Graphical abstract
Keywords: Metals, Risk assessment, Aquatic, Shrimp, Fish
Highlights
-
•
Nickel was the most dominant heavy metal in water while Iron was the most dominant in shrimp and fish.
-
•
THQ for heavy metals exposure through oral route and consumption of fish were above 1, indicating potential health risk.
-
•
TTHQ for heavy metals exposure through consumption of water and fish were above 1 indicating potential non-carcinogenic health risk.
-
•
The continuous monitoring of heavy metals in Benin River is necessary to ensure the safety of aquatic organisms and humans.
Abstract
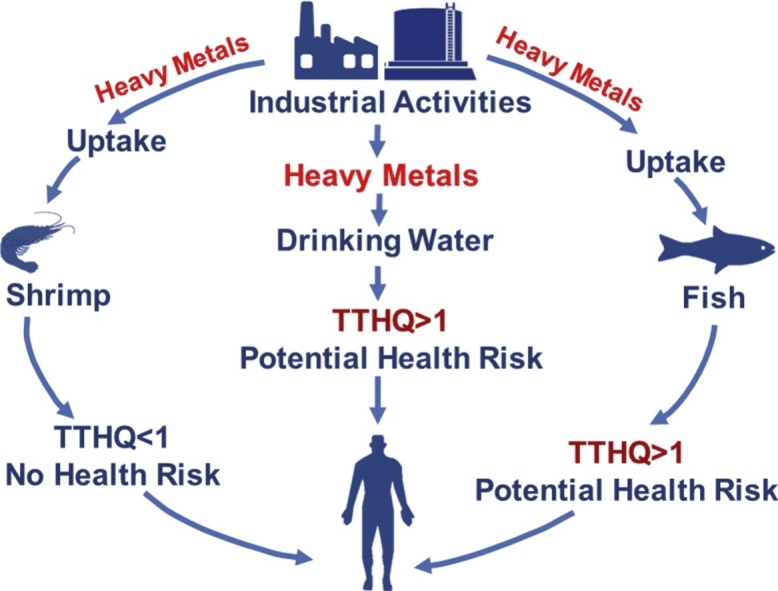
Health risk consequences of consumption of heavy metal-contaminated water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River in Nigeria were evaluated. Three stations around Koko Community (Abialegbe, Ebenco/Optima and Total Facility) with known anthropogenic activities (industrial and petrochemical installations and loading) were studied. Samples of surface water, shrimp and fish were collected and analyzed using Buck scientific atomic absorption spectrophotometer, model 210VGP. Health risk indices were reported as Estimated Daily Intake (EDI), Target Hazard Quotient (THQ) and Total Target Hazard Quotient (TTHQ). Eight heavy metals were analyzed in water, shrimp and fish. Nickel (Ni) was observed to be the most dominant heavy metal in water, while Iron (Fe) was the most dominant in shrimp and fish. Heavy metal levels in water were below recommended limits set by World Health Organization (WHO) and Standard Organization of Nigeria (SON) except for cadmium (Cd), nickel (Ni) and lead (Pb). Assessment of non-carcinogenic health risk by target hazard quotient (THQ) indicated that THQ estimated for heavy metals in water (dermal exposure) and shrimp were below the threshold value of 1. However, THQ for heavy metals in water (oral exposure) and consumption of fish were above threshold value of 1 indicating potential health risk. Total Target Hazard Quotient (TTHQ) estimated for heavy metals in water (oral exposure) and consumption of fish were above 1 indicating potential non-carcinogenic health risk to consumers. The continuous monitoring of heavy metals in Benin River is of necessity in order to ensure the safety of aquatic organisms and humans who rely heavily on aquatic resources.
1. Introduction
Rapid urbanization and industrial development in the last decade have triggered some serious concerns for the safety of the environment. The quest for improved livelihoods and energy for all have resulted in anthropogenic campaigns which have led to continuous release of chemical pollutants including heavy metals into virtually all environmental matrices.
Globally, heavy metals have been a serious threat to human health and ecosystem integrity [[1], [2], [3]]. Water quality has become a major challenge in the world today as it is being polluted by industrial and urban wastes generated largely by human activities [4].
The main anthropogenic sources of heavy metals contamination of water, sediment and aquatic animals are industrial activities, mining and disposal of untreated and partially treated effluents containing toxic metals [[5], [6], [7]]. More attention should be given to the monitoring of toxic heavy metals due to inherent bioaccumulation and biomagnification potentials and their long term persistence in environmental compartments [8]. Monitoring of the integrity of surface waters is significant in order to be assured of the sustainability of desired ecosystem functions of aquatic environments [9]. Heavy metal contamination is a major problem in growing cities of developing countries primarily due to uncontrolled pollution levels driven by causative factors like industrial growth and heavy increase in traffic using petroleum fuels [10].
After introduction into the environment from a plurality of anthropogenic sources, heavy metals may accumulate in aquatic life, enter the food chain and cause serious harm to human health where contamination and exposure are significant [[10], [11], [12], [13], [14]]. Consequently, they have been listed in United States Environmental Protection Authority (USEPA) based on their potential for human exposure and health risks [15]. The degree of environmental contamination depends on type of heavy metal, aquatic species, trophic level and feeding pattern [16]. Monitoring of heavy metal contamination in river systems by using fish tissues aids in the assessment of the quality of aquatic ecosystems [17]. Furthermore, fish populations in most cases are stable and easy to collect [18]. A great percentage of metals accumulated in fish come from polluted water through water borne exposure [19,20].
Aquatic animals especially fish has been reported by FAO statistics to account for about 16% of the world population intake of animal protein and 6% of all protein consumed [21]. Metals in fish tissues can be manifold times higher than their corresponding waterborne values. Consumption of aquatic fauna has been reported as an important route of human exposure to a variety of chemical contaminants [22]. Studies have reported that dietary intake is the major route of exposure to heavy metals for most individuals [23]. Fish assimilate metals by ingestion of particulate material suspended in water, ingestion of food, ion-exchange across lipophilic membranes (e.g., the gills), and adsorption on tissue and membrane surfaces. Levels of toxic heavy metals in aquatic biota are of particular interest because of the potential risk to humans who consume them. The accumulation of heavy metals in the tissues can result in chronic illness and cause potential damage to the population [24]. Chronic intake of heavy metals above their safe threshold in humans and animals have detrimental effects and can cause non carcinogenic hazards such as neurologic problems, headache and liver and kidney disease [25]. Target hazard quotients (THQ) were developed by the United States Environmental Protection Agency for the estimation of potential health risks associated with long term exposure to chemical pollutants. THQ is a ratio between the measured concentration and the oral reference dose, weighed by the length and frequency of exposure, amount ingested and body weight. THQ value is a dimensionless index of risk associated with long term exposure, amount ingested and body weight.
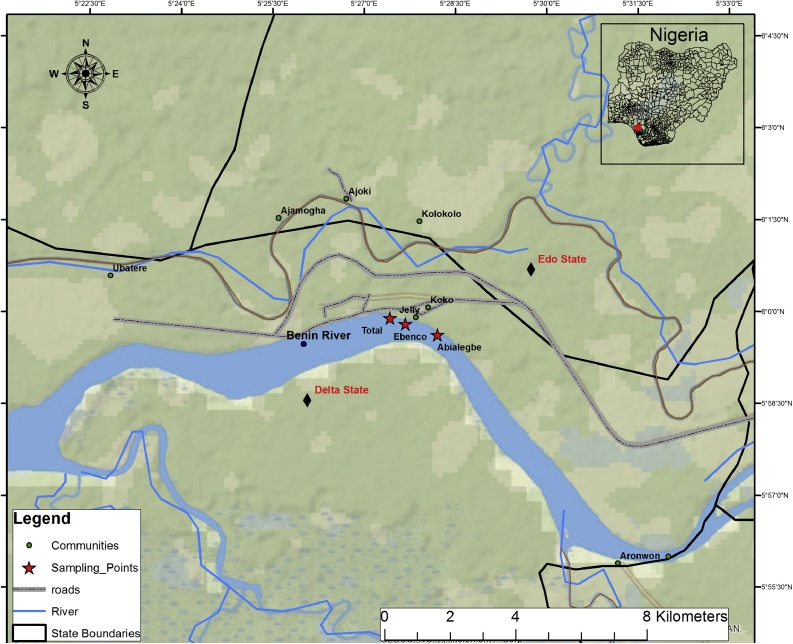
Benin River is located in the coastal belt of southern Nigeria and the western boundary of the upper delta and lowlands. This river drains rivers Ethiope, Ossiomo, Osse and Siluko into the Atlantic Ocean. It is approximately 93 km long with an average width of 3.0 km and 1.4 km in its downstream and upstream section, respectively. It is an important channel for small ships and other water crafts. For this study, areas of Benin River around Koko community with known anthropogenic activities like asphalt, boat transport, petrochemical installations, petroleum tank farms and loading activities were used.
The aim of this study was to assess heavy metal concentrations in surface water, shrimp (M. macrobrachion) and fish (B. longipinnis) from Benin River and quantify health risk consequences from exposure through consumption of contaminated shrimp and fish. The species of shrimp and fish studied are abundant in the area studied and provide a continuous source of protein for the populace of Koko Community.
2. Materials and methods
2.1. Description of study area
Koko River is located in the coastal belt of Southern Nigeria at the Western boundary of the upper Delta and the lowlands (Latitudes 05°059′43.6′' – 05°059′35.7′'N; Longitudes 005°028′06.7′'- 005°025′56.2′'E). This River drains the major rivers Ethiope, Ossiomo, Osse and Siluko into the Atlantic Ocean [26]. It is approximately 93 km long with an average width of 3.0 km and 1.4 km in its downstream and upstream section, respectively. It is an important channel for small ships and other watercrafts like speed boat, yacht and canoe. Three distinct longitudinal zones can be recognized in this river; the upper freshwater zone, the middle transitional zone with salinity fluctuations and the lower coastal zone which is predominately saline.
Samples of water for characterization of heavy metal concentrations were obtained from three stations designated along stretch of Benin River at Koko town in Delta State.
2.2. Field sampling
The sampling duration was from December 2014 to May 2015. The sampling stations were visited monthly for the study period. Water, fish and shrimp samples were collected in the early hours of the morning between 7am and 10am at the sampling stations.
Water samples for heavy metal determination were collected in acid washed polyethylene bottles. The bottles were rinsed thoroughly with deionised water after being washed in dilute nitric acid (HNO3). In the field the bottles were rinsed several times with the river water and 1 L of water sample was then collected at about 20 cm below the water surface. The water samples were acidified with concentrated nitric acid for preservation. The acid pretreatment ensured that heavy metals did not get absorbed to the surface of the container during transportation and storage.
Water samples for DO determination were collected using 250 ml reagent bottles with glass stoppers. The reagent bottles were immersed into the water and the stopper removed below the water surface to fill the bottle. The stopper was then replaced under water and the bottles brought out. This was done to keep out air from mixing with the sampled water. The water sample was immediately fixed by adding 1 ml each of Winkler’s solutions A (manganous sulphate) and B (potassium hydroxide in potassium iodide). DO was later determined in the laboratory using Winkler’s method [27].
Fish and shrimp samples were also collected at random across the various stations of the river with the help of local fishermen using local fishing tools such as gill nets, hooks, lines and local traps. The samples were preserved in ice and taken to the laboratory for identification and analysis. They were kept frozen in the refrigerator pending heavy metals analysis in the laboratory.
2.3. Sample preparation for heavy metal analysis
Water samples were filtered through whatman No. 42 filter paper and made up to 50 ml with distilled water. They were then analyzed using Buck Scientific Atomic Absorption Spectrophotometer.
Whole Fish and shrimp samples were weighed and oven dried at 105 . The weights after drying were also taken and recorded. Drying continued until a constant weight was achieved. The dried samples were homogenized into fine powder using ceramic mortar and pestle after which 0.5 g was measured for digestion. Samples were digested with tri-acid mixture (HNO3:HClO4:H2SO4 = 10:4:1) at a rate of 5 ml per 0.5 g sample and was placed on hot plate at 100 . [28] Digestion was continued until liquor was clear. All digested liquor were allowed to cool and filtered through whatman No.42 filter paper and diluted to 25 ml with distilled water [25]. They were then analyzed using Buck Scientific Atomic Absorption Spectrophotometer, model VGP 210.
2.4. Quality assurance and control
The equipment was first calibrated using buck certified atomic absorption standards for the respective heavy metals to obtain calibration curves. Reagent blanks were first run at intervals of every ten sample analysis to eliminate equipment drift. All samples were analyzed in duplicates for reproducibility, accuracy and precision.
2.5. Human health risk assessment
2.5.1. Estimated daily intake and target hazard quotient
Estimated daily intake of heavy metals via ingestion route (water, fish and shrimp) was calculated using Eq. (1) while exposure through dermal contact was calculated using Eq. (2). The target hazard quotient (THQ) was finally calculated using Eq. (3).
| (1) |
| (2) |
| (3) |
Assumptions for the health risk calculations were;
-
1
Ingested dose is equal to the absorbed pollutant dose [29].
-
2
Cooking has no effect on the pollutants [30].
-
3
The average body weight of a Nigerian is assumed to be 70 kg
Where C is the concentration of the metals in the sample mg/L or mg/kg, IR is the ingestion rate (kg/day) or (L/day), EF is exposure frequency (365 days/year). ED is the exposure duration in years; RfD is the reference dose (mg/kg/day). BW is the average adult body weight (kg), AT is the averaging time, SA is the exposed skin area (cm3). ET is the exposure time (h/day), CF is the volumetric unit conversion factor. KP is the dermal permeability coefficient. Since exposure to two or more pollutants may result in additive and/or interactive effects, total THQ in this study was treated as the arithmetic sum of the individual metal THQ values, derived by the method of Ref. [31].
2.6. Statistical analysis
Descriptive statistics, determination of one-way, analysis of variance (ANOVA) and post hoc tests were conducted using Microsoft Excel 2010 and IBM SPSS Statistics 20. Statistica was used for box and whisker plots while JMP version 10 was used for principal components analysis (Fig. 1).
Fig. 1.
Map of study area showing the sampling stations.
3. Results/discussion
3.1. Variation in dissolved oxygen across stations
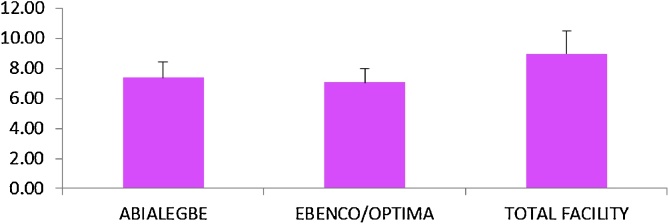
The mean values for dissolved oxygen across the stations are shown in Fig. 2. Mean DO values ranged from (7.05 mg/l to 8.96 mg/l). There was no significant difference (P > 0.05) in the spatial variation of DO across stations in Benin river.
Fig. 2.
Mean DO values for Abialegbe, Ebenco/Optima and Total plant.
The concentration of dissolved oxygen is an important indicator of the health of the aquatic ecosystem. Mean dissolved oxygen values ranged between 7.05 mg/l in Ebenco/Optima to 8.96 mg/l in Total facility. Dissolved oxygen is important as a respiratory gas and it acts as a water quality indicator as well as an indicator of health and productivity of a river [32]. Persistently low dissolved oxygen will harm most aquatic life because there will not be enough for them to use.
3.2. Heavy metal concentrations in water, fish and shrimp
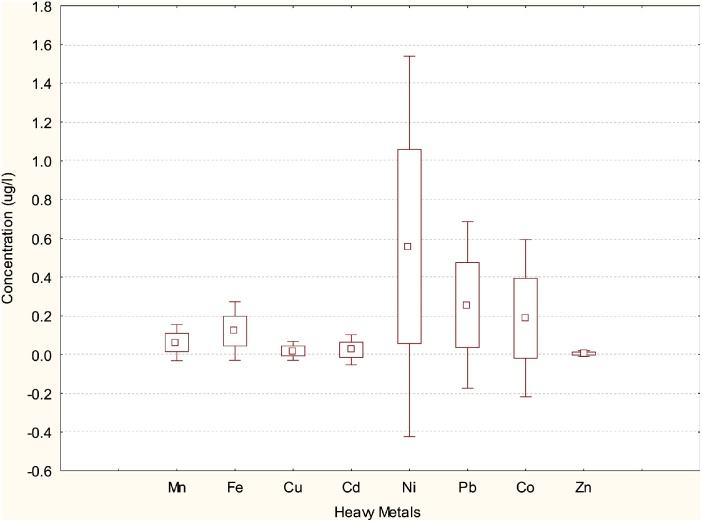
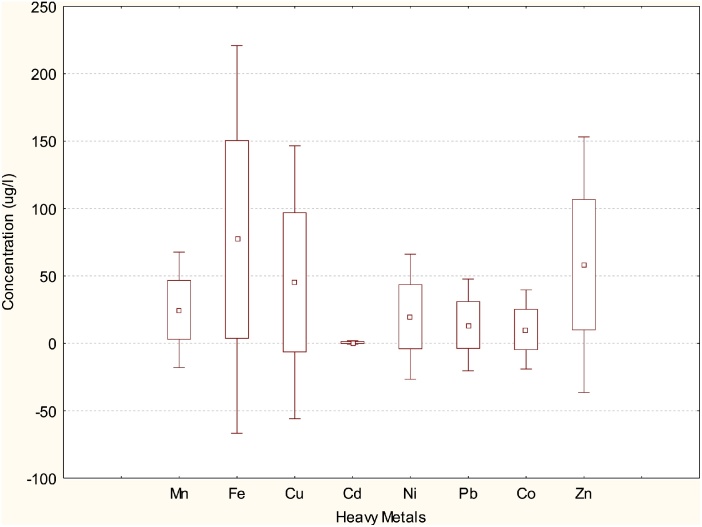
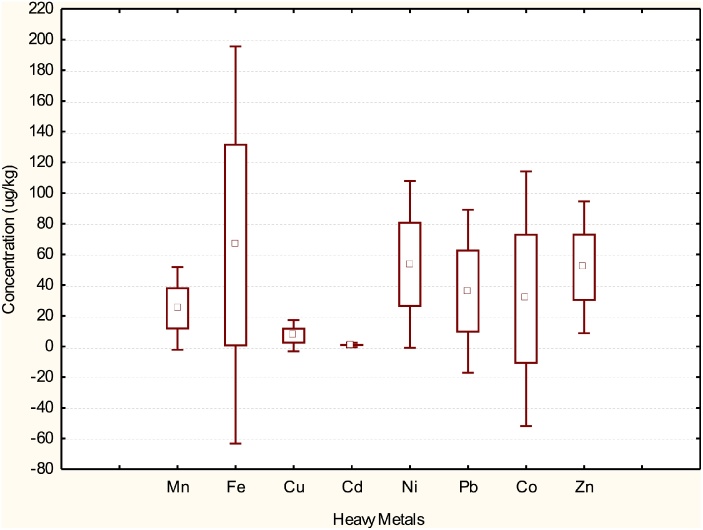
Fig. 3, Fig. 4, Fig. 5 depict box and whisker plots showing basic statistics for concentrations of heavy metals in water, M. macrobrachion and B. longipinnis from Benin River. In Fig. 2 Ni concentrations in water showed a wide spread while Pb and Co showed a moderate spread. The spread for Mn, Fe Cu Cd Zn were narrow. The lowest and highest concentrations were also observed for Ni. In Fig. 4, Fe, Cu and Zn distribution in M. macrbrachium showed a wide spread while Cd had a narrow spread. The spread for Mn, Ni and Pb were moderate. The lowest and highest concentrations were observed for Fe. Fe exhibited the highest variation in concentration for all the metals quantified in M. macrobrachion. In Fig. 5 Cd showed a narrow distribution in B. longipinnis while Fe, Cu and Zn showed a wider distribution. The lowest and highest metal concentrations were observed for Fe. The median for all the metals in water, M. macrobrachion and B. longipinnis were dissimilar.
Fig. 3.
Box-and-whisker plots showing the concentrations of heavy metals in surface water from Benin River. The boxes extend from the 25th percentile to the 75th percentile; the whiskers represent the lowest and highest coefficients and the small red box indicates the concentration median; (n = 54).
Fig. 4.
Box plot for concentrations of heavy metals in M. macrobrachion from Benin River. The boxes extend from the 25th percentile to the 75th percentile; the whiskers represent the lowest and highest coefficients and the small red box indicates the concentration median; (n = 54).
Fig. 5.
Box plot for concentrations of heavy metals in B. longipinnis from Benin River. The boxes extend from the 25th percentile to the 75th percentile; the whiskers represent the lowest and highest coefficients and the small red box indicates the concentration median; (n = 54).
Mean concentrations of Mn in water, fish and shrimp in this study were 0.06 mg/l, 39.45mk/kg, and 24.78 mg/kg respectively. The high values of Mn recorded in both shrimp and fish might be due to the occurrence of the phenomenon of bioaccumulation. Elevated concentrations of heavy
metals in tissues of aquatic organisms reflect accumulative exposure through water and/or food [33]. It is known that aquatic organisms take up contaminants from water and accumulate them in their tissues to much higher levels than that of their surrounding milieu. Similarly Ref [34] reported high levels of Mn in the gills of pelagic fish in the red sea of Egypt. The higher levels in fish compared with water were also attributed to biological accumulation.
The mean concentration of Fe in water was 0.14 mg/l. Fe was highly bioaccumulated in shrimp and fish with mean values of 114.78 mg/kg and 66.13 mg/kg. Fe is an important metal in both plants and animals, especially in the cellular processes [35]. Some of the metals found in the fish might be fundamental as they play important roles in biological system of the fish as well as in human beings but some of them may however, be toxic and may cause serious damage to human and animal health if present in excess to optimal limits for metabolism [36].
Fe is found in natural fresh- and groundwater, but have no health-based guideline value, although high concentrations give rise to consumer complaints due to its ability to discolour aerobic waters at concentrations above 0.3 mg/l of Ref. [37].
Mean concentrations of Cu during the study period were 0.02 mg/l (water), 60.83 mg/kg (shrimp) and 7.05 mg/kg (fish). Concentration of Cu in shrimp did not compare favourably with FAO guidelines [38]. The same trend was observed for levels of Cu in fish. The heavy metal may have found its way into the water body through surface runoff and the degradation of abandoned vessels close to the shorelines adjacent to the petrochemical farms.
The mean concentration of Cd in water samples was 0.01 mg/l which is above maximum permissible values for drinking water set by Ref. [37] and Ref. [39]. This is an indication that Benin River is contaminated, but not polluted with Cd. Cd was also detected in shrimp and fish with mean values 0.93 mg/kg and 0.98 mg/kg. Levels in shrimp and fish were above the recommended Ref. [40] set limit. Cd is one of the most toxic elements with widespread carcinogenic effects in humans [41]. High concentrations of Cd lead to chronic kidney dysfunction, inducing cell injury and death by interfering with Calcium regulation in biological systems in man, fish and other aquatic organisms [42].
Ni was detected throughout the sampling period, with a mean concentration of 0.42 mg/L in water. Concentrations in shrimp and fish had mean values of 33.03 mg/kg and 53.57 mg/kg which is beyond the safe limit set by Ref. [40]. Although Ni is considered an essential element to plants and some animals, its importance to man is yet to be demonstrated [43]. According to McKenzie and Symthe [44], more attention has been focused on the toxicity of Ni in low concentration. Ni can cause allergic reactions and certain Ni compounds may be carcinogenic. Nonetheless, Ni related health effects such as renal, cardio vascular, reproductive and immunological effects have been reported in man [45].
Mean concentration of Pb in water in this study was 0.18 mg/l. The value was above Ref. [37] and Ref. [39] recommended limits for drinking water quality (Fig. 5). Pb was bioaccumulated in shrimp and fish with mean values of 22.39 mg/kg and 36.04 mg/kg. The United States Environmental Protection Agency has classified Pb as being potentially hazardous and toxic to most forms of life [46]. Su et al. [47] reported high lead bioaccumulation in the muscles, and a degree of histopathologic disintegration in the muscle fibers of all the fish sampled from Manila Bay, Phillpines. Increasing contamination of aquatic ecosystems by metals has caused various morphological, physiological and biochemical changes in aquatic organisms [48].
Co was detected in all samples during the study period with mean values of 0.14 mg/l in water, 18.01 mg/kg in shrimp and 31.14 mg/kg in fish. There was a progressive decrease in the concentration of Cobalt throughout the sampling period. This decrease could be as a result of onset of rainy season causing a dilution effect as a result of increase the volume of the water body.
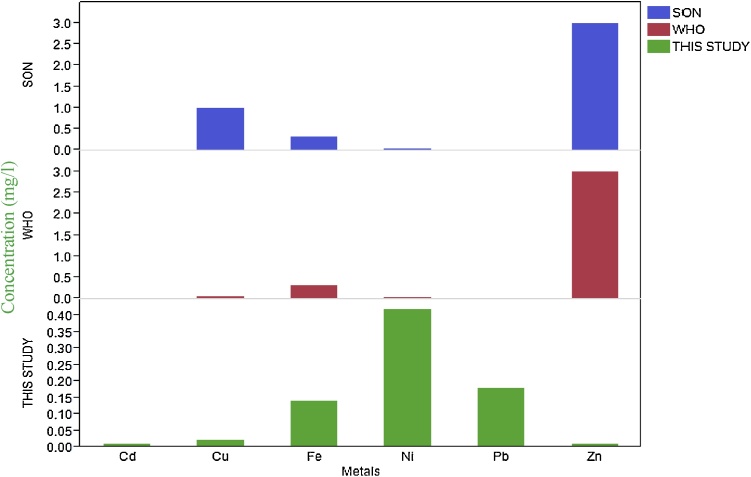
The mean concentration of Zn (0.01 mg/l) in water was below the recommended limit set by Refs. [37] and [39] for unpolluted water (Fig. 6). Mean Zn levels in shrimp (84.02 mg/kg) was within the regulatory limit set by FAO and the USEPA. Fish recorded mean Zn level of 51.64 mg/kg which was within the Ref. [37] set limit of 75 mg/kg. Zn is a cofactor in several enzyme systems including carbonic anhydrase found in red blood cells. Chance of being poisoned with Zn is rare because salts of alkaline earth element reduce toxicity of Zn. High temperature and low dissolved oxygen concentration lead to increase in toxicity of Zn. Its toxicity to fish according to Refs. [49] and [50] can be greatly influenced by both water hardness and pH. It has been found to have low toxicity effect in man. However, the prolonged consumption of large doses can result in some health complications such as fatigue, dizziness and neutropenia [51].
Fig. 6.
Mean metal levels in water in this study compared with SON [39] and WHO [37] standards.
3.3. Metal distribution patterns by principal component analysis
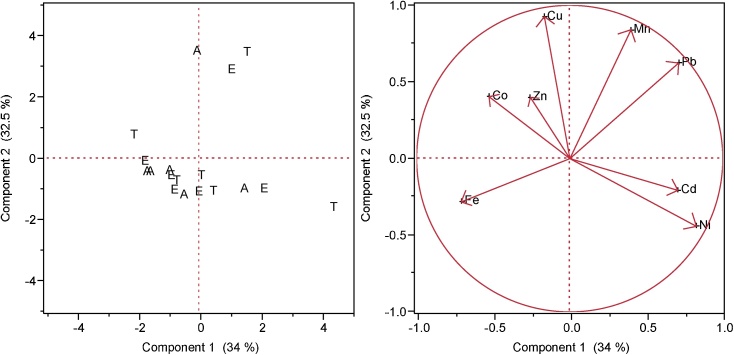
PCA has been documented as a useful statistical tool for distinguishing heavy metal sources [52]. In Fig. 7, the score plot of the PCA shows a scatterplot of metal distribution patterns for water in components 1 and 2. Components 1 and 2 accounted for 66.5% of the variation in the data set. Component 1 (PC1) made up 34% of the variation while component 2 (PC2) made up 32.5% of the total variance accounted for in the dataset. The loading plot shows the correlation between metal distribution and components 1 and 2. Cd and Ni although having dissimilar distribution spreads, were positively correlated with component 1. The positive factor loads for Cd and Ni, suggests that these metals come from similar sources. These metals are likely generated from anthropogenic and natural sources mostly from chemical weathering at the boat and vessel maintenance facility, operational discharges and cleanup of cargo by the bitumen processing facility all along the catchment of the study area. Co, Zn, Fe and Cu were negatively correlated with component 1 but positively correlated with component 2. Mn and Pb are also positively correlated with component 2. High levels of Pb and Ni reported in water in this study could be traced to the activities of the petroleum based industries located in the study area. High levels of Pb and Ni have been reported to occur in Nigerian Crude oil (Bonny Light) [53].
Fig. 7.
PCA showing metal distribution in surface water in Abialegbe (A), Ebenco (E) and Total (T).
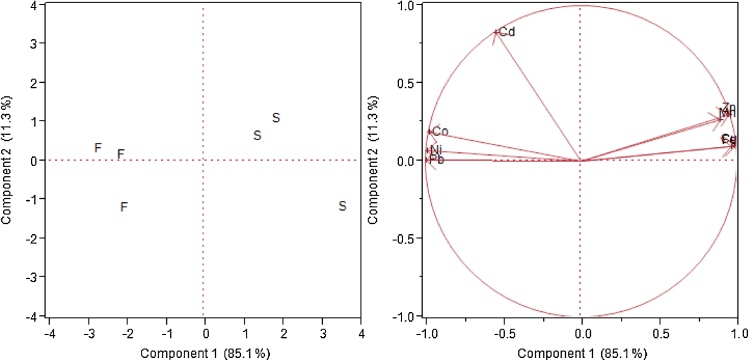
Fig. 8 shows the PCA for metal distribution water fish and shrimp in the stations sampled. The score plot of the PCA shows a scatterplot of metal distribution for environmental matrices in components 1 and 2. Component 1 accounted for 85.1% the variation in the dataset while component 2 accounted for 11.3% making a total of 96.4%. The loading plot shows that Mn, Fe, Cu and Zn were clustered together and positively correlated with component 1 while Co, Ni and Pb were also clustered together and positively correlated with component 2. Cd formed no cluster with any other metal but is positively correlated with component 2. The metals in component 1 (Mn, Fe, Cu and Zn) also showed a strong association with shrimp while the metals in component 2 (Co, Ni and Pb) showed strong association with fish.
Fig. 8.
PCA showing the relationship for metal distribution in Shrimp (S) and Fish (F).
3.4. Health risk assessments
The results of human health risk assessment from Benin River are presented in Table 1, Table 2. Human health risk assessment of heavy metals in water showed that the estimated daily intake (EDI) for heavy metals in water from dermal and oral exposure were below the reference doses except for Pb which was above the reference dose for oral exposure. The THQ showed that for health risk through ingestion, Zn had the lowest THQ value and Pb had the highest potential for risk with a value >1. Individual metals posed no health risk from their THQ values except for Pb.
Table 1.
Summary of health risk assessment for metals in water from Benin River.
| ELEMENT | RfDingestion | RfDdermal | EDIingestion | EDI dermal | THQingestion | THQdermal |
|---|---|---|---|---|---|---|
| Mn | 0.14 | 0.96 | 1.89E-03 | 1.44E-05 | 1.35E-02 | 1.50E-05 |
| Fe | 0.3 | 140 | 4.40E-03 | 3.36E-05 | 1.47E-02 | 2.40E-07 |
| Cu | 0.04 | 8 | 6.29E-04 | 4.80E-06 | 1.57E-02 | 6.00E-07 |
| Cd | 0.001 | 0.025 | 3.14E-04 | 2.40E-06 | 3.14E-01 | 9.60E-05 |
| Ni | 0.02 | 0.42 | 1.32E-02 | 4.03E-04 | 6.60E-01 | 9.60E-04 |
| Pb | 0.004 | 60 | 5.66E-03 | 2.59E-05 | 1.41E+00 | 4.32E-07 |
| Co | 0.02 | 1.4 | 4.40E-03 | 3.36E-05 | 2.20E-01 | 2.40E-05 |
| Zn | 0.3 | 200 | 3.14E-04 | 2.40E-06 | 1.05E-03 | 1.20E-08 |
| TTHQ | 2.65E+00 | 1.10E-03 |
Table 2.
Summary of health risk assessment for metals in shrimp and fish from Benin River.
| ELEMENT | CONCENTRATION IN SHRIMP |
CONCENTRATION IN FISH |
RfDo | EDI SHRIMP | EDI FISH | THQ SHRIMP | THQ FISH |
|---|---|---|---|---|---|---|---|
| Mn | 39.45 | 24.78 | 0.14 | 9.67E-03 | 5.31E-03 | 6.91E-02 | 3.79E-02 |
| Fe | 114.78 | 66.13 | 0.3 | 2.81E-02 | 1.42E-02 | 9.38E-02 | 4.72E-02 |
| Cu | 60.83 | 7.05 | 0.04 | 1.49E-02 | 1.51E-03 | 3.73E-01 | 3.78E-02 |
| Cd | 0.93 | 0.98 | 0.001 | 2.28E-04 | 2.10E-04 | 2.28E-01 | 2.10E-01 |
| Ni | 33.03 | 53.57 | 0.02 | 8.10E-03 | 1.15E-02 | 4.05E-01 | 5.74E-01 |
| Pb | 22.39 | 36.04 | 0.004 | 5.49E-03 | 7.72E-03 | 2.64E-02 | 1.93E + 00 |
| Co | 18.01 | 31.14 | 0.02 | 4.42E-03 | 6.67E-03 | 2.21E-01 | 3.34E-01 |
| Zn | 84.02 | 51.64 | 0.3 | 2.06E-02 | 1.11E-02 | 6.87E-02 | 3.69E-02 |
| TTHQ | 2.83E+00 | 3.21E+00 |
A summation of the individual THQs (TTHQ) values was >1 indicating possible health risk from consumption of water from the Benin river. Calculated health risk through dermal exposure showed that Zn had the minimum THQ while Ni had the highest THQ. Individual THQs presented no possible health risk as they all had values <1. This agrees with the work of Ref. [54]. The TTHQ value which is gotten from the summation of the individual metal THQ was also <1 indicating that no potential risks exists from dermal exposure to water from Benin River.
Results of health risk assessments from consumption of shrimps and fish showed that the EDI values estimated for heavy metals in shrimps were below the reference doses while in fish only Pb recorded values above the reference dose. THQs of Pb were the highest in shrimp and fish with the values of 2.64E-02 and 1.93E + 00 respectively. However, only consumption of fish from the study area entailed health risks due to Pb as shown in the high association of Pb with Fish in the PCA. Moreover, the THQ values of the other individual metals were <1 in both shrimp and fish which showed that the concentrations of other individual metals in shrimps and fish from Benin River posed no health risk to consumers. Ref. [55] reported values <1 from his study of exposure through fish consumption from selected rivers in Kuatan. Compared to the present study, Ref. [56] from their study of exposure through fish consumption in Andhra Pradesh India reported THQ values >1 except for Cd which was <1. Ref. [57] in their study of Human health risk assessment of metal contamination through consumption of Sesarma angolense and M. macrobrachion from Benin River, Nigeria reported THQ values less <1 for shrimp and crabs. Nonetheless, TTHQ for M. macrobrachion in that study, was greater than 1, indicating risk to human health from consumption of M. macrobrachion. TTHQ in S.angolense was less than unity. In this study, TTHQ values were >1 for both shrimps and fish indicating potential risk from consuming fish and shrimp from Benin River. This result is a source of concern because of the potential health risk consequences from the intake of heavy metals through consumption of water, of fish and shrimp from Benin River.
3.5. Conclusion
The study has shown that the surface water and commonly consumed shrimp (M. macrobrachion) and fish (Brycinus longipinnis) species sampled from Benin River had various concentrations of heavy metals and the level of accumulation varied among the species studied. This evokes health risk concerns for consumers. Mn, Fe, Cu and Zn showed a strong association with shrimp while Cd, Co, Ni and Pb showed strong association with fish as profiled by the principal components analysis. Pb showed high levels in shrimp and fish and were above Ref. [36] standards of 0.3 mg/kg for fish. THQs of Pb were the highest in shrimp and fish. THQ estimated for heavy metals in water (dermal exposure) and shrimp were below the threshold value of 1 showing no potential health risk. However, THQ for heavy metals in water (oral exposure) and consumption of fish were above threshold value of 1 indicating potential health risk. Total Target Hazard Quotient (TTHQ) estimated for heavy metals in water (oral exposure) and consumption of fish were above 1 indicating potential non-carcinogenic health risk to consumers.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
References
- 1.Vrhovnik P., Arrebola J.P., Serafimovski T., Dolenec T., Smuc R.N., Dolenec M., Mutch E. Potentially toxic contamination of sediments, water and two animal species in Lake Kalimanci, FYR Macedonia: relevance to human health. Environ. Pollut. 2013;180:92–100. doi: 10.1016/j.envpol.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Ezeonyejiaku C., Nwuba L.A., Obinna O.M., Ndidi O.C. Bioaccumulation of heavy metals in fish sourced from environmentally stressed axis of River Niger: threat to ecosystem and public health. Int. J. Environ. Prot. Pol. 2014;2(4):126–131. [Google Scholar]
- 3.Ogbomida E.T., Nakayama S., Bortey-Sam N., Oroszlany B., Tongo I., Enuneku A.A., Ogbeide O., Ainerua M.O., Fasipe I.P., Ezemonye L.I., Mizukawa H., Ikenaka Y., Ishizuka M. Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria. Ecotoxicol. Environ. Saf. 2018;151:98–108. doi: 10.1016/j.ecoenv.2017.12.069. [DOI] [PubMed] [Google Scholar]
- 4.Ojutiku R.O., Okojevoh F.I. Bioaccumulation of Some Heavy Metals in three Selected Fish Species from Chanchaga River, Minna Niger State, Nigeria. Nig. J. Fish. Aquacul. 2017;5(1):44–49. [Google Scholar]
- 5.Ammann A.A., Michalke B., Schramel P. Speciation of heavy metals in environmental water by ion chromatography coupled to ICP-MS. Anal. Bioanal. Chem. 2002;372(3):448–452. doi: 10.1007/s00216-001-1115-8. [DOI] [PubMed] [Google Scholar]
- 6.Nouri J., Mahvi A.H., Jahed G.R., Babaei A.A. Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environ. Geol. 2008;55(6):1337–1343. [Google Scholar]
- 7.Dahunsi S.O., Oranusi S.U., Ishola R.O. Differential bioaccumulation of heavy metals in selected biomarkers of Clarias gariepinus (Burchell, 1822) exposed to chemical additives effluent. J. Res. Environ. Sci. Toxicol. 2012;1(5):100–106. [Google Scholar]
- 8.Koki I.B., Bayero A.S., Umar A., Yusuf S. Health risk assessment of heavy metals in water, air, soil and fish. Afr. J. Pure Appl. Chem. 2015;9(11):204–210. [Google Scholar]
- 9.Wang Y., Teng E., Liu T., Lv Y., Jin X., Giesy J.P., Hollert H. A national pilot scheme for monitoring and assessment of ecological integrity of surface waters in China. Environ. Dev. 2014;10:104–107. [Google Scholar]
- 10.Maigari A.U., Ekanem E.O., Garba I.H., Harami A., Akan J.C. Health risk assessment for exposure to some selected heavy metals via drinking water from Dadinkowa Dam and river gombe abbain gombe state, Northeast Nigeria. World J. Anal. Chem. 2016;4(1):1–5. [Google Scholar]
- 11.Goyer A.R. Toxic metals and essential metal interactions. Annu. Rev. Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Papagiannis I., Kagalou I., Leonardos J., Petridis D., Kalfakakou V. Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece) Environ. Int. 2004;30(3):357–362. doi: 10.1016/j.envint.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Türkmen M., Tepe Y., Akyurt I. Heavy metals in three commercially valuable fish species from İskenderun Bay, Northern East Mediterranean Sea, Turkey. Food Chem. 2005;91(1):167–172. [Google Scholar]
- 14.Fernandes C., Fontaínhas-Fernandes A., Peixoto F., Salgado M.A. Bioaccumulation of heavy metals in Liza saliens from the Esmoriz–Paramos coastal Lagoon, Portugal. Ecotoxicol. Environ. Saf. 2007;66(3):426–431. doi: 10.1016/j.ecoenv.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Birungi Z., Masola B., Zaranyika M.F., Naigaga I., Marshall B. Active biomonitoring of trace heavy metals using fish (Oreochromis niloticus) as bioindicator species. The case of Nakivubo wetland along Lake Victoria. Phys. Chem. Earth Parts A/B/C. 2007;32(15–18):1350–1358. [Google Scholar]
- 16.Asuquo F.E., Ewa-Oboho M., Asuquo E.F., Udoh P.J. Fish species used as biomarkers for heavy metals and hydrocarbon contamination from the Cross River, Nigeria. Environmentalist. 2004;24:29–37. [Google Scholar]
- 17.Adam S.M. American Fisheries Society; Bethseda, MD: 2002. Biological Indicators of Aquatic Ecosystem Stress. p656. [Google Scholar]
- 18.Renieri E.A., Alegakis A.K., Kiriakakis M., Vinceti M., Ozcagli E., Wilks M.F., Tsatsakis A.M. Cd, Pb and Hg biomonitoring in fish of the Mediterranean region and risk estimations on fish consumption. Toxics. 2014;2:417–442. [Google Scholar]
- 19.Salem Z.B., Capellia N., Laffraya X., Elisea G., Ayadib H., Aleya L. Seasonal variation of heavy metals in water, sediment and roach tissues in a landfill draining system pond (Etueffont, France) Ecol. Engin. 2014;69:25–37. [Google Scholar]
- 20.Renieri E.A., Sfakianakis D.G., Alegakis A.A., Safenkova I.V., Buha A., Matović V., Boris M.T., Dzantiev B., Divanach P., Kentouri M., Tsatsakis A.M. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017;157:173–181. doi: 10.1016/j.envres.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 21.F.A.O. The international fish trade and world fisheries, http://www.fao.org/fileadmin/use_upload/newsroom/docs/fact_sheet_fish_trade_en.pd.2010.
- 22.Usero J., Izquierdo C., Morill J., Gracia I. Heavy metals in fish (Solea vulgris, Anguila anguila, Liza aurate) from marshes on the Southern Atlantic coast of Spain. Environ. Int. 2003;29(7):949–956. doi: 10.1016/S0160-4120(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang P., Bride M., Xia H., Li N., Li Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009;407:1551–1561. doi: 10.1016/j.scitotenv.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Amirah M.N., Afiza A.S., Faizal W.I.W., Nurliyana M.H., Laili S. Human health risk assessment of metal contamination through consumption of fish. J. Environ. Pollut. Hum. Hlth. 2013;1(1):1–5. [Google Scholar]
- 25.John G.G., Andrew B.A. Lead isotopic study of the human bioaccessibility of lead in urban soils from Glassgow, Scotland. Sci. Tot. Environ. 2011;409:4958–4965. doi: 10.1016/j.scitotenv.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 26.Francis O.A., Robert B.I., Efe C.O. The impact of sawmill wood wastes on the water quality and fish communities of Benin River, Niger Delta area, Nigeria. Wood J. Zool. 2006;1:94–102. [Google Scholar]
- 27.APHA 1998. Standard methods for the examination of water and waste water (20th ed). New York: American Public Health Association (APHA), American Water Works Association (AWWA), AND Water Pollution Control Federation (WPCF).
- 28.A.O.A.C (Association of official analytical chemists) 15th edition. 1990. Official Methods of Analysis. Washington D.C.858pp. [Google Scholar]
- 29.USEPA . vol. I. United States Environmental Protection Agency; Washington DC: 1989. Risk assessment guidance for superfund. (Human Health Evaluation Manual Part A, Interim Final). [Google Scholar]
- 30.Cooper C.B., Doyle M.E., Kipp K. Risk of consumption of contaminated seafood, the Quincy Bay case study. Environ. Health Perspect. 1991;90:133–140. doi: 10.1289/ehp.90-1519503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien L.C., Hung T.C., Choang K.Y., Yeh C.Y., Meng P.J., Shieh M.J., Han B.C. Daily intake of TBT, Cu, Zn, Cd and as for fishermen in Taiwan. Sci. Total Environ. 2002;285(1):177–185. doi: 10.1016/s0048-9697(01)00916-0. [DOI] [PubMed] [Google Scholar]
- 32.Moshood K.M. Assessment of the water quality of Oyun reservoir, Offa, Nigeria, using selected physicochemical parameters. Turk. J. Fish. Aquat. Sci. 2008;8:309–319. [Google Scholar]
- 33.Abdel-Mohsien H.S., Mahmoud M.A. Accumulation of some heavy metals in Oreochromis niloticus from the Nile in Egypt: potential hazards to fish and consumers. J. Environ. Prot. 2015;6:1003–1013. [Google Scholar]
- 34.El-Moselhy K.M., Othman A.I., Abd El-Azem H., El-Metwally M.E.A. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea. Egypt. J. Basic Appl. Sci. 2015;97:e105. [Google Scholar]
- 35.Whalley C., Rowlatt S., Bennet M., Lovell D. Total arsenic in sediments from the western North Sea and the Humber estuary. Mar. Pollut. Bull. 1999;38:394–400. [Google Scholar]
- 36.Ahmed Q., Khan D., Elahi N. Concentrations of heavy metals (Fe, Mn, Zn, Cd, Pb, and Cu) in muscles, liver and gills of adult Sardinella albella (Valenciennes, 1847) from Gwadar water of Balochistan, Pakistan. Fuuast J. Biol. 2014;4(2):195–204. [Google Scholar]
- 37.WHO . 3rd Edition. Vol. 1. Recommendation World Health Organization; Geneva: 2004. (Guidelines for Drinking Water Quality). pp.130. [Google Scholar]
- 38.FAO (Food and Agriculture Organization) 1983. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products. FAO Fishery Circular No. 464. 100pp. [Google Scholar]
- 39.Standards Organisation of Nigeria . Standards Organisation of Nigeria (SON); Abuja, Nigeria: 2007. Nigerian Standard for Drinking Water Quality. Nigerian Industrial Standard (NIS 554) pp. 14–17. [Google Scholar]
- 40.FAO/WHO (2011) Codex Alimentarius Commission, Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme, Alinorm 01/12A2001, (2011) 1–28.
- 41.Goering P.L., Waalkes M.P., Klaassen C.D. Toxicology of Cadmium. In: Goyer R.A., Cherian M.G., editors. Vol. 115. 1994. pp. 189–214. (Handbook of Experimental Pharmacology; Toxicology of Metals. Biochemical Effects). [Google Scholar]
- 42.Woodworth J.C., Pascoe V. Cadmium toxicity to rainbow trout (Salmon gairdneri). A study of eggs and alevins. J. Fish Biol. 1982;21:47–57. [Google Scholar]
- 43.Teo K.C., Chen Determination of cobalt and nickel in water using atomic absorption spectrometry after cloud point extraction. Anal. Chem. Acta. 2001;434(3):325–330. doi: 10.1039/b008717n. [DOI] [PubMed] [Google Scholar]
- 44.McKenzie H.A., Symthe L.E. Elsevier; Amsterdam: 1998. Quantitative Trace of Analysis of Biological Materials; pp. 511–525. [Google Scholar]
- 45.Salnikow K., Denkhaus E. Nickel essentiality, toxicity and carcinogenicity. Crit. Rev. Oncol. Haematol. 2002;42(1):35–56. doi: 10.1016/s1040-8428(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 46.United State Environmental Protection Authority (USEPA) 1986. Quality Criteria for Water.EPA-440/5-86-001, Office of Water Regulations Standards. Washington DC, USA. [Google Scholar]
- 47.Su G.S., Ramos G.B., Su M.L. Bioaccumulation and histopathological alteration of total lead in selected fishes from Manila Bay, Philippines. Saudi J. Biol. Sci. 2013:353–355. doi: 10.1016/j.sjbs.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos D.C., Matta S.L., Oliveira J.A., Santos J.A. Histological alterations in gills of Astyanax aff. bimaculatus caused by acute exposition to zinc. Exp. Toxicol. Pathol. 2012;64:861–866. doi: 10.1016/j.etp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Alabaster J.S., Lloyds R. second edition. Butterworths publication; London: 1982. Water Quality Criteria for Freshwater Fish; p. 361. [Google Scholar]
- 50.Everall N.C., MacFarlane N.A., Sedgwick R.W. The interactions of water hardness and pH with acute toxicity of zinc to brown trout, S. trutta, L. J. Fish Biol. 1989;35:27–36. [Google Scholar]
- 51.Hess R., Schmid B. Zinc supplement overdose can have toxic effects. J. Paediatr. Haematol./Oncol. 2002;24:582–584. [Google Scholar]
- 52.Wu C., Zhang L. Heavy metal concentrations and their possible sources in paddy soils of a modern agricultural zone, south eastern China. Environ. Earth Sci. 2010;60:45–56. [Google Scholar]
- 53.Oti W.J.O. Levels of heavy metal in bonny light crude oil. IOSR J. Appl. Chem. 2016;9(7):86–88. [Google Scholar]
- 54.Naveedullah N., Hashmi M.Z., Yu C.H., Shen H., Duan D., Shen C.H., Lou L., Chen Y. Risk assessment of heavy metals pollution in agricultural soils of Siling Reservoir Watershed in Zhejiang Province, China. J. Biomed. Biotechnol. 2013:1–10. doi: 10.1155/2013/590306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amirah M.N., Afiza A.S., Faizal W.I.W., Nurliyana M.H., Laili S. Human health risk assessment of metal contamination through consumption of fish. J. Environ. Pollut. Hum. Health. 2013;1(1):1–5. [Google Scholar]
- 56.Krishna P.V., Jyothirmayi V., Rao K.M. Human health risk assessment of heavy metal accumulation through fish consumption, from Machilipatnam Coast, Andhra Pradesh, India. Int. Res. J. Pub. Environ. Health. 2014;1(5):121–125. [Google Scholar]
- 57.Enuneku A.A., Ezemonye L.I., Ainerua M.O. Human health risk assessment of metal contamination through consumption of Sesarma angolense and Macrobrachium macrobrachion from Benin River, Nigeria. Eur. Int. J. Sci. Technol. 2014;3(6):77–86. [Google Scholar]