.2013.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Technical reports series No. 461. Relative Biological Effectiveness in Ion Beam Therapy. Jointly sponsored by the IAEA and ICRU. 2008
- 18.Sorensen B.S., Horsman M.R., Alsner J., Overgaard J., Durante M., Scholz M. Relative biological effectiveness of carbon ions for tumor control, acute skin damage and late radiation-induced fibrosis in a mouse model. Acta Oncol. 2015;54:1623–1630. doi: 10.3109/0284186X.2015.1069890. [DOI] [PubMed] [Google Scholar]
- 19.Wambersie A. RBE, reference RBE and clinical RBE: applications of these concepts in hadron therapy. Strahlenther Onkol. 1999;175(Suppl 2):39–43. doi: 10.1007/BF03038886. [DOI] [PubMed] [Google Scholar]
- 20.Schulz-Ertner D., Jakel O., Schlegel W. Radiation therapy with charged particles. Seminars Radiat Oncol. 2006;16:249–259. doi: 10.1016/j.semradonc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Baumann M., Krause M., Overgaard J., Debus J., Bentzen S.M., Daartz J. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C., Jones B., Moustafa M., Schwager C., Bauer J., Yang B. Quantitative assessment of radiation dose and fractionation effects on normal tissue by utilizing a novel lung fibrosis index model. Radiat Oncol. 2017;12:172. doi: 10.1186/s13014-017-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kallman P., Agren A., Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol. 1992;62:249–262. doi: 10.1080/09553009214552071. [DOI] [PubMed] [Google Scholar]
- 24.Dale R.G., Jones B. The assessment of RBE effects using the concept of biologically effective dose. Int J Radiat Oncol. 1999;43:639–645. doi: 10.1016/s0360-3016(98)00364-2. [DOI] [PubMed] [Google Scholar]
- 25.Jones B., Carabe-Fernandez A., Dale R.G. Calculation of high-LET radiotherapy dose required for compensation of overall treatment time extensions. British J Radiol. 2006;79:254–257. doi: 10.1259/bjr/49977661. [DOI] [PubMed] [Google Scholar]
- 26.Carabe-Fernandez A., Dale R.G., Jones B. The incorporation of the concept of minimum RBE (RBEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int J Radiat Biol. 2007;83:27–39. doi: 10.1080/09553000601087176. [DOI] [PubMed] [Google Scholar]
- 27.Jones B., Dale R.G., Deehan C., Hopkins K.I., Morgan D.A. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol. 2001;13:71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- 28.Barendsen G.W. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8:1981–1997. doi: 10.1016/0360-3016(82)90459-x. [DOI] [PubMed] [Google Scholar]
- 29.Combs S.E., Debus J. Treatment with heavy charged particles: systematic review of clinical data and current clinical (comparative) trials. Acta Oncol. 2013;52:1272–1286. doi: 10.3109/0284186X.2013.818254. [DOI] [PubMed] [Google Scholar]
- 30.Karger C.P., Peschke P. RBE and related modeling in carbon-ion therapy. Phys Med Biol. 2017 doi: 10.1088/1361-6560/aa9102. [DOI] [PubMed] [Google Scholar]
- 31.Jones B. Why RBE must be a variable and not a constant in proton therapy. British J Radiol. 2016;89:20160116. doi: 10.1259/bjr.20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benveniste M.F., Welsh J., Godoy M.C., Betancourt S.L., Mawlawi O.R., Munden R.F. New era of radiotherapy: an update in radiation-induced lung disease. Clin Radiol. 2013;68:e275–e290. doi: 10.1016/j.crad.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox J.D. Fractionation: a paradigm for clinical research in radiation oncology. Int J Radiat Oncol Biol Phys. 1987;13:1271–1281. doi: 10.1016/0360-3016(87)90215-x. [DOI] [PubMed] [Google Scholar]
- 34.Tsujino K., Hirota S., Kotani Y., Kado T., Yoden E., Fujii O. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: Dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006;64:1100–1105. doi: 10.1016/j.ijrobp.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins P., D’Amico K., Benstead K., Elyan S. Radiation pneumonitis following treatment of non–small-cell lung cancer with continuous hyperfractionated accelerated radiotherapy (CHART) Int J Radiat Oncol*Biol*Phys. 2003;56:360–366. doi: 10.1016/s0360-3016(02)04491-7. [DOI] [PubMed] [Google Scholar]
- 36.Roach M., 3rd, Gandara D.R., Yuo H.S., Swift P.S., Kroll S., Shrieve D.C. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol Official J Am Soc Clin Oncol. 1995;13:2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 37.Dubray B., Henry-Amar M., Meerwaldt J.H., Noordijk E.M., Dixon D.O., Cosset J.M. Radiation-induced lung damage after thoracic irradiation for Hodgkin's disease: the role of fractionation. Radiother. Oncol J Eur Soc Therap Radiol Oncol. 1995;36:211–217. doi: 10.1016/0167-8140(95)01606-h. [DOI] [PubMed] [Google Scholar]
- 38.Karube M., Yamamoto N., Shioyama Y., Saito J., Matsunobu A., Okimoto T. Carbon-ion radiotherapy for patients with advanced stage non-small-cell lung cancer at multicenters. J Radiat Res. 2017;58:761–764. doi: 10.1093/jrr/rrx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto N., Miyamoto T., Nakajima M., Karube M., Hayashi K., Tsuji H. A Dose Escalation Clinical Trial of Single-Fraction Carbon Ion Radiotherapy for Peripheral Stage I Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:673–680. doi: 10.1016/j.jtho.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Hauswald H., Rieken S., Dienemann H.C., Thomas M., Kieser M., Debus J. Ion therapy within the trimodal management of superior sulcus tumors: the INKA trial. BMC Cancer. 2015;15:192. doi: 10.1186/s12885-015-1163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakel O., Schulz-Ertner D., Debus J. Specifying carbon ion doses for radiotherapy: the heidelberg approach. J Radiat Res. 2007;48 Suppl A doi: 10.1269/jrr.48.a87. A87–A95. [DOI] [PubMed] [Google Scholar]
- 42.van Luijk P., Novakova-Jiresova A., Faber H., Schippers J.M., Kampinga H.H., Meertens H. Radiation damage to the heart enhances early radiation-induced lung function loss. Cancer Res. 2005;65:6509–6511. doi: 10.1158/0008-5472.CAN-05-0786. [DOI] [PubMed] [Google Scholar]
- 43.Wiegman E.M., Meertens H., Konings A.W.T., Kampinga H.H., Coppes R.P. Loco-regional differences in pulmonary function and density after partial rat lung irradiation. Radiother Oncol. 2003;69:11–19. doi: 10.1016/s0167-8140(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 44.Chiblak S., Tang Z., Campos B., Gal Z., Unterberg A., Debus J. Radiosensitivity of Patient-Derived Glioma Stem Cell 3-Dimensional Cultures to Photon, Proton, and Carbon Irradiation. Int J Radiat Oncol Biol Phys. 2016;95:112–119. doi: 10.1016/j.ijrobp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Winter M., Dokic I., Schlegel J., Warnken U., Debus J., Abdollahi A. Deciphering the acute cellular phosphoproteome response to irradiation with X-rays, protons and carbon ions. Mol Cellular Proteomics: MCP. 2017 doi: 10.1074/mcp.M116.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niklas M., Abdollahi A., Akselrod M.S., Debus J., Jakel O., Greilich S. Subcellular spatial correlation of particle traversal and biological response in clinical ion beams. Int J Radiat Oncol Biol Phys. 2013;87:1141–1147. doi: 10.1016/j.ijrobp.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 47.Dokic I., Mairani A., Niklas M., Zimmermann F., Chaudhri N., Krunic D. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget. 2016;7:56676–56689. doi: 10.18632/oncotarget.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.